The Half-Heading Stage May Represent the Optimal Harvest Time for the First Cut of Tall Wheatgrass
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth
2.2. DM and Forage Nutritive Value Measurement
2.3. RNA Extraction and First Strand cDNA Synthesis
2.4. Gene Expression Analysis
2.5. Data Summary and Statistics
3. Results
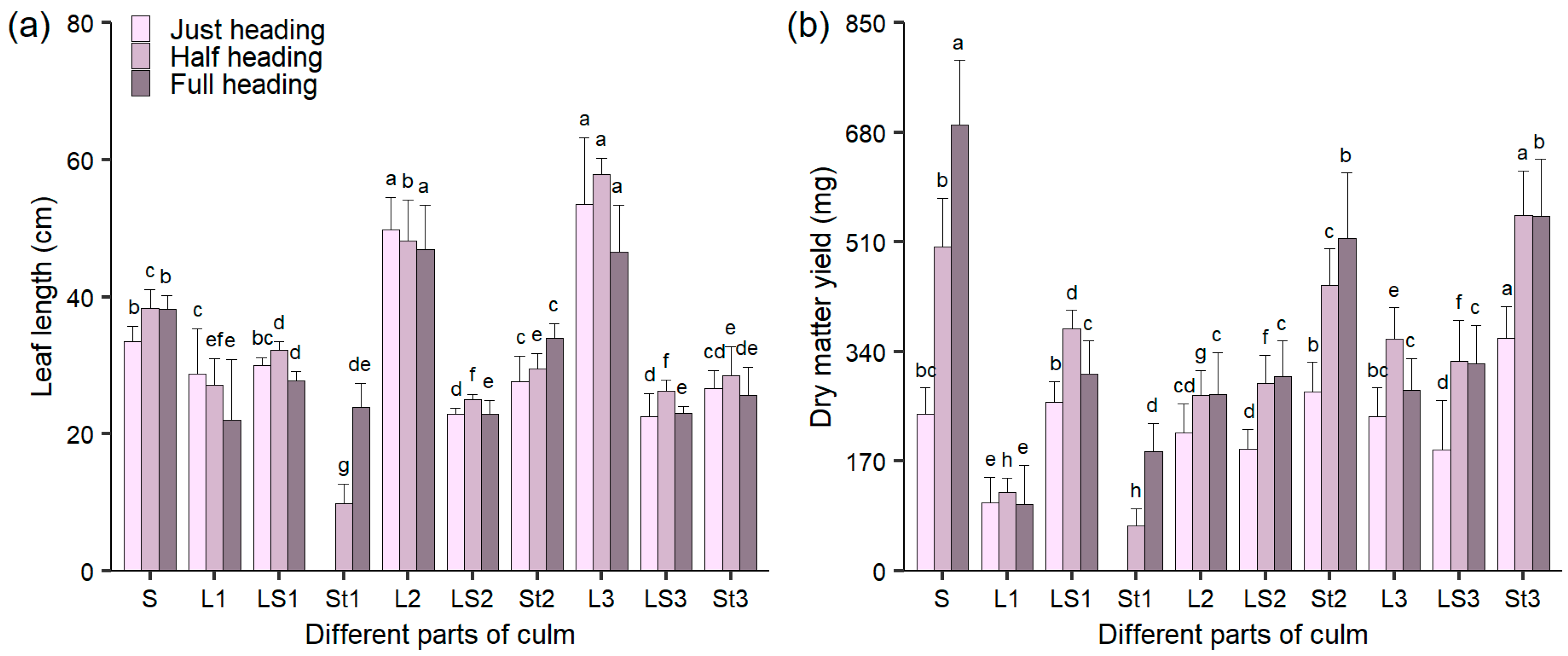
3.1. The Length and DM in Different Culm Parts During Heading Stage
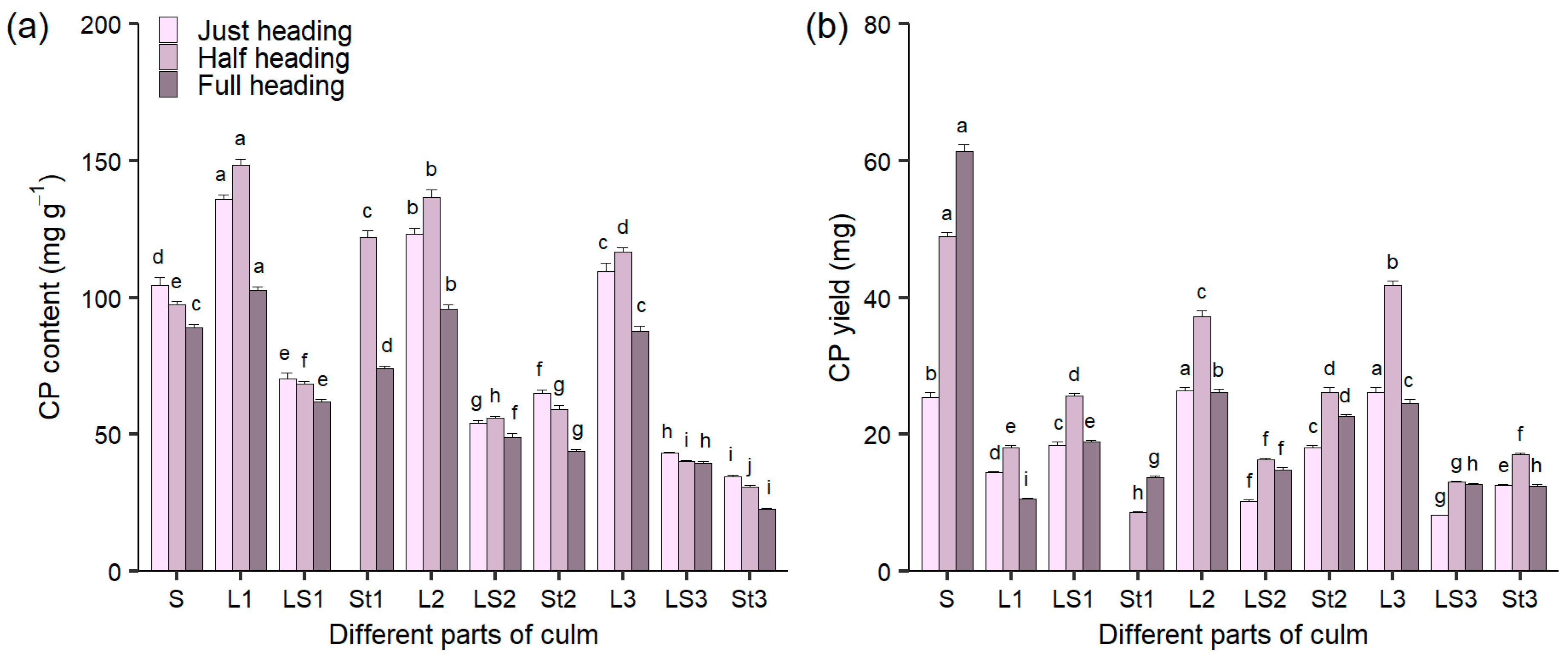
3.2. The Content and Yield of CP in Different Culm Parts During Heading Stage
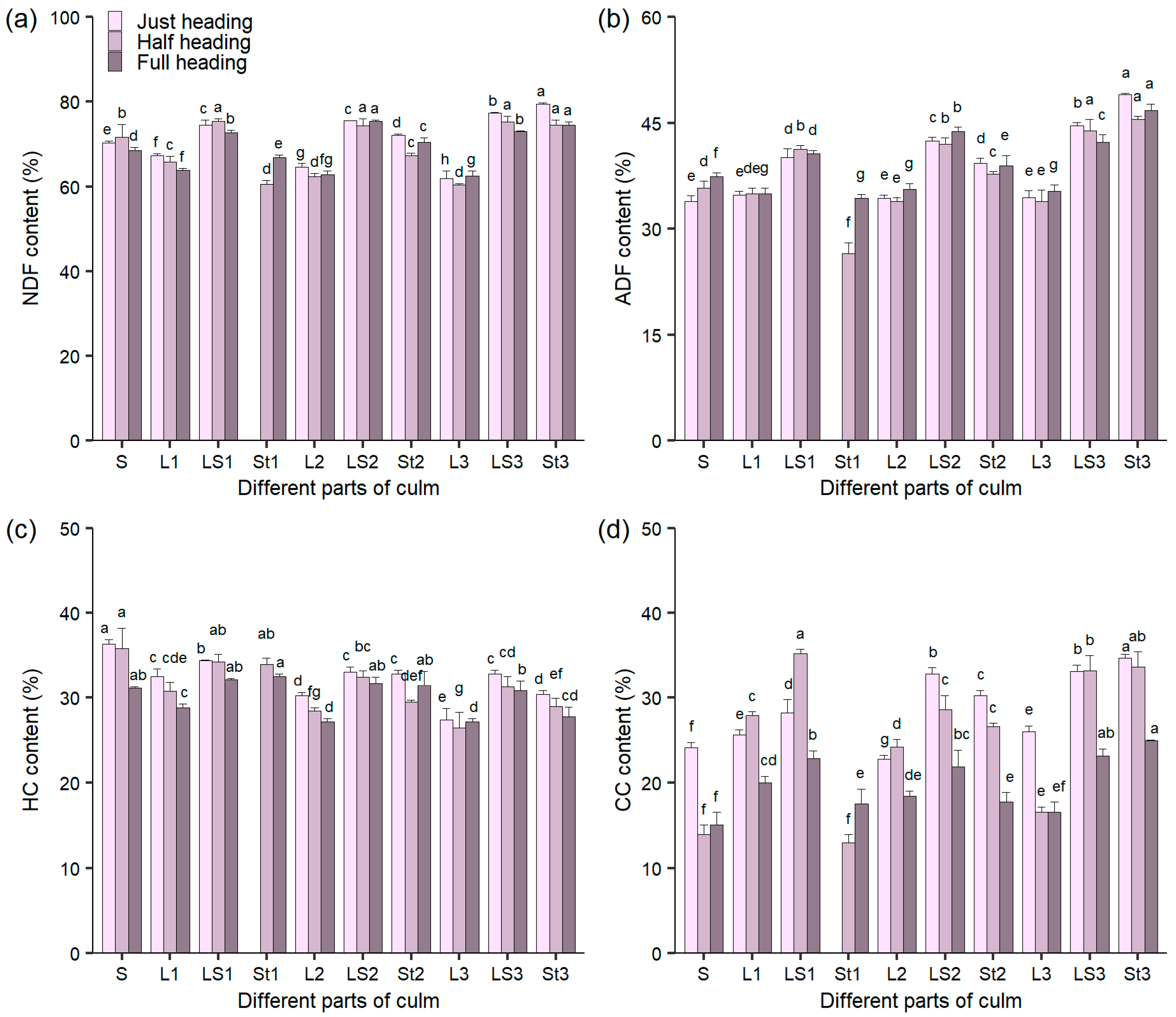
3.3. The Content of Fiber and Cellulose in Different Culm Parts During Heading Stage
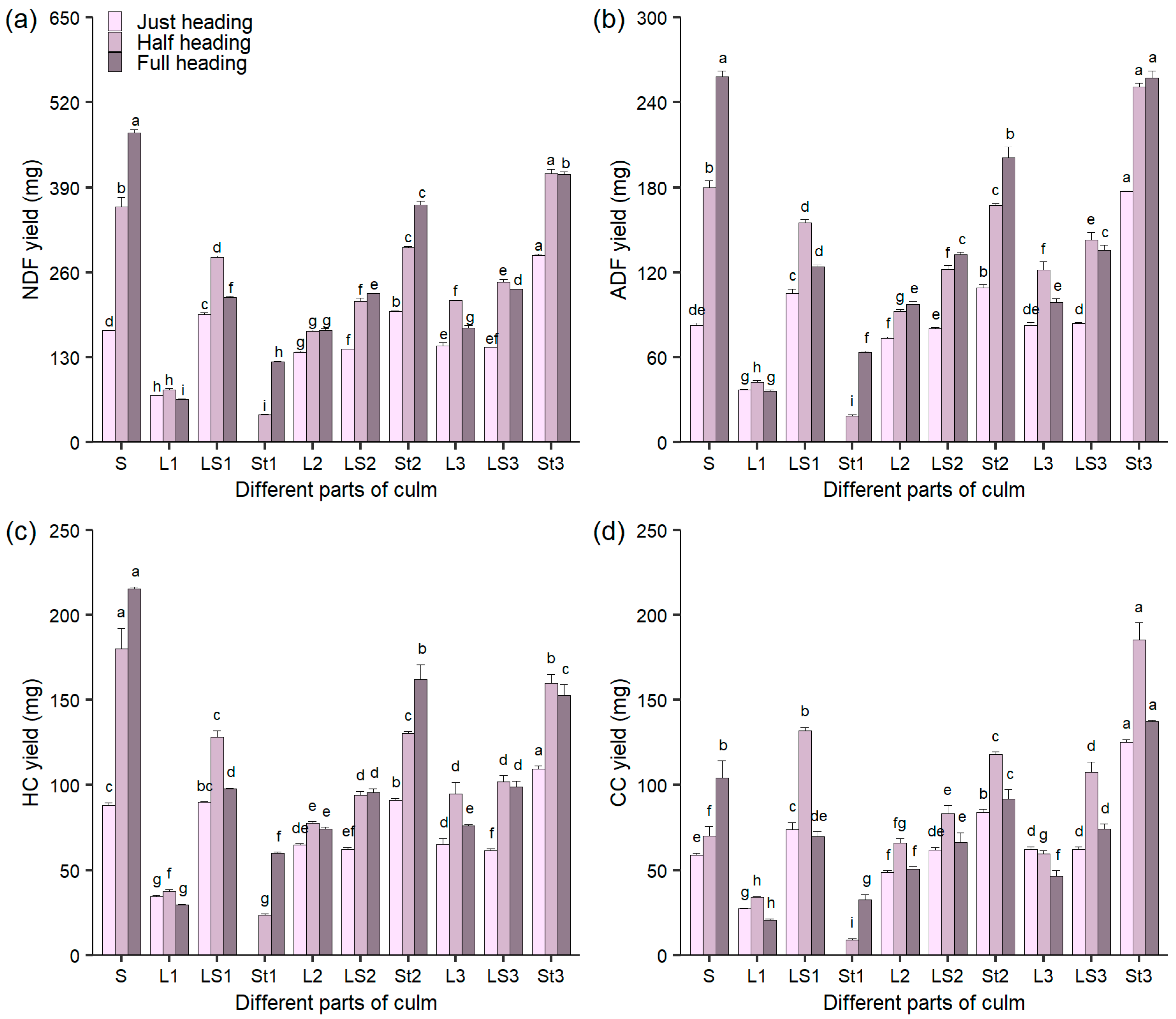
3.4. The Yields of Fiber and Cellulose in Different Culm Parts During Heading Stage
3.5. The Content and Yield of ADL in Different Culm Parts During Heading Stage
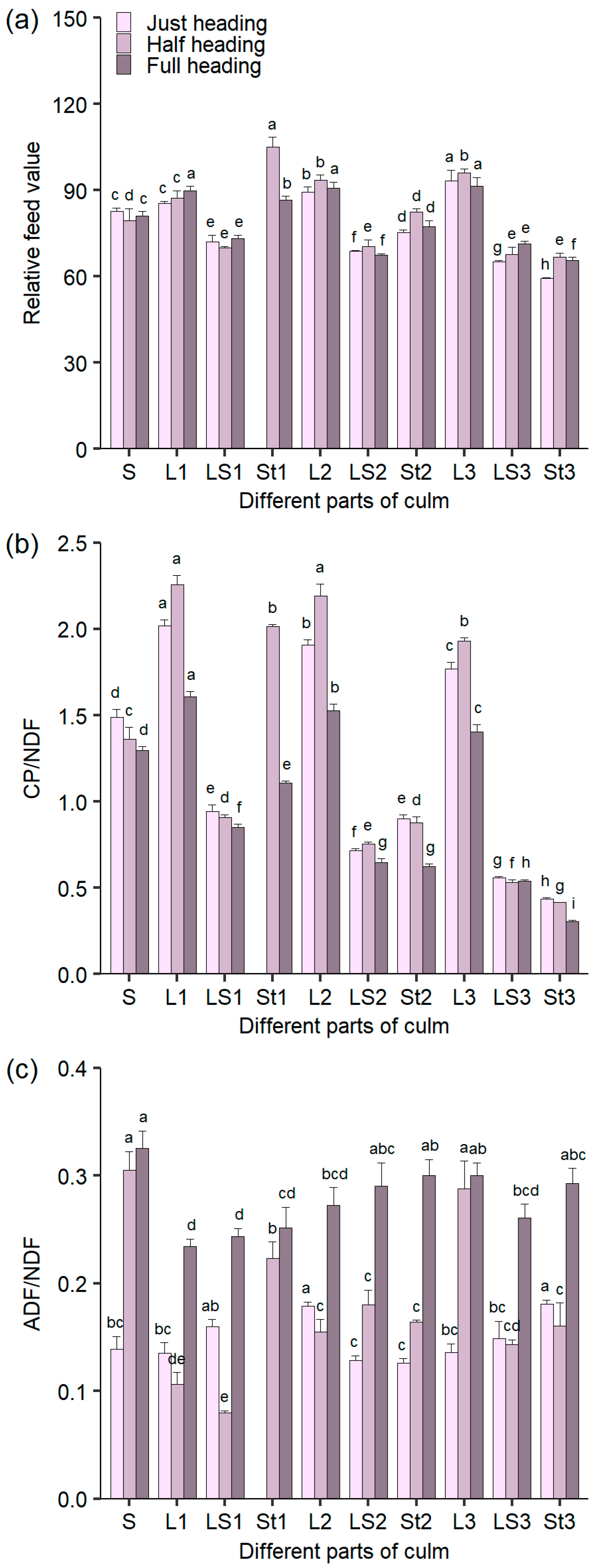
3.6. RFV and the Ratios of CP/NDF and ADL/NDF in Different Parts of Culms During the Heading Stage
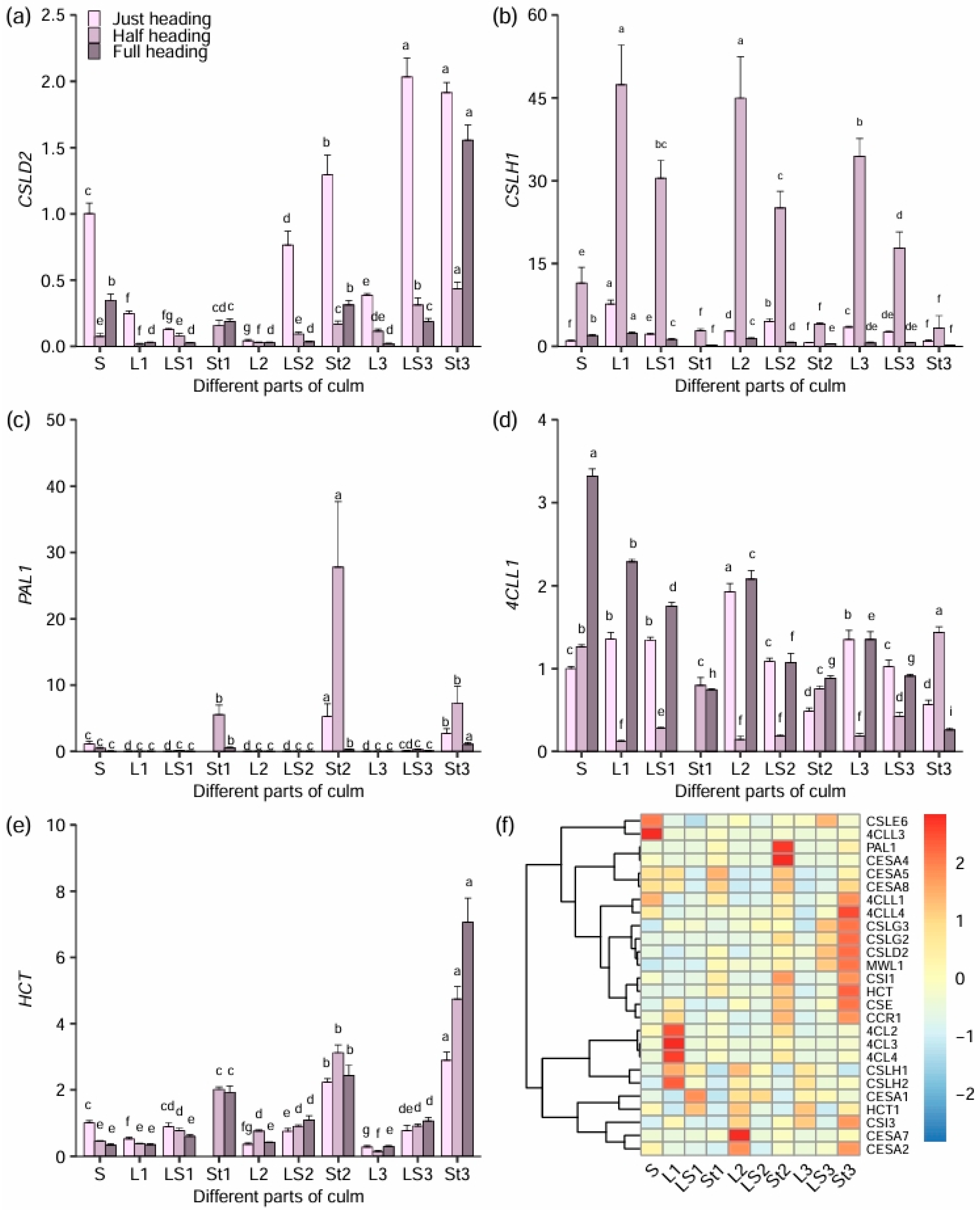
3.7. Expressional Responses of the Lignin and Cellulose Biosynthesis Genes in Different Culm Parts to Heading Stage
