Understanding the Wood Pocket Physiopathy in Persian Lime Through Its Physiological Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Physiological Variables and Data Analysis
2.3. Chlorophyll and Fluorescence Variables
2.4. Gas Exchange Variables
2.5. Calculation of Water Use and Carboxylation Efficiency
3. Results
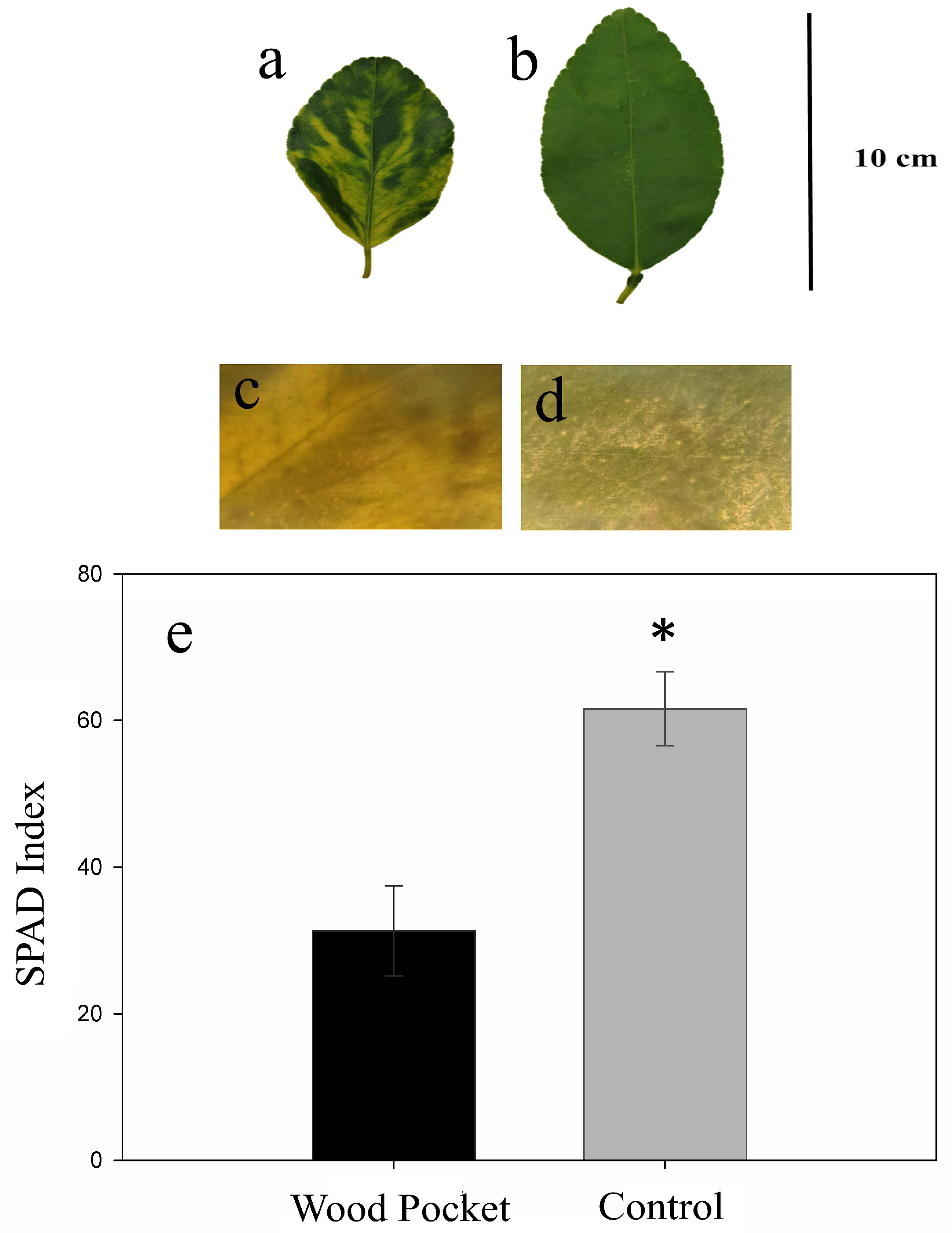
3.1. Wood Pocket Symptoms and SPAD Index
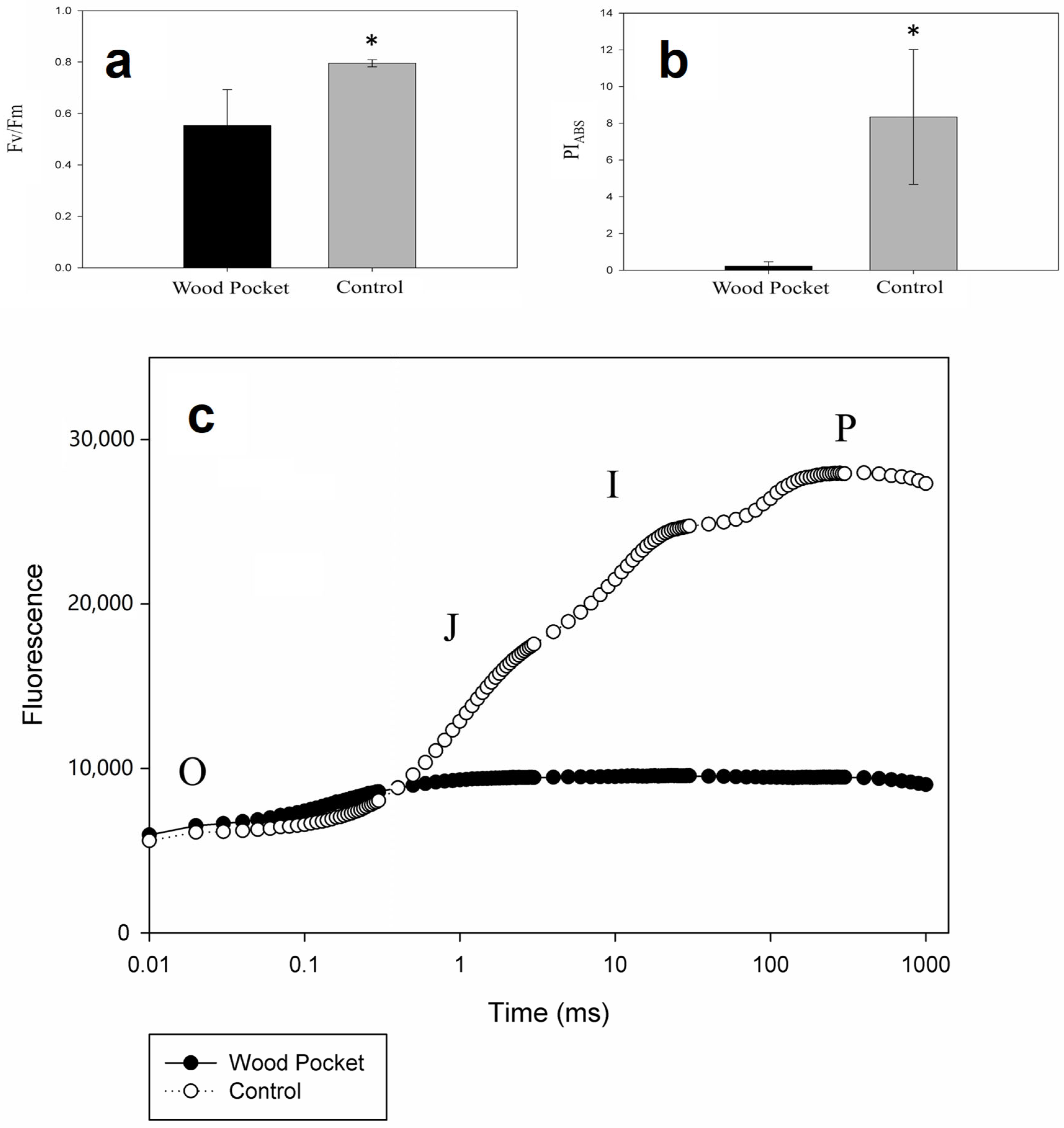
3.2. Fluorescence Parameters and OJIP Curves
3.3. Gas Exchange Parameters and Efficiencies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo-Martínez, S.I.; Díaz-José, J.; Herrera-Corredor, J.A.; Cabal-Prieto, A.; Leyva-Ovalle, O.R.; Murguía-González, J.; Dimas-García, C.H.; Prinyawiwatkul, W.; Ramírez-Rivera, E.J. Sensory, cognitive perception, and consumer liking of Mexican Persian lime (Citrus latifolia Tanaka): An online survey with pictures comparing consumer types and sale contexts. Int. J. Food Sci. Technol. 2023, 58, 6485–6495. [Google Scholar] [CrossRef]
- Padilla-de la Rosa, J.D.; Manzano-Alfaro, M.D.; Gómez-Huerta, J.R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Cardador-Martínez, A.; Estarrón-Espinosa, M. Innovation in a continuous system of distillation by steam to obtain essential oil from Persian lime juice (Citrus latifolia Tanaka). Molecules 2021, 26, 4172. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Tornez, R.; Marquez-Berber, S.R.; Almaguer-Vargas, G.; Aguilar-Avila, J.; Gardezi, A.K.; Ayala-Garay, A.V. Value network of the Persian lime in Mexico. Agrofor Int. J. 2016, 1, 39–45. [Google Scholar] [CrossRef]
- Fernández-Lambert, G.; Aguilar-Lasserre, A.; Azzaro-Pantel, C.; Miranda-Ackerman, M.A.; Purroy-Vázquez, R.; Pérez-Salazar, M.R. Behavior patterns related to the agricultural practices in the production of Persian lime (Citrus latifolia Tanaka) in the seasonal orchard. Comput. Electron. Agric. 2015, 116, 162–172. [Google Scholar] [CrossRef]
- Martínez-Ardila, H.; Corredor-Clavijo, A.; Rojas-Castellanos, V.P.; Contreras, O.; Lesmes, J.C. The technology life cycle of Persian lime. A patent based analysis. Heliyon 2022, 8, e11781. [Google Scholar] [CrossRef]
