Developmental Stages of Bell Pepper Influence the Response to Far-Red Light Supplements in a Controlled Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Growth Conditions
2.2. Treatments
2.3. Photosynthetic Activity
2.4. Evaluation of Growth Parameters
2.5. Flower Monitoring and Production Yield Determination
2.6. Volume, Dry Matter, Thickness
2.7. Soluble Sugar Contents
2.8. Phenolic Content
2.9. Ascorbic Acid Content
2.10. Lycopene Content
2.11. Antioxidant Activities
2.12. Statistical Analysis
3. Results
3.1. Photosynthesis Rate Remains Similar for FR Supplements During the Vegetative and the Reproductive Phases
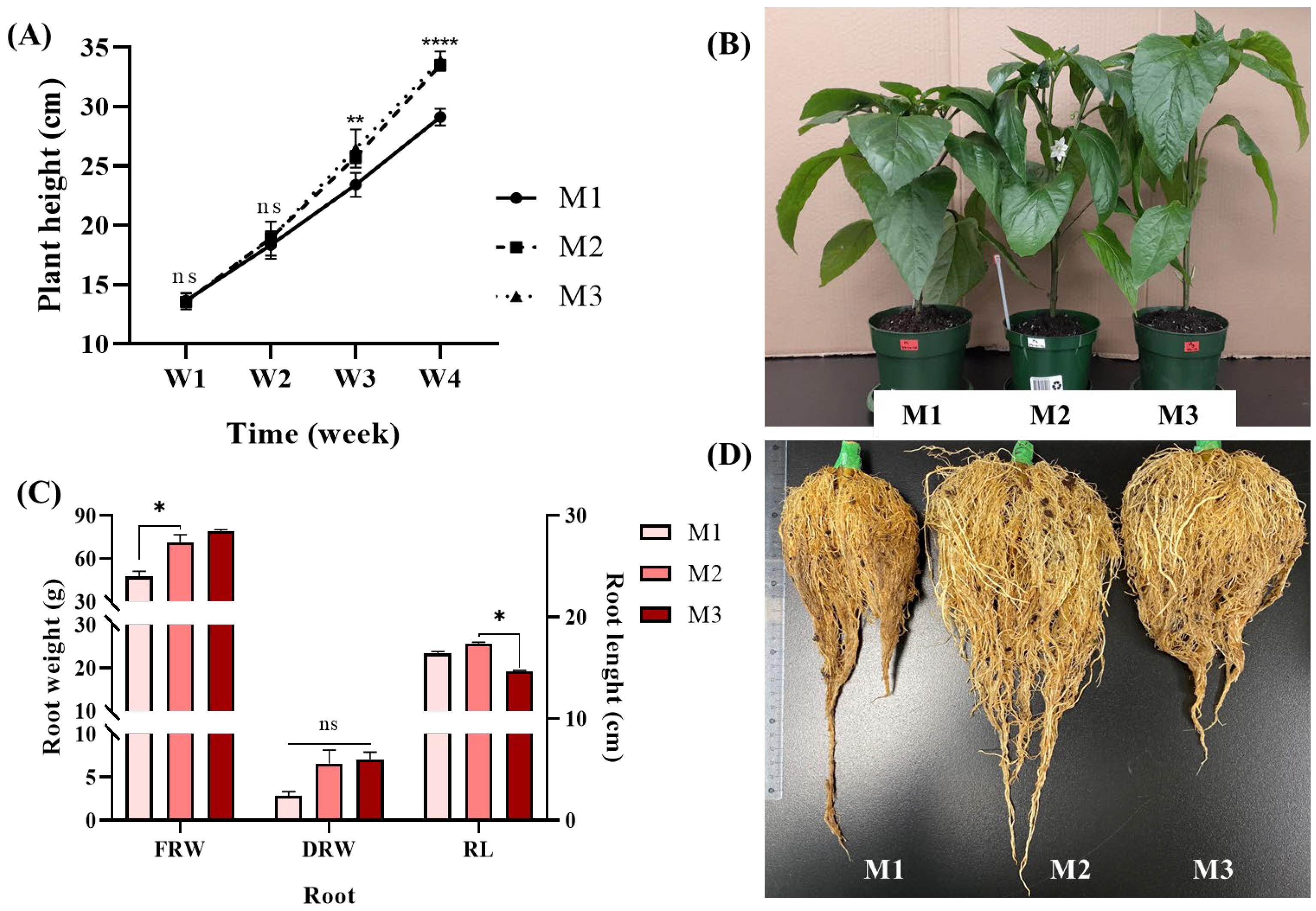
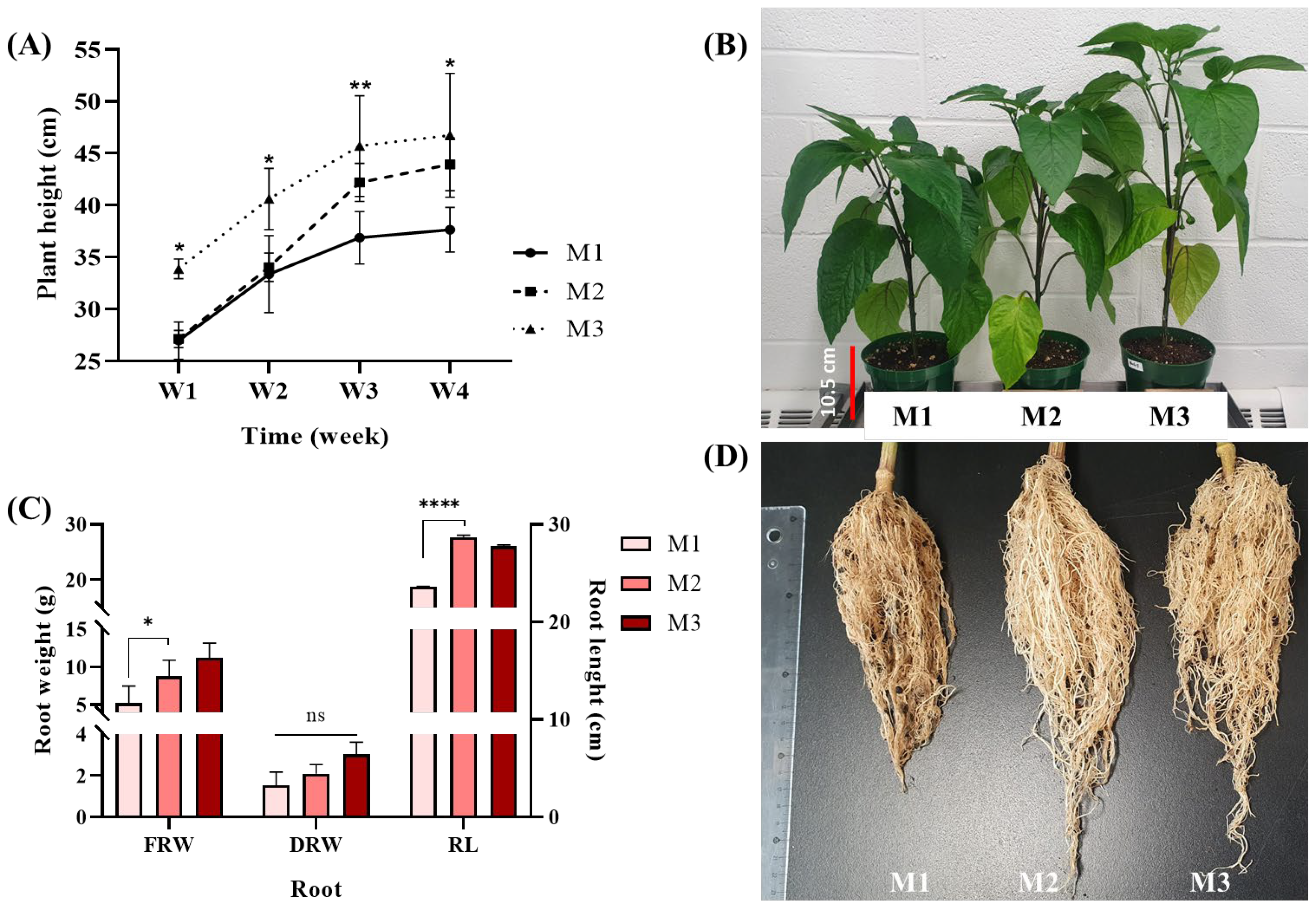
3.2. FR Supplements to the Lighting Stimulated Sweet Pepper Growth Both During the Vegetative Growth and the Reproductive Growth Phases
3.3. The Plant Developmental Stage Influences Fruit Yield and Time to Maturation in Response to FR Supplements
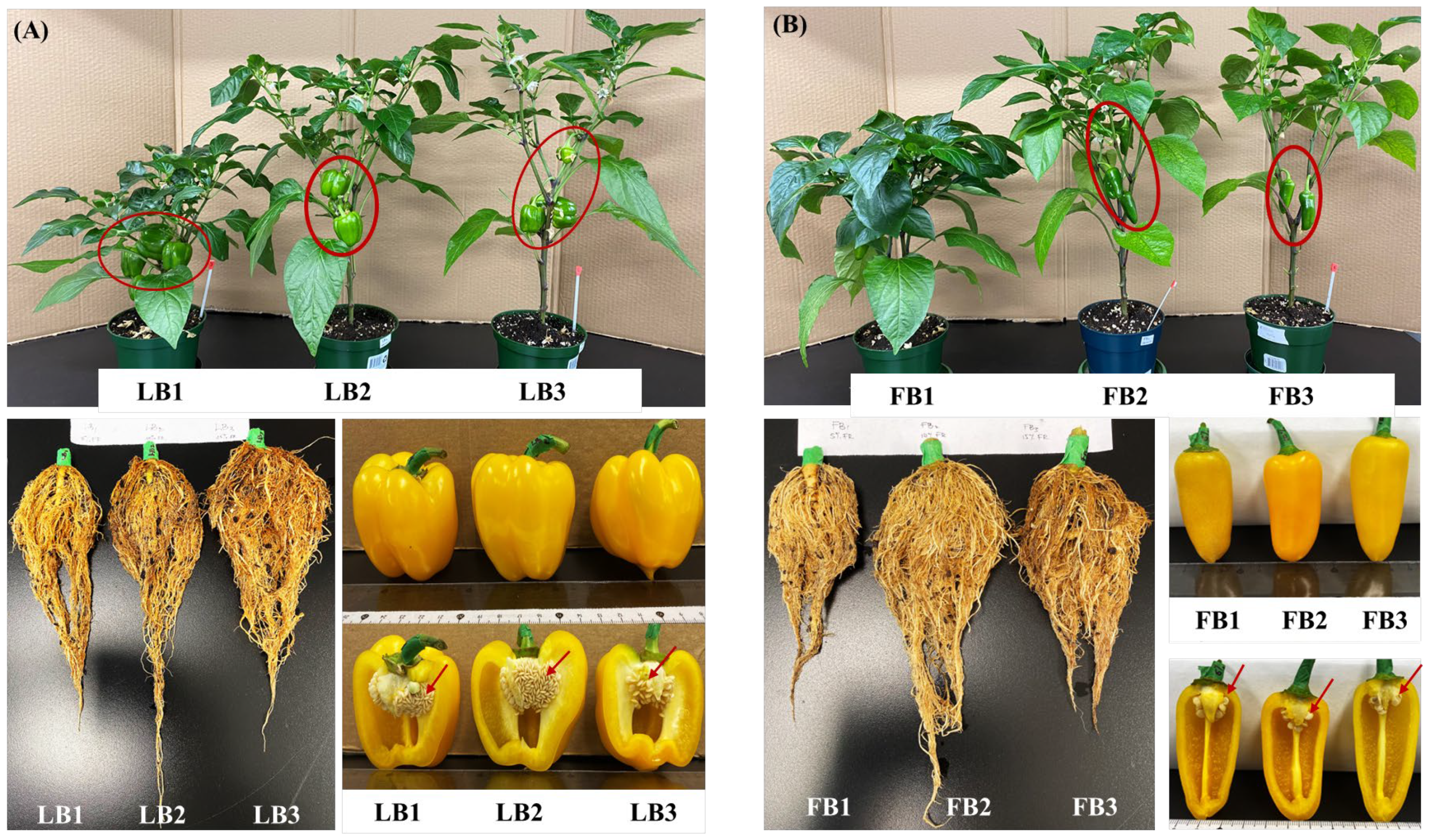
3.4. FR Modulates Bell Pepper Fruit Size and Morphology
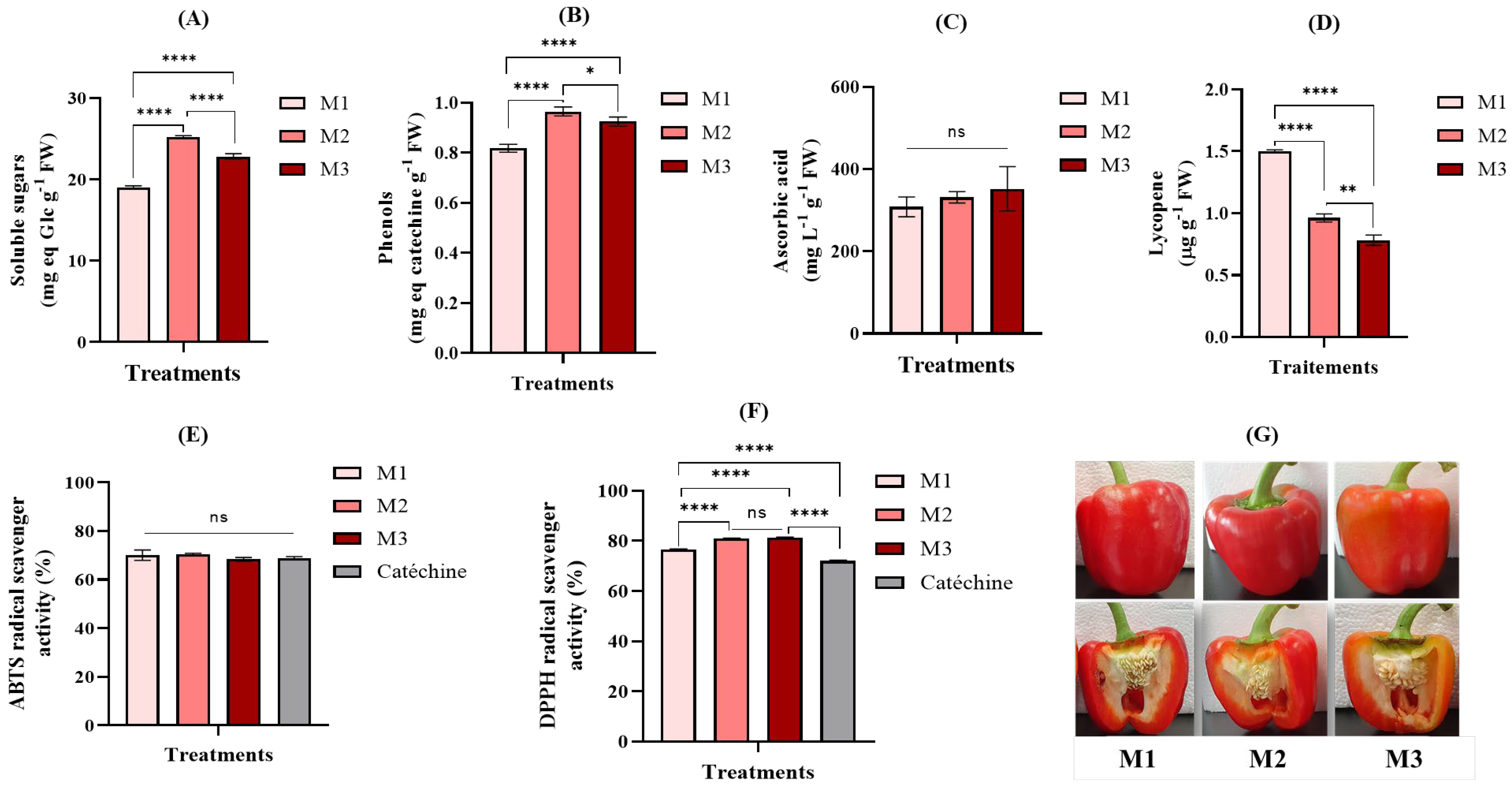
3.5. FR Increased Sugar, Phenolic Levels, and Vitamin C in Sweet Pepper Fruits Independently of the Plant Growth Phase at the Time of FR Addition to the Lighting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Assimilation |
| ABTS•+ | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) free radicals |
| Amax | Maximum assimilation |
| Ci | Intracellular CO2 |
| DCP | Dark compensation point |
| DLI | Daily light integral |
| DPPH | 2,2-diphenyl-1-picryhydrazyl |
| DR | Dark respiration |
| DRW | Dry root weight |
| FB | Fresh Bites |
| FR | Far-red |
| FRW | Fresh root weight |
| HPLC | High Performance Liquid Chromatography |
| LA | Leaf area |
| LB | Liberty Belle |
| LCP | Light compensation point |
| LEDs | Light emitting diodes |
| LR | Light respiration |
| M1 | 5.5 µmol m−2 s−1 far-red supplement |
| M2 | 12 µmol m−2 s−1 far-red supplement |
| M3 | 18.1 µmol m−2 s−1 far-red supplement |
| PFD | Photon flux density |