3.8. Correlations Between Expression Levels of Cellulose and Lignin Biosynthesis Genes and Forage Nutrient Value During Heading Stage
4. Discussion
4.1. Half Heading Stage May Represent the Optimal Harvesting Time for the First Cut of Tall Wheatgrass
4.2. Leaf/Stem Ratio Reflects Forage Nutritive Value of Tall Wheatgrass
4.3. Cellulose and Lignin Biosynthesis Genes Expressed Differentially in Different Culm Parts of Tall Wheatgrass During Heading Stage
4.4. Low Lignin Content May Be an Index for Tall Wheatgrass Genetic Improvement for High-Quality Forage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | crude protein |
| NDF | neutral detergent fiber |
| ADF | acid detergent fiber |
| ADL | acid detergent lignin |
| CC | crude cellulose |
| HC | hemicellulose |
| DM | dry matter |
| RFV | relative feed value |
| DDM | digestible dry matter |
| DMI | dry matter intake |
| DMY | dry matter yield |
| NDFY | neutral detergent fiber yield |
| ADFY | acid detergent fiber yield |
| HCY | hemicellulose yield |
| CCY | crude cellulose yield |
References
- Asay, K.H.; Jensen, K.B. Wheatgrasses. In Cool-Season Forage Grasses; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy (ASA): Madison, WI, USA, 1996; pp. 691–724. [Google Scholar] [CrossRef]