- Santillán-Mendoza, R.; Estrella-Maldonado, H.; Marín-Oluarte, L.; Matilde-Hernández, C.; Rodriguez-Alvarado, G.; Fernández-Pavia, S.P.; Flores-de la Rosa, F.R. Phylogenetic and pathogenic evidence reveals novel host–pathogen interactions between species of Lasiodiplodia and Citrus latifolia dieback disease in southern Mexico. J. Fungi 2022, 10, 484. [Google Scholar] [CrossRef]
- Volkers, R.J.M.; van Doorn, B.B.J.A.; Blom, N.I.; van de Bilt, J.L.J.; Gorkink-Smits, P.M.A.; Landman, M.N.M.; Teunissen, M.; Pel, M.J.C.; Raaymakers, T.M.; Bergsma-Vlami, M. Xanthomonas citri pv. citri findings in citrus fruits imported in the Netherlands. Plant Health Prog. 2024, 25, 237–243. [Google Scholar] [CrossRef]
- Rivera-Hernández, B.; González-Jiménez, V.; Carillo-Ávila, E.; Garruña-Hernández, R.; Andrade, J.L.; Quej-Chi, V.H.; Arreola-Enríquez, J. Yield, physiology, fruit quality and water footprint in Persian lime (Citrus latifolia tan.) in response to soil moisture tension in two phenological stages in Campeche, Mexico. Water 2022, 14, 1011. [Google Scholar] [CrossRef]
- Raddatz-Mota, D.; Barbosa-Martínez, C.; Jacuinde-Guzmán, J.K.; Alia-Tejacal, I.; Soriano-Melgar, L.l.A.A.; Rivera-Cabrera, F. Oleocellosis development in Persian lime (Citrus latifolia T.) fruit influenced by citrus rootstock. Sci. Horiculturae 2020, 271, 109461. [Google Scholar] [CrossRef]
- Alanís-Martínez, I.; Cora-Valencia, E.; Robles-García, P.L.; Silva-Rojas, H.V.; López-Buenfil, A. Mancha Sectorial (Wood Pocket) y Huanglongbing (HLB), reto para la producción de lima persa en Morelos. Rev. Mex. Fitopatol. 2016, 34, 115–116. [Google Scholar] [CrossRef]
- Fawcett, H.S.; Calavan, E.C. Wood pocket, a newly reported disease of lemons. Phtytopathology 1947, 37, 843. [Google Scholar]
- Knorr, L.C.; Childs, J.F.L. Ocurrence of Wood Pocket (Blotch), chimeric breakdown, and endoxerosis in Florida, with particular reference to Tahiti lime. Proc. Fla. State Hortic. Soc. 1957, 70, 75–81. [Google Scholar]
- Calavan, E.C. Wood pocket disease of lemons and seedless limes. Calif. Citrogr. 1957, 42, 265–268. [Google Scholar]
- Santillán-Mendoza, R.; Flores-de la Rosa, F.R.; Rodríguez-Quibrera, C.G.; Domínguez-Monge, S.; Matilde-Hernández, C. La sintomatología del Wood Pocket en limón persa (Citrus latifolia Tan.) está asociada a la represión de genes antioxidantes. In Ciencia y Tecnología para el Campo Mexicano: Retos y Oportunidades, 1st ed.; Zetina Lezama, R., Tosquy Valle, O.H., del Ángel Pérez, A.L., Ríos-Utrera, A., Esqueda-Esquivel, V.A., Eds.; INIFAP: Veracruz, Mexico, 2021; pp. 525–531. [Google Scholar]
