Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Gaseous Emission Measurements
2.2. Physicochemical Analyses
2.3. Statistical Analyses
3. Results and Discussion
3.1. Composting Trial
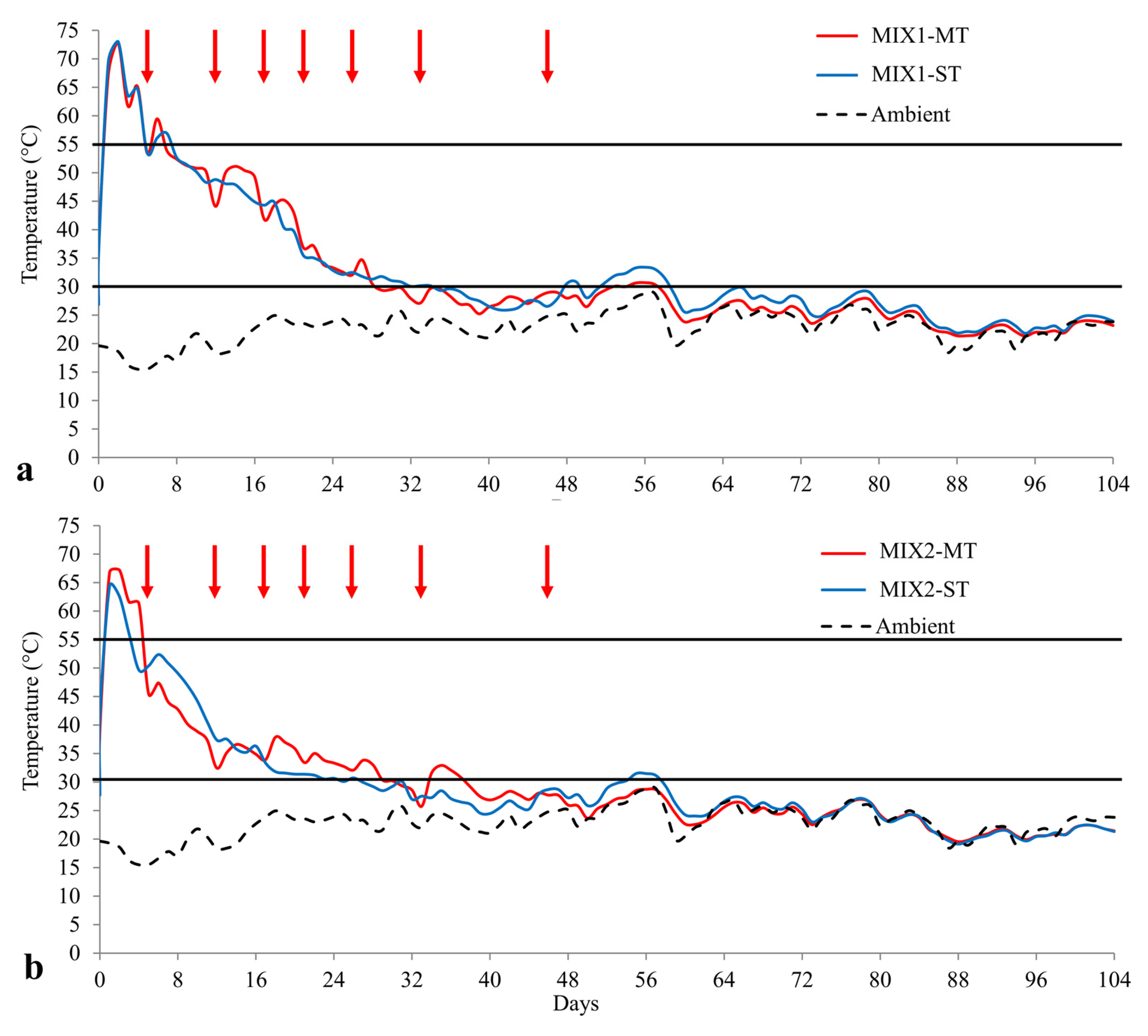
3.1.1. Temperature Trends
3.1.2. Physicochemical Properties
3.2. Greenhouse Gas Emissions
3.2.1. CO2 Emissions
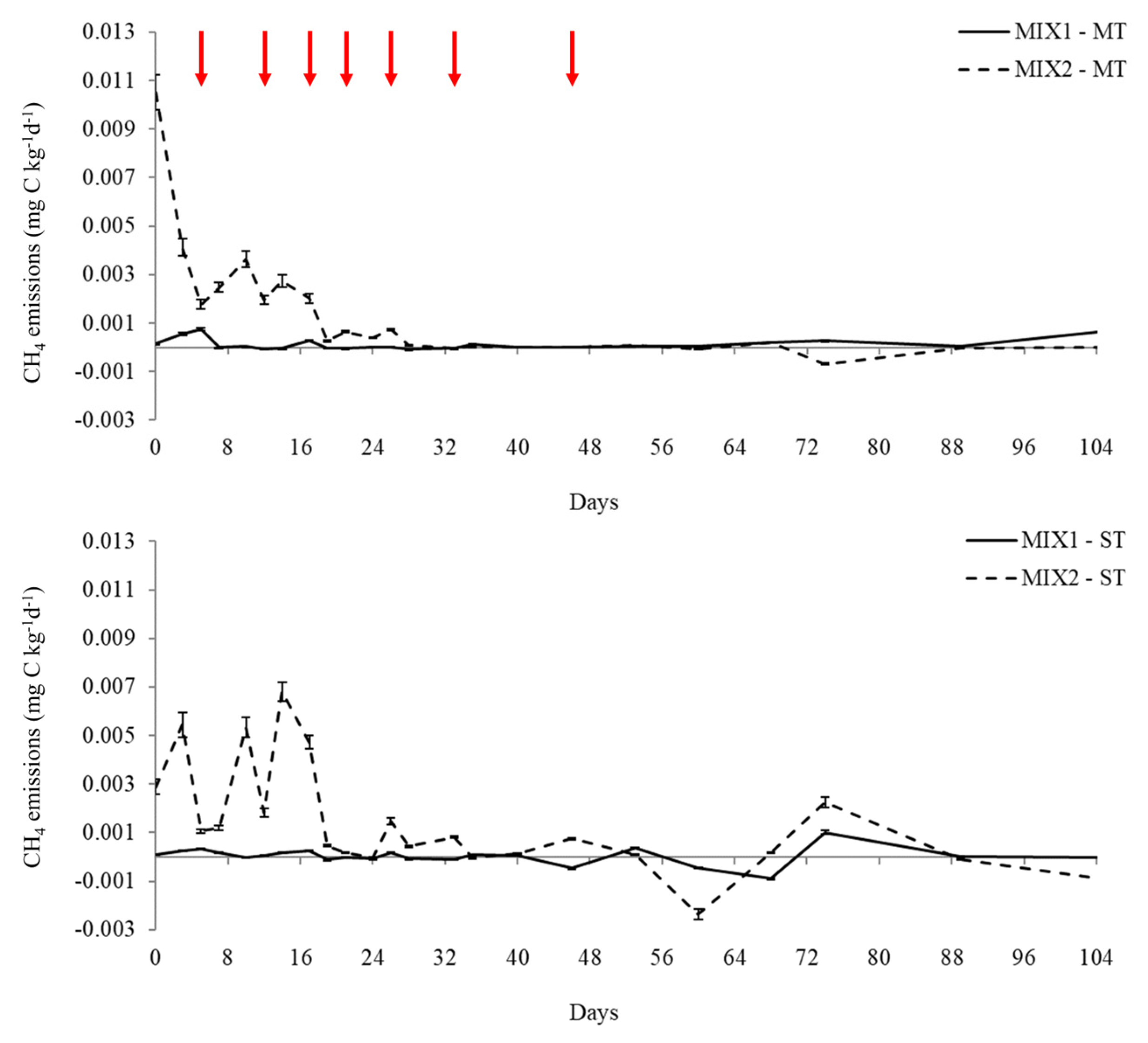
3.2.2. CH4 Emissions
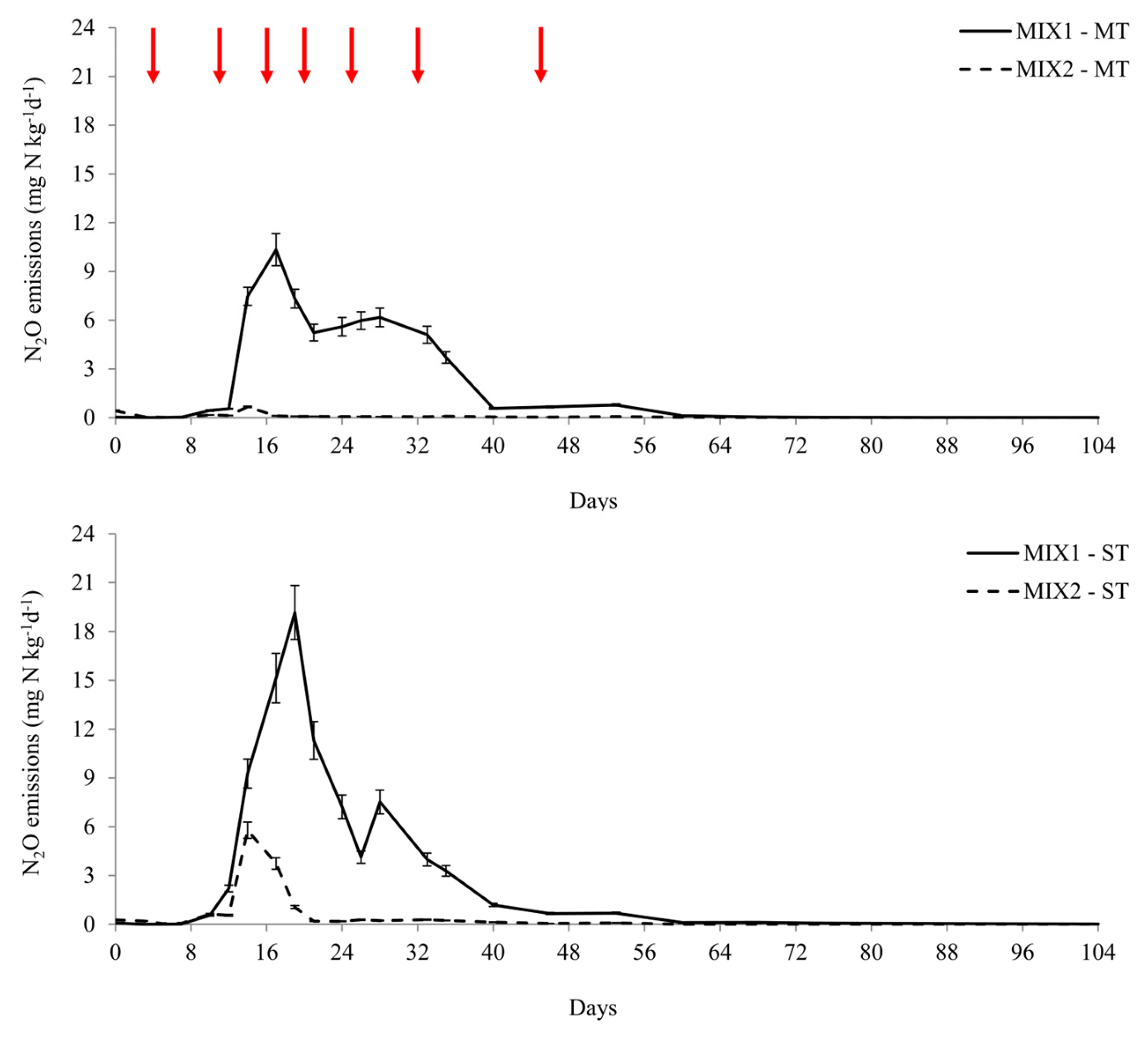
3.2.3. N2O Emissions
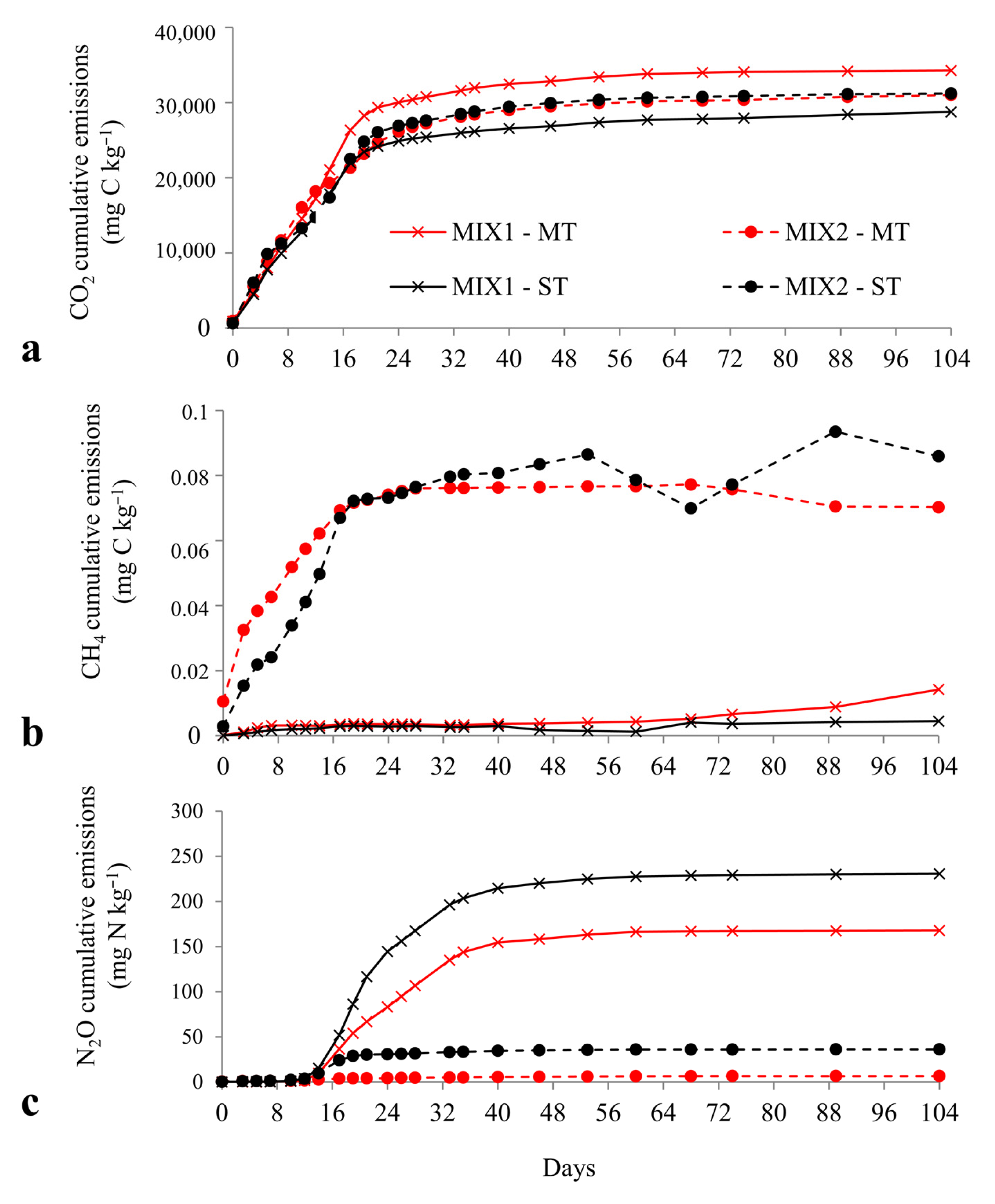
3.2.4. Cumulative Gas Emissions