- Andrioli, R.J. Adaptive mechanisms of tall wheatgrass to salinity and alkalinity stress. Grass Forage Sci. 2023, 78, 23–36. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Q.; Wang, J.; Sun, H.; Zhang, K.; Fang, H.; Xing, X.; Yang, W.; Cao, X.; Liu, X. Industrialization of tall wheatgrass for construction of “Coastal Grass Belt”. Bull. Chin. Acad. Sci. 2023, 38, 622–631. [Google Scholar] [CrossRef]
- Hou, R.; Ouyang, Z.; Liu, Z.; Lai, J.; Sun, Z.; Li, Y.; Li, H.; Li, Z.; Li, J. “Coastal Grass Belt” as paradigm for grass-based livestock husbandry around Bohai bay. Bull. Chin. Acad. Sci. 2021, 36, 652–659. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Q.; Li, B.; Zhao, M.; Li, Z. Progress in research on tall wheatgrass as a salt-alkali tolerant forage grass. Acta Pratacult. Sin. 2022, 31, 190–199. [Google Scholar] [CrossRef]
- Wang, T.; Cao, L.; Liu, Z.; Yang, Q.; Chen, L.; Chen, M.; Jing, H. Forage grass basic biology of constructing Coastal Grass Belt. Chin. Bull. Bot. 2022, 57, 837–847. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Liu, X.; Xie, Q.; Yang, W.; Cao, X.; Li, Z. Scientific and technological reasons, contents and corresponding policies of constructing “Coastal Grass Belt”. Bull. Chin. Acad. Sci. 2022, 37, 238–245. [Google Scholar] [CrossRef]