| PPFD | Photosynthetic photon flux density (μmol m−2 s−1) |
| R:FR | Photonic irradiance (666–775 nm)/photonic irradiance (725–735 nm) |
| RL | Root length |
| α | Apparent quantum yield |
| Ɛ | Carboxylation efficiency |
References
- Dueck, T.; Ieperen, V.W.; Taulavuori, K. Light perception, signalling and plant responses to spectral quality and photoperiod in natural and horticultural environments. Env. Exp. Bot. 2016, 121, 1–3. [Google Scholar] [CrossRef]
- De Wit, M.; Galvão, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Ann. Rev. Plant. Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef]
- Mccree, K.J. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 444–453. [Google Scholar] [CrossRef]
- Zhen, S.; Iersel, M.V.; Bugbee, B. Why Far-Red Photons Should Be Included in the Definition of Photosynthetic Photons and the Measurement of Horticultural Fixture Efficacy. Front. Plant Sci. 2021, 12, 693445. [Google Scholar] [CrossRef]
- Zhen, S.; Iersel, V.M.; Bugbee, B. Photosynthesis in sun and shade: The surprising importance of far-red photons. New Phytol. 2022, 236, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Haidekker, M.; Iersel, V.M. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Iersel, V.M. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Mitchell, C.A. Academic Research Perspective of LEDs for the Horticulture Industry. HortScience 2015, 50, 1293–1296. [Google Scholar] [CrossRef]
- Kim, D.; Moon, T.; Kwon, S.; Hwang, I.; Son, J.E. Supplemental inter-lighting with additional far-red to red and blue light increases the growth and yield of greenhouse sweet peppers (Capsicum annuum L.) in winter. Hortic. Environ. Biotechnol. 2023, 64, 83–95. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Gao, M.; Liu, R.H.; Su, W.; Liu, H. Far-Red-Light-Induced Morphology Changes, Phytohormone, and Transcriptome Reprogramming of Chinese Kale (Brassica alboglabra Bailey). Int. J. Mol. Sci. 2023, 24, 5563. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant. Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Ieperen, W.V.; Harbinson, J.; Meer, M.V.D.