3.2.5. Global Warming Potential (GWP)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSG | Brewers’ spent grain |
| C | Carbon |
| C/N | Carbon to nitrogen ratio |
| CH4 | Methane |
| CNR | Italian National Research Council |
| CO2 | Carbon dioxide |
| GHG | Greenhouse gases |
| GWP | Global warming potential |
| H2SO4 | Sulfuric acid |
| IPCC | Intergovernmental panel on climate change |
| MT | Manual turning composting |
| N2 | Molecular nitrogen |
| N2O | Nitrous oxide |
| NH3 | Ammonia |
| NH4+-N | Ammoniacal nitrogen |
| NO2− | Nitrite |
| NO3− | Nitrate |
| NO3−-N | Nitrate nitrogen |
| O2 | Molecular oxygen |
| OM | Organic matter |
| PSF | Pig slurry solid fraction |
| SD | Standard deviation |
| SM | Sheep manure |
| ST | Static composting |
| STEMS | Institute of Sciences and Technologies for Sustainable Energy and Mobility |
| TKN | Total Kjeldahl nitrogen |
| TOC | Total organic carbon |
| US | The United States |
| WS | Wheat straw |
References
- Vincast Is Wine Losing to Beer? Available online: https://www.forbes.com/sites/auctionforecast/2018/03/02/is-wine-losing-to-beer/ (accessed on 1 January 2025).