- Rumery, M.G.; Ramig, R.E.; Somerhalder, B.R. Irrigated Bromegrass, Intermediate, and Tall Wheatgrass Pastures for Dairy Cows; University of Nebraska: Lincoln, NE, USA, 1964. [Google Scholar]
- Warren, B.E.; Casson, T. Performance of sheep grazing salt tolerant forages on revegetated salt land. Proc. Aust. Soc. 1992, 19, 237. [Google Scholar]
- Harmoney, K.; Jaeger, J. Tall wheatgrass and western wheatgrass used for complementary cool-season forage systems. Crop Forage Turfgrass Manag. 2019, 5, 180065. [Google Scholar] [CrossRef]
- Castellaro, G.; Giorgio, L.; Urra, A.; Hernán, A.; Hidalgo, A.; Javier, A.; Orellana, M.; Carla, L.; Escanilla, C.; Juan, P. Sheep and goat grazing diets on an annual Mediterranean grassland containing tall wheatgrass (Thinopyrum ponticum (PODP.)). Cienc. Investig. Agrar. 2018, 45, 240–250. [Google Scholar] [CrossRef]
- Conard, E.C.; Clanton, D.C. Cool-season, warm-season pastures needed. In Beef Cattle Progress Report; Animal Husbandry Department, College of Agriculture, University of Nebraska: Lincoln, NE, USA, 1963; pp. 11–13. [Google Scholar]