- Strasser, A.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation, 1st ed.; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Chauhan, J.; Prathiba, M.D.; Singh, P.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; Mehta, B.; Dey, P.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; de Oliveira, T.R.; Santa-Catarina, C.; Gómez-Cadenas, A. Reduction of heat stress pressure and activation of photosystem II repairing system are crucial for citrus tolerance to multiple abiotic stress combination. Physiol. Plant. 2022, 174, e13809. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Lin, X.; Grierson, D.; Yin, X.; Chen, K. Citrus heat shock transcription factor CitHsfA7-mediated citric acid degradation in response to heat stress. Plant Cell Environ. 2021, 45, 95–104. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Otero, A.; Goni, C.; Jifon, J.L.; Syvertsen, J.P. High temperature effects on citrus orange leaf gas exchange, flowering, fruit quality and yield. Acta Hortic. 2011, 903, 1069–1075. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of two reflective materials on gas exchange, yield, and fruit quality of sweet orange tree Citrus sinensis (L.) Osb. Eur. J. Agron. 2020, 118, 126071. [Google Scholar] [CrossRef]
- Aroca, A.; García-Díza, I.; García-Calderón, M.; Gotor, C.; Márquez, A.J.; Betti, M. Photorespiration: Regulation and new insights on the potential role of persulfidation. J. Exp. Bot. 2023, 74, 6023–6039. [Google Scholar] [CrossRef]
- Betti, M.; Bauwe, H.; Busch, F.A.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry, M.A.J.; Sage, R.; Timm, S.; et al. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Paudel, I.; Shaviv, A.; Bernstein, N.; Heuer, B.; Shapira, O.; Lukyanov, V.; Bar-Tal, A.; Rotbart, N.; Ephrath, J.; Cohen, S. Lower leaf gas-exchange and higher photorespiration of treated wastewater irrigated Citrus trees is modulated by soil type and climate. Physiol. Plant. 2016, 156, 478–496. [Google Scholar] [CrossRef]