- Reid, N.; McLaughlin, R.B.; Moore, M.S. From Yellow Fizz to Big Biz: American Craft Beer Comes of Age. Focus Geogr. 2014, 57, 114–125. [Google Scholar] [CrossRef]
- Barth-Haas Group. The Barth Report Hops 2023/2024; Barth-Haas: Nürnberg, Germany, 2024. [Google Scholar]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Devolli, A.; Shahinasi, E.; Stafasani, M.; Feta, D.; Dara, F. Evaluation of Brewery Waste and Its Reduction Methods. Albanian J. Agric. Sci. 2018, 506–513. Available online: https://www.researchgate.net/profile/Ariola-Devolli/publication/329529123_Evaluation_of_brewery_waste_and_its_reduction_methods/links/5c0e3bf44585157ac1b7312c/Evaluation-of-brewery-waste-and-its-reduction-methods.pdf (accessed on 18 March 2025).
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The Role of Microorganisms on Biotransformation of Brewers’ Spent Grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef]
- Cacace, C.; Cocozza, C.; Traversa, A.; Coda, R.; Rizzello, C.G.; Pontonio, E.; De Mastro, F.; Brunetti, G.; Verni, M. Potential of Native and Bioprocessed Brewers’ Spent Grains as Organic Soil Amendments. Front. Sustain. Food Syst. 2022, 6, 1010890. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological Potential of Brewers Spent Grain and Its Recent Applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Cavallo, E.; Budroni, M. Suitability of Composting Process for the Disposal and Valorization of Brewer’s Spent Grain. Agriculture 2020, 11, 2. [Google Scholar] [CrossRef]
- Olajire, A.A. The Brewing Industry and Environmental Challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- Lao, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The Prebiotic Potential of Brewers’ Spent Grain on Livestock’s Health: A Review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Gupta, S.; Jaiswal, A.K.; Abu-Ghannam, N. Optimization of Fermentation Conditions for the Utilization of Brewing Waste to Develop a Nutraceutical Rich Liquid Product. Ind. Crops Prod. 2013, 44, 272–282. [Google Scholar] [CrossRef]
- Hassan, S.S.; Tiwari, B.K.; Williams, G.A.; Jaiswal, A.K. Bioprocessing of Brewers’ Spent Grain for Production of Xylanopectinolytic Enzymes by Mucor sp. Bioresour. Technol. Rep. 2020, 9, 100371. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s Spent Grain: A Review of Its Potentials and Applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Bianco, A.; Fancello, F.; Garau, M.; Deroma, M.; Atzori, A.S.; Castaldi, P.; Zara, G.; Budroni, M. Microbial and Chemical Dynamics of Brewers’ Spent Grain during a Low-Input Pre-Vermicomposting Treatment. Sci. Total Environ. 2022, 802, 149792. [Google Scholar] [CrossRef]
- Stocks, C.; Barker, A.J.; Guy, S. The Composting of Brewery Sludge. J. Inst. Brew. 2002, 108, 452–458. [Google Scholar] [CrossRef]
- Kakabouki, I.; Folina, A.; Efthimiadou, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Kapsalis, N.C.; Katsenios, N.; Travlos, I. Evaluation of Processing Tomato Pomace after Composting on Soil Properties, Yield, and Quality of Processing Tomato in Greece. Agronomy 2021, 11, 88. [Google Scholar] [CrossRef]
- Nolan, T.; Troy, S.M.; Healy, M.G.; Kwapinski, W.; Leahy, J.J.; Lawlor, P.G. Characterization of Compost Produced from Separated Pig Manure and a Variety of Bulking Agents at Low Initial C/N Ratios. Bioresour. Technol. 2011, 102, 7131–7138. [Google Scholar] [CrossRef]
- Moretti, B.; Bertora, C.; Grignani, C.; Lerda, C.; Celi, L.; Sacco, D. Conversion from Mineral Fertilisation to MSW Compost Use: Nitrogen Fertiliser Value in Continuous Maize and Test on Crop Rotation. Sci. Total Environ. 2020, 705, 135308. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential Benefits and Risks for Soil Health Derived From the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Kim, K.-H.; Das, S.; Uchimiya, M.; Jeon, B.H.; Kwon, E.; Szulejko, J.E. A Review on the Role of Organic Inputs in Maintaining the Soil Carbon Pool of the Terrestrial Ecosystem. J. Environ. Manag. 2016, 167, 214–227. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Saridakis, C.; Tzortzakis, N. Use of Municipal Solid Waste Compost as Growing Medium Component for Melon Seedlings Production. J. Plant Biol. Soil Health 2013, 1, 5. [Google Scholar] [CrossRef]
- Papamichalaki, M.; Papadaki, A.; Tzortzakis, N. Substitution of Peat with Municipal Solid Waste Compost in Watermelon Seedling Production Combined with Fertigation. Chil. J. Agric. Res. 2014, 74, 452–459. [Google Scholar] [CrossRef]
- Ermolaev, E.; Sundberg, C.; Pell, M.; Jönsson, H. Greenhouse Gas Emissions from Home Composting in Practice. Bioresour. Technol. 2014, 151, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Barker, X.; Shannon, K.B.; Kyaw Tha Paw, U.; Burger, M.; Horwath, W.R. Greenhouse Gas Emissions from Green Waste Composting Windrow. Waste Manag. 2017, 59, 70–79. [Google Scholar] [CrossRef]