- Newell, L.C.; Moline, W.J. Forage quality evaluations of twelve grasses in relation to season of grazing. Agric. Food Sci. 1978. [Google Scholar]
- Ma, Z. An investigation on the economic characteristic and nutrient components of grasses. Chin. J. Grassl. 1986, 2, 48–51. [Google Scholar]
- Gillen, R.; Berg, W. Response of perennial cool-season grasses to clipping in the southern plains. Agron. J. 2005, 97, 125–130. [Google Scholar] [CrossRef]
- Jafari, A.A.; Anvari, H.; Nakhjavan, S.; Rahmani, E. Effects of phenological stages on yield and quality traits in 22 populations of tall wheatgrass Agropyron elongatum grown in Lorestan, Iran. J. Rangel. Sci. 2010, 1, 9–16. [Google Scholar] [CrossRef]
- Vogel, K.P.; Moore, K.J. Forage yield and quality of tall wheatgrass accessions in the USDA germplasm collection. Crop Sci. 1998, 38, 509–512. [Google Scholar] [CrossRef]
- Holechek, J.L.; Estell, R.E.; Kuykendall, C.B.; Valdez, R.; Cardenas, M.; Hernández, G.H. Seeded wheatgrass yield and nutritive quality on New Mexico big sagebrush range. J. Range Manag. 1989, 42, 118–122. [Google Scholar] [CrossRef]