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lin, M.Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Kasperbauer, M.J.; Tso, T.C.; Soromn, T.P. Effects of end-of-day red and far-red radiation on free sugars, organic acids and amino acids of tobacco. Phytochemistry 1970, 9, 2091–2095. [Google Scholar] [CrossRef]
- Lanoue, J.; Little, C.; Hao, X. The Power of Far-Red Light at Night: Photomorphogenic, Physiological, and Yield Response in Pepper During Dynamic 24-Hour Lighting. Front. Plant Sci. 2022, 13, 857616. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs Make It Resilient: Effects on Plant Growth and Defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED Lighting: A Grower’s Guide to Light Spectra. Hort. Sci. 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Chen, S.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation increases flower and fruit abortion in sweet pepper (Capsicum annuum L.). Sci. Hortic. 2022, 305, 111386. [Google Scholar] [CrossRef]
- Chen, S.; Marcelis, L.F.; Offringa, R.; Kohlen, W.; Heuvelink, E. Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper. Plant Physiol. 2024, 195, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ouzounis, T.; Courbier, S.; Kaisier, E.; Nguyen, P.T.; Schouten, H.J.; Visser, R.G.; Pierick, R.; Marcelis, L.F.; Heuvelink, E. Far-red radiation increases dry mass partitioning to fruits but reduces Botrytis cinerea resistance in tomato. Environ. Exp. Bot. 2019, 168, 103889. [Google Scholar] [CrossRef]
- Courbier, S.; Snoek, B.L.; Kajala, K.; Li, L.; Wees, V.S.; Pierik, R. Mechanisms of far-red light-mediated dampening of defense against Botrytis cinerea in tomato leaves. Plant Physiol. 2021, 187, 1250–1266. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Schimmel, B.C.; Zhang, Y.; Wang, L.; Robert, C.A.; Glauser, G.; Ballare, C.L.; Erb, M. Far-red light increases maize volatile emissions in response to volatile cues from neighbouring plants. Plant Cell Environ. 2024, 47, 3979–3998. [Google Scholar] [CrossRef]