- Cavanagh, A.P.; South, P.F.; Bernacchi, C.J.; Ort, D.R. Alternative pathway to photorespiration protects growth and productivity at elevated temperatures in a model crop. Plant Biotechnol. J. 2021, 20, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Osei-Bonsu, I.; McClain, A.M.; Walker, B.J.; Sharkey, T.D.; Kramer, D.M. The roles of photorespiration and alternative electron acceptors in the responses of photosynthesis to elevated temperatures in cowpea. Plant Cell Environ. 2021, 44, 2290–2307. [Google Scholar] [CrossRef]
- Cuervo-Robayo, A.P.; Ureta, C.; Gómez-Albores, M.A.; Meneses-Mosquera, A.K.; Téllez-Valdés, O.; Martínez-Meyer, E. One hundred years of climate change in Mexico. PLoS ONE 2020, 15, e0209808. [Google Scholar] [CrossRef]
- Rouiss, H.; Bakry, F.; Froelicher, Y.; Navarro, L.; Aleza, P.; Ollitrault, P. Origin of C. latifolia and C. aurantiifolia triploid limes: The preferential disomic inheritance of doubled-diploid ‘Mexican’ lime is consistent with an interploid hybridization hypothesis. Ann. Bot. 2018, 121, 571–585. [Google Scholar] [CrossRef]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Brat, P.; Gros, O.; Ollitrault, P.; Morillon, R. Specific physiological and anatomical traits associated with polyploidy and better detoxification processes contribute to improved Huanglongbing tolerance of the persian lime compared with the mexican lime. Front. Plant Sci. 2021, 12, 685679. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid citrus genotypes have a better tolerance to natural chilling conditions of photosynthetic capacities and specific leaf volatile organic compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 4, 615114. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, P.; Zhang, X.; Wang, D.; Wang, Y.; Ma, X.; Yang, Y.; Liu, X.; Chang, X.; Sui, P.; et al. Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review. Agronomy 2022, 12, 1579. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Purves, R.W.; Bueckert, R.; Taran, B.; Warkentin, T.D. Comparative analysis of heat-stress-induced abscisic acid and heat shock protein responses among pea varieties. Crop Sci. 2023, 63, 139–150. [Google Scholar] [CrossRef]
- Trueba, S.; Pan, R.; Scoffoni, C.; John, G.P.; Davis, S.D.; Sack, L. Thresholds for leaf damage due to dehydration: Declines of hydraulic function, stomatal conductance and cellular integrity precede those for photochemistry. New Phytol. 2019, 223, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Rios-Rojas, L.; Chaali, N.; Jaramillo-Barrios, C.I.; Ouazaa, S.; Correa, J.F. Irrigation and nutrition as criteria for adequate management of Tahiti acid lime trees affected by a physiological disorder in tropical conditions. Sci. Hortic. 2020, 270, 109438. [Google Scholar] [CrossRef]