- Li, W.; Xiao, Q.; Li, H.; Chang, H.; Zheng, Q.; Li, B.; Li, Z. Two cut system suggested for tall wheatgrass to balance herbage yield and quality in the coastal saline–alkaline land around the Bohai Sea. Grassl. Res. 2024, 3, 132–146. [Google Scholar] [CrossRef]
- Chen, H. Chemical Composition and Structure of Natural Lignocellulose In Biotechnology of Lignocellulose: Theory and Practice; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. [Google Scholar] [CrossRef]
- Lemaire, G.; Belanger, G. Allometries in Plants as Drivers of Forage Nutritive Value: A Review. Agriculture 2020, 10, 5. [Google Scholar] [CrossRef]
- Weimer, P.J. Why Don’t Ruminal Bacteria Digest Cellulose Faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
- Jung, H.; Samac, D.A.; Sarath, G. Modifying crops to increase cell wall digestibility. Plant Sci. 2012, 185–186, 65–77. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Grev, A.M.; Wells, M.S.; Catalano, D.N.; Martinson, K.L.; Jungers, J.M.; Sheaffer, C.C. Stem and leaf forage nutritive value and morphology of reduced lignin alfalfa. Agron. J. 2020, 112, 406–417. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lam, P.Y.; Lee, M.H.; Jeon, H.S.; Tobimatsu, Y.; Park, O.K. The Arabidopsis R2R3 MYB Transcription Factor MYB15 Is a Key Regulator of Lignin Biosynthesis in Effector-Triggered Immunity. Front. Plant Sci. 2020, 11, 583153. [Google Scholar] [CrossRef]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.P.; Vuylsteke, M.; et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef]
- Chen, F.; Srinivasa Reddy, M.S.; Temple, S.; Jackson, L.; Shadle, G.; Dixon, R.A. Multi-site genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wall-bound ferulic acid in alfalfa (Medicago sativa L.). Plant J. 2006, 48, 113–124. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Wang, J.P.; Matthews, M.L.; Williams, C.M.; Shi, R.; Yang, C.; Tunlaya-Anukit, S.; Chen, H.C.; Li, Q.; Liu, J.; Lin, C.Y.; et al. Improving wood properties for wood utilization through multi-omics integration in lignin biosynthesis. Nat. Commun. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y. Expression of brown-midrib in a spontaneous sorghum mutant is linked to a 5′-UTR deletion in lignin biosynthesis gene SbCAD2. Sci. Rep. 2017, 7, 11664. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps, R Package Version 1.0.12. 2018. Available online: https://github.com/raivokolde/pheatmap (accessed on 16 March 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 16 March 2025).