- Pampuro, N.; Busato, P.; Cavallo, E. Gaseous Emissions after Soil Application of Pellet Made from Composted Pig Slurry Solid Fraction: Effect of Application Method and Pellet Diameter. Agriculture 2018, 8, 119. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Bianco, A.; Cavallo, E.; Budroni, M. Co-Composting of Brewers’ Spent Grain with Animal Manures and Wheat Straw: Influence of Two Composting Strategies on Compost Quality. Agronomy 2021, 11, 1349. [Google Scholar] [CrossRef]
- Vallini, G.; Di Gregorio, S.; Pera, A.; Cunha Queda, A.C. Exploitation of Composting Management for Either Reclamation of Organic Wastes or Solid-Phase Treatment of Contaminated Environmental Matrices. Environ. Rev. 2002, 10, 195–207. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Peyron, M.; Bertora, C.; Pelissetti, S.; Said-Pullicino, D.; Celi, L.; Miniotti, E.; Romani, M.; Sacco, D. Greenhouse Gas Emissions as Affected by Different Water Management Practices in Temperate Rice Paddies. Agric. Ecosyst. Environ. 2016, 232, 17–28. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; Hill, J.E.; Six, J.; van Kessel, C.; Linquist, B.A. Yield-Scaled Global Warming Potential of Annual Nitrous Oxide and Methane Emissions from Continuously Flooded Rice in Response to Nitrogen Input. Agric. Ecosyst. Environ. 2013, 177, 10–20. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-Based Measurement of Trace Gas Exchange: Applications and Sources of Error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Matson, P.A., Harris, R.C., Eds.; Blackwell Sci. Ltd.: Oxford, UK, 1995; pp. 14–51. [Google Scholar]
- Hutchinson, G.L.; Mosier, A.R. Improved Soil Cover Method for Field Measurement of Nitrous Oxide Fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Christensen, T.H.; Scheutz, C. Greenhouse Gas Emissions from Home Composting of Organic Household Waste. Waste Manag. 2010, 30, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Bertora, C.; Peyron, M.; Pelissetti, S.; Grignani, C.; Sacco, D. Assessment of Methane and Nitrous Oxide Fluxes from Paddy Field by Means of Static Closed Chambers Maintaining Plants Within Headspace. J. Vis. Exp. 2018, 2018, e56754. [Google Scholar] [CrossRef]
- Rochette, P.; Eriksen-Hamel, N.S. Chamber Measurements of Soil Nitrous Oxide Flux: Are Absolute Values Reliable? Soil Sci. Soc. Am. J. 2008, 72, 331–342. [Google Scholar] [CrossRef]
- Santos, C.; Goufo, P.; Fonseca, J.; Pereira, J.L.S.; Ferreira, L.; Coutinho, J.; Trindade, H. Effect of Lignocellulosic and Phenolic Compounds on Ammonia, Nitric Oxide and Greenhouse Gas Emissions During Composting. J. Clean. Prod. 2018, 171, 548–556. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2023; ISBN 9781009157896. [Google Scholar]
- Pampuro, N.; Bertora, C.; Sacco, D.; Dinuccio, E.; Grignani, C.; Balsari, P.; Cavallo, E.; Bernal, M.P. Fertilizer Value and Greenhouse Gas Emissions from Solid Fraction Pig Slurry Compost Pellets. J. Agric. Sci. 2017, 155, 1646–1658. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Santos, A.; Bustamante, M.A.; Tortosa, G.; Moral, R.; Bernal, M.P. Gaseous Emissions and Process Development during Composting of Pig Slurry: The Influence of the Proportion of Cotton Gin Waste. J. Clean. Prod. 2016, 112, 81–90. [Google Scholar] [CrossRef]
- ANPA. Metodi Di Analisi Del Compost; ANPA: Rome, Italy, 2001; ISBN 88-448-0258-9. [Google Scholar]
- de Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Liu, K.; Price, G.W. Evaluation of Three Composting Systems for the Management of Spent Coffee Grounds. Bioresour. Technol. 2011, 102, 7966–7974. [Google Scholar] [CrossRef]
- Arrigoni, J.P.; Paladino, G.; Garibaldi, L.A.; Laos, F. Inside the Small-Scale Composting of Kitchen and Garden Wastes: Thermal Performance and Stratification Effect in Vertical Compost Bins. Waste Manag. 2018, 76, 284–293. [Google Scholar] [CrossRef]
- Luo, W.; Chen, T.B.; Zheng, G.D.; Gao, D.; Zhang, Y.A.; Gao, W. Effect of Moisture Adjustments on Vertical Temperature Distribution during Forced-Aeration Static-Pile Composting of Sewage Sludge. Resour. Conserv. Recycl. 2008, 52, 635–642. [Google Scholar] [CrossRef]
- Isobaev, P.; McCartney, D.; Wichuk, K.M.; Scott, C.; Neumann, N.F. An Enhanced Direct Process Temperature Validation Framework in Composting: Case Study of a Full-Scale Covered Aerated Static Pile. Compost Sci. Util. 2014, 22, 164–178. [Google Scholar] [CrossRef]
- Khalib, S.N.B.; Zakarya, I.A.; Izhar, T.N.T. The Effect of Low Initial C:N Ratio during Composting of Rice Straw Ash with Food Waste in Evaluating the Compost Quality. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012144. [Google Scholar] [CrossRef]