- Huber, M.; De Boer, H.J.; Romanowski, A.; Veen, H.V.; Buti, S.; Kahlon, P.S.; Meijden, J.V.; Koch, J.; Pierick, R. Far-red light enrichment affects gene expression and architecture as well as growth and photosynthesis in rice. Plant Cell Environ. 2024, 47, 2936–2953. [Google Scholar] [CrossRef]
- Wang, J.L.; Evers, J.B.; Anten, N.P.; Yang, L.Y.; Douma, J.C.; Schneider, H.M. Far-red light perception by the shoot influences root growth and development in cereal-legume crop mixtures. Plant Soil 2024, 1–18. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Funct. Plant Biol. 2000, 27, 87. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Ehleringer, J.R.; Mooney, H.A.; Rundel, P.W. Field Methods and Intrumentation. In Plant Physiological Ecology; Chapman and Hall: London, UK, 1989; p. 457. Available online: https://ecophys.utah.edu/uploads/3/1/8/3/31835701/106.pdf (accessed on 23 December 2024).
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Marigo, G. Méthode de fractionnement et d’estimation des composés phénoliques chez les végétaux. Analysis 1973, 2, 106–110. [Google Scholar]
- Benakmoum, A. Effet du lycopène sur certains paramètres structuraux et fonctionnels chez le rat en croissance. Ph.D. Thesis, École Nationale Supérieure Agronomique El Harrach Alger, Oued Smar, Algeria, 2009; p. 114. [Google Scholar]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Samaniego, S.C.; González, T.A.; García-Parrilla, M.C.; Granados, Q.J.; López García de la Serrana, H.; Martínez, L. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Xia, J.; Mattson, N. Daily Light Integral and Far-Red Radiation Influence Morphology and Quality of Liners and Subsequent Flowering and Development of Petunia in Controlled Greenhouses. Horticulturae 2024, 10, 1106. [Google Scholar] [CrossRef]