- Smolikova, G.; Dolgikh, E.; Vikhnina, M.; Frolov, A.; Medvedev, S. Genetic and hormonal regulation of chlorophyll degradation during maturation of seeds with green embryos. Int. J. Mol. Sci. 2017, 18, 1993. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kitaya, Y.; Seoka, M.; Yahata, M.; Kato, M. Effects of light and plant hormones on regreening in flavedos of Ponkan mandarin in vitro. Sci. Hortic. 2024, 338, 113641. [Google Scholar] [CrossRef]
- Yamburenko, M.V.; Zubo, Y.O.; Börner, T. Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: Repression by guanosine-3′-5′-bisdiphosphate and activation by sigma factor 5. Plant J. 2015, 82, 1030–1041. [Google Scholar] [CrossRef]

| Technical Fluorescence Parameters | ||
|---|---|---|
| Quantum efficiencies | ||
| TR0/ABS | =Fv/Fm | Maximum quantum yield for primary photochemistry. |
| ET0/ABS | =Fv/Fm × (1 − Vj) | Probability that an absorbed photon will move an electron into electron transport chain further than QA−. |
| ET0/TR0 | =(1 − Vj) | Efficiency by which a trapped exciton, having triggered the reduction of QA to QA−, can move an electron further than QA− into the electron transport chain. |
| Specific fluxes | ||
| ABS/RC | =M0 × (1/Vj) × [1/(TR0/ABS)] | Chlorophyll antenna’s absorption flux per RC. |
| TR0/RC | =M0 × (1/Vj) | Trapped energy flux that leads to QA reduction per RC. |
| ET0/RC | =M0 × (1/Vj) × (ET0/TR0) | Electron transport flux, further than QA, per RC. |
| Phenomenological fluxes | ||
| ABS/CS0 | =ABS/CSChl =Chl/CS0 or ABS/CS0 = F0 or ABS/CSm = Fm | Absorbed energy flux per excited cross-section of leaf sample at F0. |
| TR0/CS0 | =ABS/CS0 × TR0/ABS | Trapped energy flux that leads to QA reduction per excited cross-section of leaf sample. |
| ET0/CS0 | =ABS/CS0 × (TR0/ABS) × (ET0/TR0) | Electron transport flux (further than QA) per excited cross-section of leaf sample. |
| Density of PSII reaction center | ||
| RC/CS0 | =(ET0/TR0) × (Vj/M0) × (ABS/CS0) | Reaction center per cross-section at F0. |
| RC/CSm | =(ET0/TR0) × (Vj/M0) × (ABS/CSm) | Reaction center per cross-section at Fm. |
| Control | Wood Pocket | |
|---|---|---|
| Quantum efficiencies | ||