- Zhu, N. Composting of High Moisture Content Swine Manure with Corncob in a Pilot-Scale Aerated Static Bin System. Bioresour. Technol. 2006, 97, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, E.I. Composting Control: Principles and Practice. In The Science of Composting; De Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 49–59. [Google Scholar] [CrossRef]
- Tang, J.C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of Temperature on Reaction Rate and Microbial Community in Composting of Cattle Manure with Rice Straw. J. Biosci. Bioeng. 2007, 104, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of Aeration Rate, C/N Ratio and Moisture Content on the Stability and Maturity of Compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Vázquez, M.A.; De La Varga, D.; Plana, R.; Soto, M. Integrating Liquid Fraction of Pig Manure in the Composting Process for Nutrient Recovery and Water Re-Use. J. Clean. Prod. 2015, 104, 80–89. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and Carbohydrate Drive Microbial Responses in Diverse Ways during Different Animal Manures Composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef]
- Pan, I.; Dam, B.; Sen, S.K. Composting of Common Organic Wastes Using Microbial Inoculants. 3 Biotech 2012, 2, 127–134. [Google Scholar] [CrossRef]
- Wan, J.; Wang, X.; Yang, T.; Wei, Z.; Banerjee, S.; Friman, V.-P.; Mei, X.; Xu, Y.; Shen, Q. Livestock Manure Type Affects Microbial Community Composition and Assembly During Composting. Front. Microbiol. 2021, 12, 621126. [Google Scholar] [CrossRef]
- Doublet, J.; Francou, C.; Poitrenaud, M.; Houot, S. Sewage Sludge Composting: Influence of Initial Mixtures on Organic Matter Evolution and N Availability in the Final Composts. Waste Manag. 2010, 30, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Nigusie, A.; Entele, T.; Van Gerven, T.; Van Der Bruggen, B. Effect of Turning Frequencies on Composting Biodegradable Municipal Solid Waste Quality. Resour. Conserv. Recycl. 2012, 65, 79–84. [Google Scholar] [CrossRef]
- Ahmed, M.; Idris, A.; Omar, S.R.S. Physicochemical Characterization of Compost of the Industrial Tannery Sludge. J. Eng. Sci. Technol. 2007, 2, 81–94. [Google Scholar]
- Ros, M.; García, C.; Hernández, T. A Full-Scale Study of Treatment of Pig Slurry by Composting: Kinetic Changes in Chemical and Microbial Properties. Waste Manag. 2006, 26, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Navarro, A.F.; Roig, A.; Cegarra, J.; Garcìa, D. Carbon and Nitrogen Transformation during Composting of Sweet Sorghum Bagasse. Biol. Fertil. Soils 1996, 22, 141–148. [Google Scholar] [CrossRef]
- Huang, G.F.; Fang, M.; Wu, Q.T.; Zhou, L.X.; Liao, X.D.; Wong, J.W.C. Co-Compositing of Pig Manure with Leaves. Environ. Technol. 2001, 22, 1203–1212. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen Transformation during Organic Waste Composting by the Rutgers System and Its Effects on PH, EC and Maturity of the Composting Mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Brito, L.M.; Coutinho, J.; Smith, S.R. Methods to Improve the Composting Process of the Solid Fraction of Dairy Cattle Slurry. Bioresour. Technol. 2008, 99, 8955–8960. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.C.; Wu, Q.T.; Nagar, B.B. Effect of C/N on Composting of Pig Manure with Sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Abdoli, M.A.; Omrani, G.; Safa, M.; Samavat, S. Comparison between Aerated Static Piles and Vermicomposting in Producing Co-Compost from Rural Organic Wastes and Cow Manure. Int. J. Environ. Sci. Technol. 2019, 16, 1551–1562. [Google Scholar] [CrossRef]
- Paradelo, R.; Prieto, B.; Sandu, R.G.; Barral, M.T.; Moldes, A.B. Can Stability and Maturity Be Evaluated in Finished Composts from Different Sources? Compost Sci. Util. 2010, 18, 22–31. [Google Scholar] [CrossRef]
- Huang, D.; Gao, L.; Cheng, M.; Yan, M.; Zhang, G.; Chen, S.; Du, L.; Wang, G.; Li, R.; Tao, J.; et al. Carbon and N Conservation during Composting: A Review. Sci. Total Environ. 2022, 840, 156355. [Google Scholar] [CrossRef] [PubMed]
- Assandri, D.; Bianco, A.; Pampuro, N.; Cavallo, E.; Zara, G.; Bardi, L.; Coronas, R.; Budroni, M. Enhancing Fertilizer Effect of Bioprocessed Brewers’ Spent Grain by Microbial Consortium Addition. Agronomy 2023, 13, 2654. [Google Scholar] [CrossRef]
- Bianco, A.; Melito, S.; Garau, M.; Giannini, V.; Zara, G.; Assandri, D.; Oufensou, S.; Coronas, R.; Pampuro, N.; Budroni, M. The Potential Use of Brewers’ Spent Grain-Based Substrates as Horticultural Bio-Fertilizers. Front. Sustain. Food Syst. 2024, 8, 1404914. [Google Scholar] [CrossRef]
- Montemurro, F.; Ciaccia, C.; Leogrande, R.; Ceglie, F.; Diacono, M. Suitability of Different Organic Amendments from Agro-Industrial Wastes in Organic Lettuce Crops. Nutr. Cycl. Agroecosyst. 2015, 102, 243–252. [Google Scholar] [CrossRef]