- Owen, W.G.; Meng, Q.; Lopez, R.G. Promotion of Flowering from Far-red Radiation Depends on the Photosynthetic Daily Light Integral. HortScience Horts 2018, 53, 465–471. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Su, Y.S.; Lagarias, J.C. Phytochrome structure and signaling mechanisms. Ann. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.; Yanofsky, M.F.; Coupland, G. Distinct Roles of CONSTANS Target Genes in Reproductive Development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Maier, A.; Lee, J.-H.; Laubinger, S.; Saijo, Y.; Wang, H.; Qu, L.-J.; Hoecker, U.; Deng, X.W. Biochemical characterization of Arabidopsis complexes containing constitutively photomorphogenic1 and suppressor of PHYA proteins in light control of plant development. Plant Cell 2008, 20, 2307–2323. [Google Scholar] [CrossRef]
- Lian, H.L.; He, S.-B.; Zhang, Y.-C.; Zhu, D.-M.; Zhang, J.-Y.; Jia, K.-P.; Sun, S.-X.; Li, L.; Yang, H.-Q. Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev. 2011, 25, 1023–1028. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, H.; Liu, B.; Liu, X.; Lin, C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 2011, 21, 841–847. [Google Scholar] [CrossRef]
- Hajdu, A.; Adam, E.; Sheerin, D.J.; Dobos, O.; Bernula, P.; Hiltbrunner, A.; Kozma-Bognar, L.; Nagy, F. High-level expression and phosphorylation of phytochrome B modulates flowering time in Arabidopsis. Plant J. 2015, 83, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor Regulation of CONSTANS Protein in Photoperiodic Flowering. Science 1979, 303, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Sheerin, D.J.; Menon, C.; Oven-Krockhaus, S.Z.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.-D.; Huq, E.; Hiltbrunner, A. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.L.; Schreuder, M.E.; Smith, H.; Koornneef, M.; Kendrick, R.E. Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. Plant J. 2000, 24, 345–356. [Google Scholar] [CrossRef]
- Bianchetti, E.R.; Lira, B.S.; Monteiro, S.S.; Demarco, D.; Purgatto, E.; Rothan, C.; Rossi, M.; Freschi, L. Fruit-localized phytochromes regulate plastid biogenesis, starch synthesis, and carotenoid metabolism in tomato. J. Exp. Bot. 2018, 69, 3573–3586. [Google Scholar] [CrossRef]