| TR0/ABS | 0.796 ± 0.012 a | 0.572 ± 0.134 b |
| ET0/ABS | 0.495 ± 0.069 a | 0.157 ± 0.105 b |
| ET0/TR0 | 0.621 ± 0.084 a | 0.250 ± 0.147 b |
| Specific fluxes | ||
| ABS/RC | 0.864 ± 0.058 a | 3.012 ± 0.771 b |
| TR0/RC | 0.839 ± 0.374 a | 1.931 ± 0.350 b |
| ET0/RC | 0.416 ± 0.074 a | 0.443 ± 0.220 a |
| DI0/RC | 0.176 ± 0.021 a | 2.301 ± 1.810 b |
| Phenomenological fluxes | ||
| ABS/CS0 | 5808.83 ± 301.95 a | 6683.25 ± 1068.03 b |
| TR0/CS0 | 4620.65 ± 183.27 a | 4399.00 ± 1628.54 a |
| ET0/CS0 | 2878.56 ± 419.70 a | 1325.13 ± 755.22 b |
| Density of PSII reaction center | ||
| RC/CS0 | 4724.44 ± 301.67 a | 1137.99 ± 898.57 b |
| RC/CSm | 25,536.79 ± 3985.89 a | 3928.65 ± 1820.40 b |
| Treatment | Water Use Efficiency (μmol CO2 mmol−1 H2O) | Intrinsic Water Use Efficiency (µmol CO2 mol−1 H2O) | Instantaneous Carboxylation Efficiency (mol m−2 s−1) |
|---|---|---|---|
| Healthy | 5.91 ± 1.17 a | 129.08 ± 8.59 a | 0.0231 ± 0.003 a |
| Wood Pocket | −16.05 ± 5.87 b | −150.62 ± 21.42 b | 0.006 ± 0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-de la Rosa, F.R.; Fuentes-Ortíz, G.; Santillán-Mendoza, R.; Matilde-Hernández, C.; Estrella-Maldonado, H.; Santamaría, J.M. Understanding the Wood Pocket Physiopathy in Persian Lime Through Its Physiological Characterization. Agronomy 2025, 15, 762. https://doi.org/10.3390/agronomy15040762
Flores-de la Rosa FR, Fuentes-Ortíz G, Santillán-Mendoza R, Matilde-Hernández C, Estrella-Maldonado H, Santamaría JM. Understanding the Wood Pocket Physiopathy in Persian Lime Through Its Physiological Characterization. Agronomy. 2025; 15(4):762. https://doi.org/10.3390/agronomy15040762
Chicago/Turabian StyleFlores-de la Rosa, Felipe Roberto, Gabriela Fuentes-Ortíz, Ricardo Santillán-Mendoza, Cristian Matilde-Hernández, Humberto Estrella-Maldonado, and Jorge M. Santamaría. 2025. "Understanding the Wood Pocket Physiopathy in Persian Lime Through Its Physiological Characterization" Agronomy 15, no. 4: 762. https://doi.org/10.3390/agronomy15040762
APA StyleFlores-de la Rosa, F. R., Fuentes-Ortíz, G., Santillán-Mendoza, R., Matilde-Hernández, C., Estrella-Maldonado, H., & Santamaría, J. M. (2025). Understanding the Wood Pocket Physiopathy in Persian Lime Through Its Physiological Characterization. Agronomy, 15(4), 762. https://doi.org/10.3390/agronomy15040762