- Agapios, A.; Andreas, V.; Marinos, S.; Katerina, M.; Antonis, Z.A. Waste Aroma Profile in the Framework of Food Waste Management through Household Composting. J. Clean. Prod. 2020, 257, 120340. [Google Scholar] [CrossRef]
- Antil, R.S.; Raj, D.; Abdalla, N.; Inubushi, K. Physical, Chemical and Biological Parameters for Compost Maturity Assessment: A Review. In Composting for Sustainable Agriculture; Springer: Cham, Switzerland, 2014; pp. 83–101. ISBN 9783319080048. [Google Scholar]
- Rastogi, M.; Nandal, M.; Khosla, B. Microbes as Vital Additives for Solid Waste Composting. Heliyon 2020, 6, e03343. [Google Scholar] [CrossRef]
- Bignami, C.; Reyes, F.; Saccaggi, M.; Pane, C.; Zaccardelli, M.; Ronga, D. Composts from Grapevine and Hazelnut By-Products: A Sustainable Peat Partial Replacement for the Growth of Micropropagated Hazelnut and Raspberry in Containers. Horticulturae 2023, 9, 481. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Cegarra, J.; Bernal, M.P.; Noguera, P.; Abad, M.; Antón, A. Composts as Media Constituents for Vegetable Transplant Production. Compost Sci. Util. 2004, 12, 161–168. [Google Scholar] [CrossRef]
- Ko, H.J.; Kim, K.Y.; Kim, H.T.; Kim, C.N.; Umeda, M. Evaluation of Maturity Parameters and Heavy Metal Contents in Composts Made from Animal Manure. Waste Manag. 2008, 28, 813–820. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, S.H.; Shim, J.; Park, S.J. Composting Process and Gas Emissions during Food Waste Composting under the Effect of Different Additives. Sustainability 2020, 12, 7811. [Google Scholar] [CrossRef]
- Chiumenti, A.; Da Borso, F.; Rodar, T.; Chiumenti, R. Swine Manure Composting by Means of Experimental Turning Equipment. Waste Manag. 2007, 27, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Altieri, R.; Esposito, A.; Nair, T. Novel Static Composting Method for Bioremediation of Olive Mill Waste. Int. Biodeterior. Biodegrad. 2011, 65, 786–789. [Google Scholar] [CrossRef]
- Hobson, A.M.; Frederickson, J.; Dise, N.B. CH4 and N2O from Mechanically Turned Windrow and Vermicomposting Systems Following In-Vessel Pre-Treatment. Waste Manag. 2005, 25, 345–352. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse Gas Emissions from Organic Waste Composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef]
- Nordahl, S.L.; Preble, C.V.; Kirchstetter, T.W.; Scown, C.D. Greenhouse Gas and Air Pollutant Emissions from Composting. Environ. Sci. Technol. 2023, 57, 2235–2247. [Google Scholar] [CrossRef]
- Szanto, G.L.; Hamelers, H.V.M.; Rulkens, W.H.; Veeken, A.H.M. NH3, N2O and CH4 Emissions during Passively Aerated Composting of Straw-Rich Pig Manure. Bioresour. Technol. 2007, 98, 2659–2670. [Google Scholar] [CrossRef]
- Ermolaev, E.; Pell, M.; Smårs, S.; Sundberg, C.; Jönsson, H. Greenhouse Gas Emission from Covered Windrow Composting with Controlled Ventilation. Waste Manag. Res. 2012, 30, 155–160. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Osada, T.; Hanajima, D.; Haga, K. Patterns and Quantities of NH3, N2O and CH4 Emissions during Swine Manure Composting without Forced Aeration––Effect of Compost Pile Scale. Bioresour. Technol. 2003, 89, 109–114. [Google Scholar] [CrossRef]
- Ba, S.; Qu, Q.; Zhang, K.; Groot, J.C.J. Meta-Analysis of Greenhouse Gas and Ammonia Emissions from Dairy Manure Composting. Biosyst. Eng. 2020, 193, 126–137. [Google Scholar] [CrossRef]
- Alvarez, R.; Lidén, G. Low Temperature Anaerobic Digestion of Mixtures of Llama, Cow and Sheep Manure for Improved Methane Production. Biomass Bioenergy 2009, 33, 527–533. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.X.; Yang, Q.Y.; Luo, W.H. Effect of Bulking Agents on Maturity and Gaseous Emissions during Kitchen Waste Composting. Chemosphere 2013, 93, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Posmanik, R.; Gross, A.; Nejidat, A. Effect of High Ammonia Loads Emitted from Poultry-Manure Digestion on Nitrification Activity and Nitrifier-Community Structure in a Compost Biofilter. Ecol. Eng. 2014, 62, 140–147. [Google Scholar] [CrossRef]
- Manu, M.K.; Li, D.; Luo, L.; Zhao, J.; Varjani, S.; Wong, J.W.C. A Review on Nitrogen Dynamics and Mitigation Strategies of Food Waste Digestate Composting. Bioresour. Technol. 2021, 334, 125032. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The Role of Nitrifier Denitrification in the Production of Nitrous Oxide Revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Hwang, S.; Hanaki, K. Effects of Oxygen Concentration and Moisture Content of Refuse on Nitrification, Denitrification and Nitrous Oxide Production. Bioresour. Technol. 2000, 71, 159–165. [Google Scholar] [CrossRef]
- Amlinger, F.; Peyr, S.; Cuhls, C. Green House Gas Emissions from Composting and Mechanical Biological Treatment. Waste Manag. Res. 2008, 26, 47–60. [Google Scholar] [CrossRef]