- Bianchetti, R.; Bellora, N.; De Haro, L.A.; Zuccarelli, R.; Rosado, D.; Freschi, L.; Rossi, M.; Bermudez, L. Phytochrome-Mediated Light Perception Affects Fruit Development and Ripening Through Epigenetic Mechanisms. Front Plant Sci. 2022, 13, 870974. [Google Scholar] [CrossRef]
- Wubs, A.M.; Ma, Y.; Heuvelink, E. Fruit Set and Yield Patterns in Six Capsicum Cultivars. HortScience 2009, 44, 1296–1301. [Google Scholar] [CrossRef]
- Wubs, A.M.; Ma, Y.; Heuvelink, E.; Marcelis, L.F. Genetic differences in fruit-set patterns are determined by differences in fruit sink strength and a source: Sink threshold for fruit set. Ann. Bot. 2009, 104, 957–964. [Google Scholar] [CrossRef]
- Hu, C.; Nawrocki, W.J.; Croce, R. Long-term adaptation of Arabidopsis thaliana to far-red light. Plant Cell Environ. 2021, 44, 3002–3014. [Google Scholar] [CrossRef]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef]
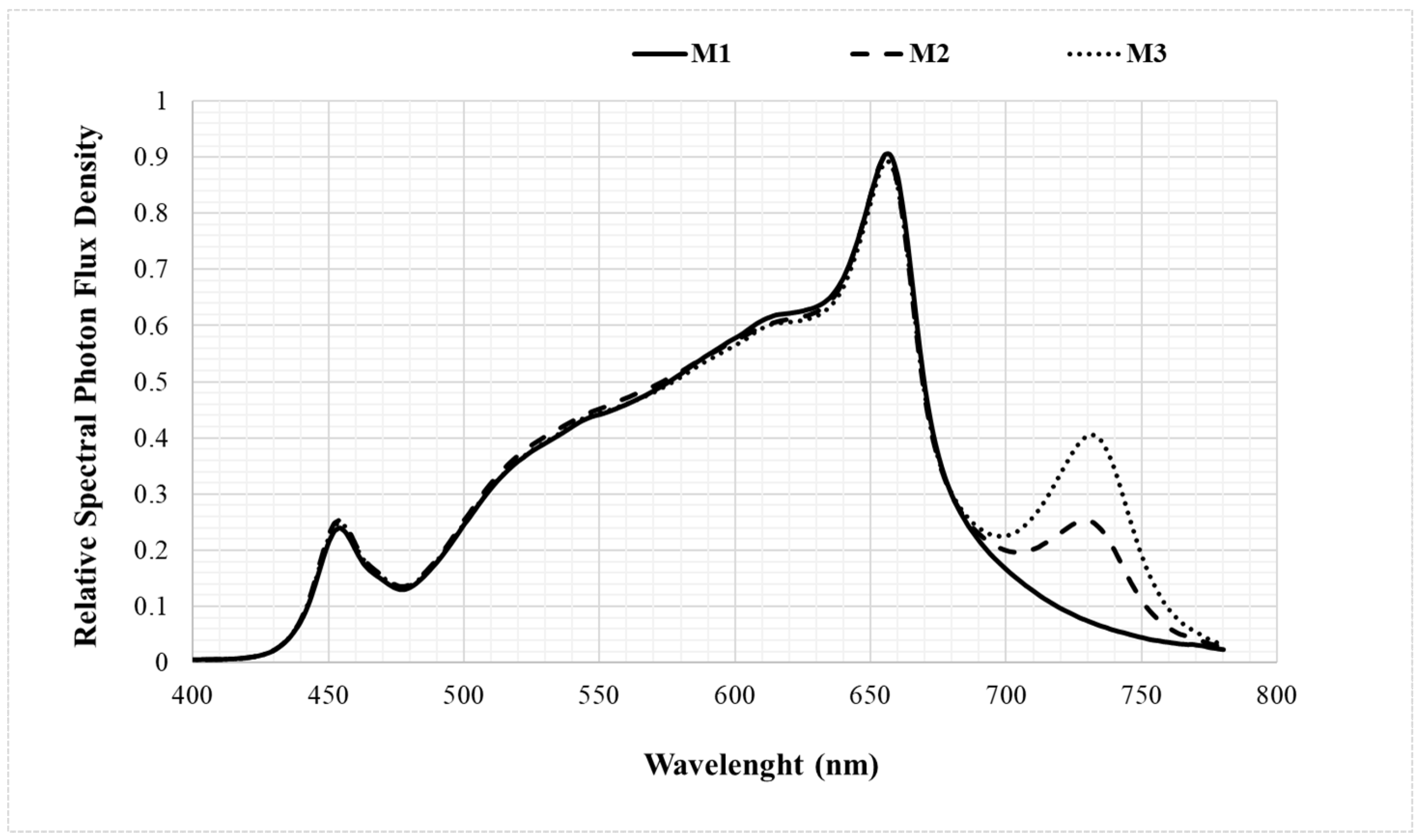

| Light Treatments | PPFD 2 (μmol m−2 s−1) | PFD 3 (μmol m−2 s−1) | PFD (μmol m−2 s−1) | R:B | R:FR 4 | R:G | |||
|---|---|---|---|---|---|---|---|---|---|
| Blue | Green | Red | FR 1 | ||||||
| M1 | 110.7 | 116.3 | 11.0 | 43.5 | 56.3 | 5.5 | 5.1 | 10.2 | 1.3 |
| M2 | 110.5 | 123.7 | 11.5 | 44.2 | 55.8 | 12.0 | 4.8 | 4.6 | 1.3 |
| M3 | 110.4 | 128.6 | 11.3 | 43.4 | 55.7 | 18.1 | 4.9 | 3.1 | 1.3 |
| Amax | LCP | LR | α | |
|---|---|---|---|---|
| Vegetative phase | ||||
| M1 | 11.32 ± 0.30a | 24.45 ± 6.70a | −1.19 ± 0.36a | 0.049 ± 0.001a |
| M2 | 13.30 ± 1.05a | 24.72 ± 3.35a | −1.22 ± 0.17a | 0.049 ± 0.001a |
| M3 | 12.11 ± 1.33a | 24.34 ± 0.84a | −1.12 ± 0.01a | 0.046 ± 0.001b |
| Reproductive phase | ||||
| M1 | 8.70 ± 1.70a | 13.61 ± 2.84a | −0.70 ± 0.14a | 0.052 ± 0.002a |
| M2 | 9.01 ± 1.64a | 08.56 ± 3.74a | −0.15 ± 3.78a | 0.039 ± 0.005b |
| M3 | 9.96 ± 0.90a | 12.65 ± 1.09a | −0.60 ± 0.11a | 0.047 ± 0.006ab |
| Amax | DCP | DR | Ɛ | |
|---|---|---|---|---|
| Vegetative phase | ||||
| M1 | 13.30 ± 1.22a | 57.76 ± 0.09a | −1.25 ± 0.14a | 0.022 ± 0.002a |
| M2 | 16.06 ± 0.00a | 70.90 ± 5.96a | −1.60 ± 0.09a | 0.023 ± 0.003a |
| M3 | 13.45 ± 2.76a | 66.74 ± 7.04a | −1.48 ± 0.10a | 0.022 ± 0.004a |