- Wilson, J. Variation of leaf characteristics with level of insertion on a grass tiller. 1. Development rate, chemical composition and dry matter digestibility. Crop Pasture Sci. 1976, 27, 343–354. [Google Scholar] [CrossRef]
- Norton, B.W. Limitations caused by chemical composition and digestibility. In Nutritional Limits to Animal Production from Pastures; Hacker, J.B., Ed.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1984; pp. 87–110. [Google Scholar]
- Albrecht, K.A.; Wedin, W.F.; Buxton, D.R. Cell-wall composition and digestibility of alfalfa stems and leaves. Crop Sci. 1987, 27, 735–741. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Martin, N.P.; Lamb, J.; Cuomo, G.R.; Jewett, J.G.; Quering, S.R. Leaf and stem properties of alfalfa entries. Agron. J. 2000, 92, 733–739. [Google Scholar] [CrossRef]
- Ghasemi, E.; Ghorbani, G.R.; Khorvash, M.; Emami, M.R.; Karimi, K. Chemical composition, cell wall features and degradability of stem, leaf blade and sheath in untreated and alkali-treated rice straw. Animal 2013, 7, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Sierra, P.; Cid, M.; Brizuela, M.; Ferri, C. Microhistological estimation of grass leaf blade percentages in pastures and diets. Rangel. Ecol. Manag. 2005, 58, 207–214. [Google Scholar] [CrossRef]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Höfte, H.; Gonneau, M.; Vernhettes, S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar] [CrossRef]
- Persson, S.; Paredez, R.A.; Carroll, A.; Palsdottir, H.; Doblin, M.; Poindexter, P.; Khitrov, N.; Auer, M.; Somerville, R.C. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 15566–15571. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Gui, J.; Shen, J.; Li, L. Functional characterization of evolutionarily divergent 4-coumarate:coenzyme a ligases in rice. Plant Physiol. 2011, 157, 574–586. [Google Scholar] [CrossRef]
- Yang, J.; Chen, F.; Yu, O.; Beachy, R.N. Controlled silencing of 4-coumarate:CoA ligase alters lignocellulose composition without affecting stem growth. Plant Physiol. Biochem. 2011, 49, 103–109. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Rajasekar, S.; Dixon, R.A.; Monteros, M.J. Agronomic Performance and Lignin Content of HCT Down-Regulated Alfalfa (Medicago sativa L.). Bioenergy Res. 2018, 11, 505–515. [Google Scholar] [CrossRef]
- Zheng, K.; Cai, Y.; Qu, Y.; Teng, L.; Wang, C.; Gao, J.; Chen, Q. Effect of the HCT Gene on Lignin Synthesis and Fiber Development in Gossypium barbadense. Plant Sci. 2023, 338, 111914. [Google Scholar] [CrossRef]
- Vignols, F.; Rigau, J.; Torres, M.A.; Capellades, M.; Puigdomènech, P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell 1995, 7, 407–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vermerris, W.; Thompson, K.J.; McIntyre, L.M. The maize Brown midrib1 locus affects cell wall composition and plant development in a dose-dependent manner. Heredity 2002, 88, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, J.M.; Moreta, D.E.; Repka, A.; Bradbury, P.; Nelson, R.J. Brown midrib mutant and genome-wide association analysis uncover lignin genes for disease resistance in maize. Plant Genome 2023, 16, e20278. [Google Scholar] [CrossRef]
- Li, X.; Chapple, C. Understanding lignification: Challenges beyond monolignol biosynthesis. Plant Physiol. 2010, 154, 449–452. [Google Scholar] [CrossRef]
- Grev, A.M.; Wells, M.S.; Samac, D.A.; Martinson, K.L.; Sheaffer, C.C. Forage accumulation and nutritive value of reduced lignin and reference alfalfa cultivars. Agron. J. 2017, 109, 2749–2761. [Google Scholar] [CrossRef]


| RFV | ADF | NDF | CC | ADL | HC | |
|---|---|---|---|---|---|---|
| CP | 0.832 ** | −0.851 ** | −0.781 ** | −0.420 ** | −0.302 ** | −0.054 |
| RFV | −0.954 ** | −0.977 ** | −0.671 ** | −0.072 | −0.271 * | |
| ADF | 0.878 ** | 0.670 ** | 0.120 | −0.021 | ||
| NDF | 0.651 ** | 0.021 | 0.460 ** | |||
| CC | −0.657 ** | 0.118 | ||||
| ADL | −0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xiao, Q.; Fang, Z.; Zheng, Q.; Li, H.; Li, Z. The Half-Heading Stage May Represent the Optimal Harvest Time for the First Cut of Tall Wheatgrass. Agronomy 2025, 15, 763. https://doi.org/10.3390/agronomy15040763
Li W, Xiao Q, Fang Z, Zheng Q, Li H, Li Z. The Half-Heading Stage May Represent the Optimal Harvest Time for the First Cut of Tall Wheatgrass. Agronomy. 2025; 15(4):763. https://doi.org/10.3390/agronomy15040763
Chicago/Turabian StyleLi, Wei, Qiang Xiao, Zhengwu Fang, Qi Zheng, Hongwei Li, and Zhensheng Li. 2025. "The Half-Heading Stage May Represent the Optimal Harvest Time for the First Cut of Tall Wheatgrass" Agronomy 15, no. 4: 763. https://doi.org/10.3390/agronomy15040763
APA StyleLi, W., Xiao, Q., Fang, Z., Zheng, Q., Li, H., & Li, Z. (2025). The Half-Heading Stage May Represent the Optimal Harvest Time for the First Cut of Tall Wheatgrass. Agronomy, 15(4), 763. https://doi.org/10.3390/agronomy15040763