| Reproductive phase | ||||
| M1 | 13.74 ± 0.40a | 51.55 ± 1.54a | −1.04 ± 0.10a | 0.020 ± 0.002a |
| M2 | 13.57 ± 1.39a | 51.32 ± 2.62a | −0.78 ± 0.28a | 0.015 ± 0.005b |
| M3 | 13.95 ± 0.78a | 51.62 ± 6.01a | −0.58 ± 1.16a | 0.021 ± 0.006a |
| Vegetative Stage | Reproductive Stage | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | |
| Plant height (cm) | 29.1 ± 0.71b | 33.48 ± 0.47a | 33.88 ± 0.76a | 37.63 ± 2.16b | 43.93 ± 2.54a | 46.73 ± 5.96a |
| Leaf number | 42.5 ± 2.38a | 41.75 ± 1.26a | 33.0 ± 0.82b | N/A | N/A | N/A |
| Leaf area (cm2) | 101.1 ± 12.52a | 96.87 ± 9.88a | 90.1 ± 9.49a | N/A | N/A | N/A |
| Stem diameter (mm) | 8.39 ± 0.35a | 8.71 ± 0.51a | 8.64 ± 0.23a | 8.36 ± 0.63a | 8.89 ± 0.45a | 8.83 ± 0.19a |
| Nodes number | 8.25 ± 0.50a | 8.5 ± 0.58a | 9.00 ± 0.0a | 20.33 ± 0.58a | 19.33 ± 2.31a | 17.0 ± 1.0a |
| Internodes length (cm) | 2.92 ± 0.07b | 3.32 ± 0.16a | 3.37 ± 0.09a | 1.78 ± 0.11a | 2.08 ± 0.28a | 2.53 ± 0.55a |
| Vegetative Stage | Reproductive Stage | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | |
| Time to flowering (days) 1 | 46.25 ± 0.50c | 49.5 ± 1.0b | 55.75 ± 1.5a | N/A | N/A | N/A |
| Number of flowers 2 | 10.25 ± 1.26a | 7.5 ± 1.29b | 3.5 ± 1.29c | 9.33 ± 0.58a | 3.33 ± 0.58c | 5.67 ± 0.58b |
| Survival time of fallen flowers (days) 2 | 7.67 ± 1.16b | 13.67 ± 1.53a | 13.67 ± 0.58a | 7.75 ± 1.26b | 12.25 ± 0.96a | 11.0 ± 3.56ab |
| Fruits set (%) 2 | 36.36 ± 8.17a | 50.0 ± 5.77a | 33.3 ± 5.0a | 33.3 ± 11.5a | 26.7 ± 5.8a | 40.0 ± 10.0a |
| Time to fruit set (days) 2 | 3.50 ± 0.58a | 4.0 ± 0.82a | 3.25 ± 0.5a | 4.67 ± 0.58a | 4.67 ± 0.58a | 4.33 ± 0.58a |
| Ripening time (days) 2 | 59.75 ± 1.26a | 59.75 ± 0.96a | 54.5 ± 1.92b | 63.67 ± 0.58b | 69.67 ± 2.52ab | 72.0 ± 2.65a |
| Fruits collected 3 | 40 | 56 | 42 | 38 | 46 | 36 |
| Yields (kg/14 plants) 3 | 4.692 | 8.226 | 6.1698 | 3.590 | 5.385 | 4.281 |
| FR Treatment: | Beginning at Vegetative Stage | Beginning at Reproductive Stage | ||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | |
| Thickness (mm) | 9.80 ± 0.44ab | 10.90 ± 0.82a | 8.80 ± 0.45b | 8.95 ± 0.34a | 8.33 ± 0.60a | 5.40 ± 0.25b |
| Volume (cm3) | 129.06 ± 24.01a | 184.20 ± 33.43a | 181.79 ± 35.72a | 121.41 ± 12.09a | 151.73 ± 13.16a | 158.77 ± 28.24a |
| Dry matter (%) | 7.85 ± 0.55a | 7.46 ± 0.56a | 6.77 ± 0.68a | 8.30 ± 0.14a | 6.64 ± 0.05a | 6.98 ± 0.37a |
| Weight (g) | 117.30 ± 2.66b | 146.89 ± 5.48a | 146.90 ± 5.59a | 94.47 ± 5.43b | 117.06 ± 2.75a | 118.91 ± 1.64a |
| FR Treatments: | Beginning at Vegetative Stage | Beginning at Reproductive Stage | ||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M1 | M2 | M3 | |
| Glucose (mg g−1 MF) | 18.96 ± 0.50b | 19.70 ± 0.18a | 18.50 ± 0.09b | 17.52 ± 1.03a | 16.77 ± 0.65a | 16.56 ± 0.01a |
| Fructose (mg g−1 MF) | 16.55 ± 0.08b | 18.49 ± 0.04a | 16.23 ± 0.10b | 14.96 ± 1.06a | 15.89 ± 0.78a | 14.26 ± 0.15a |
| Sucrose (mg g−1 MF) | 0.73 ± 0.13a | 0.77 ± 0.19a | 0.38 ± 0.02b | 0.70 ± 0.07a | 0.81 ± 0.10a | 0.55 ± 0.01b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouliom-Ntapnze, A.M.; Fangue-Yapseu, G.Y.; Missihoun, T.D. Developmental Stages of Bell Pepper Influence the Response to Far-Red Light Supplements in a Controlled Environment. Agronomy 2025, 15, 732. https://doi.org/10.3390/agronomy15030732
Mouliom-Ntapnze AM, Fangue-Yapseu GY, Missihoun TD. Developmental Stages of Bell Pepper Influence the Response to Far-Red Light Supplements in a Controlled Environment. Agronomy. 2025; 15(3):732. https://doi.org/10.3390/agronomy15030732
Chicago/Turabian StyleMouliom-Ntapnze, Awa Marina, Georges Yannick Fangue-Yapseu, and Tagnon D. Missihoun. 2025. "Developmental Stages of Bell Pepper Influence the Response to Far-Red Light Supplements in a Controlled Environment" Agronomy 15, no. 3: 732. https://doi.org/10.3390/agronomy15030732
APA StyleMouliom-Ntapnze, A. M., Fangue-Yapseu, G. Y., & Missihoun, T. D. (2025). Developmental Stages of Bell Pepper Influence the Response to Far-Red Light Supplements in a Controlled Environment. Agronomy, 15(3), 732. https://doi.org/10.3390/agronomy15030732