- He, Y.; Inamori, Y.; Mizuochi, M.; Kong, H.; Iwami, N.; Sun, T. Measurements of N2O and CH4 from the Aerated Composting of Food Waste. Sci. Total Environ. 2000, 254, 65–74. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Pell, M.; Sonesson, U.; Jönsson, H.; Kirchmann, H. Formation and Emission of N2O and CH4 from Compost Heaps of Organic Household Waste. Environ. Monit. Assess. 2000, 62, 317–331. [Google Scholar] [CrossRef]
- El Kader, N.A.; Robin, P.; Paillat, J.M.; Leterme, P. Turning, Compacting and the Addition of Water as Factors Affecting Gaseous Emissions in Farm Manure Composting. Bioresour. Technol. 2007, 98, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N Ratio, Aeration Rate and Moisture Content on Ammonia and Greenhouse Gas Emission during the Composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.; Zhu-Barker, X.; Lew, S.; Croze, B.J.; Fallan, K.R.; Horwath, W.R. Impact of Composting Food Waste with Green Waste on Greenhouse Gas Emissions from Compost Windrows. Compost Sci. Util. 2019, 27, 35–45. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Sinicco, T.; Mondini, C. Biochemical Changes and GHG Emissions during Composting of Lignocellulosic Residues with Different N-Rich by-Products. Chemosphere 2012, 88, 196–203. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting Technology in Waste Stabilization: On the Methods, Challenges and Future Prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O Production in Biological Wastewater Treatment under Nitrifying and Denitrifying Conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in Physical, Chemical, and Microbiological Properties during the Two-Stage Co-Composting of Green Waste with Spent Mushroom Compost and Biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Samuelsson, J.; Christensen, T.H.; Scheutz, C. Quantification of Greenhouse Gas Emissions from Windrow Composting of Garden Waste. J. Environ. Qual. 2010, 39, 713–724. [Google Scholar] [CrossRef]
- Christensen, T.H.; Gentil, E.; Boldrin, A.; Larsen, A.W.; Weidema, B.P.; Hauschild, M. C Balance, Carbon Dioxide Emissions and Global Warming Potentials in LCA-Modelling of Waste Management Systems. Waste Manag. Res. 2009, 27, 707–715. [Google Scholar] [CrossRef]



| Compost | Moisture * | pH | TOC | OM | TKN | NH4+-N | NO3−-N | C/N |
|---|---|---|---|---|---|---|---|---|
| % | g kg−1 | % | % | mg kg−1 | mg kg−1 | |||
| MIX1 | 73.2 ± 1.07 a | 8.3 ± 0.06 b | 515 ± 0.23 a | 88.6 ± 0.29 a | 2.8 ± 0.11 a | 1091 ± 5.03 b | 74 ± 1.66 b | 18.2 ± 0.78 a |
| MIX2 | 73.0 ± 1.52 a | 8.7 ± 0.12 a | 488 ± 2.06 a | 85.8 ± 0.58 b | 2.6 ± 0.09 b | 1623 ± 4.07 a | 103 ± 6.24 a | 19.1 ± 0.15 a |
| Treatment | Day | Moisture * (%) | pH | TOC (g kg−1) | OM (%) | OM Loss ** (%) | TKN (%) | NH4+-N (mg kg−1) | NO3-N (mg kg−1) | C/N |
|---|---|---|---|---|---|---|---|---|---|---|
| MIX1-MT | 13 | 73.6 ± 1.57 a | 8.1 ± 0.15 b | 497 ± 1.7 ab | 85.8 ± 0.30 ab | 22.2 ± 0.55 ab | 3.0 ± 0.02 a | 706 ± 9.90 c | 75 ± 1.09 b | 16.6 ± 0.04 bc |
| 46 | 70.5 ± 0.87 a | 6.4 ± 0.00 c | 465 ± 0.4 a | 80.2 ± 0.08 c | 47.6 ± 1.56 a | 3.5 ± 0.06 a | 471 ± 2.91 c | 100 ± 1.11 c | 13.2 ± 0.21 d | |
| 74 | 67.4 ± 0.29 a | 6.1 ± 0.00 b | 457 ± 3.9 a | 78.8 ± 0.65 a | 52.0 ± 0.64 a | 3.8 ± 0.11 a | 380 ± 6.81 b | 460 ± 9.15 c | 12.2 ± 0.44 b | |
| 104 | 40.6 ± 0.63 b | 6.2 ± 0.06 c | 424 ± 1.3 b | 76.9 ± 0.67 ab | 56.9 ± 1.73 a | 3.7 ± 0.10 a | 187 ± 3.92 a | 490 ± 3.05 bc | 11.6 ± 0.29 c | |
| MIX2-MT | 13 | 75.6 ± 2.04 a | 8.7 ± 0.00 a | 488 ± 4.0 b | 84.1 ± 0.68 b | 12.1 ± 1.10 ab | 2.7 ± 0.15 b | 728 ± 2.08 b | 108 ± 6.24 a | 18.1 ± 0.96 b |
| 46 | 45.2 ± 2.52 b | 9.0 ± 0.06 a | 471 ± 1.3 a | 81.1 ± 0.22 b | 28.7 ± 4.39 c | 3.0 ± 0.06 c | 684 ± 3.76 b | 155 ± 1.51 ab | 15.5 ± 0.29 b | |
| 74 | 37.0 ± 1.37 b | 8.8 ± 0.44 a | 445 ± 4.2 a | 76.8 ± 0.73 a | 45.3 ± 0.46 a | 3.2 ± 0.03 b | 363 ± 6.73 b | 503 ± 1.97 c | 14.0 ± 0.15 a | |
| 104 | 18.4 ± 0.73 d | 9.4 ± 0.00 a | 425 ± 9.5 b | 73.6 ± 1.61 b | 53.9 ± 1.64 ab | 3.1 ± 0.13 b | 281 ± 7.70 b | 560 ± 1.73 a | 13.9 ± 0.28 b | |
| MIX1-ST | 13 | 74.8 ± 0.57 a | 8.5 ± 0.12 a | 509 ± 3.7 a | 88.0 ± 0.81 a | 4.7 ± 9.22 b | 3.1 ± 0.06 a | 834 ± 1.40 ab | 79 ± 8.54 b | 16.4 ± 0.41 c |
| 46 | 71.2 ± 0.04 a | 6.5 ± 0.06 c | 475 ± 0.9 a | 82.0 ± 0.08 a | 41.2 ± 1.47 ab | 3.3 ± 0.03 b | 820 ± 6.76 a | 114 ± 1.73 c | 14.3 ± 0.11 c | |
| 74 | 68.6 ± 3.14 a | 6.1 ± 0.06 b | 456 ± 11.3 a | 79.1 ± 1.79 a | 50.8 ± 6.75 a | 3.7 ± 0.10 a | 328 ± 6.73 c | 572 ± 1.21 b | 12.5 ± 0.67 ab | |
| 104 | 46.0 ± 1.76 a | 6.0 ± 0.12 c | 449 ± 0.3 a | 77.4 ± 0.05 a | 55.7 ± 1.37 a | 3.5 ± 0.17 a | 247 ± 3.00 a | 836 ± 9.64 c | 12.9 ± 0.64 bc | |
| MIX2-ST | 13 | 58.4 ± 3.97 b | 8.7 ± 0.12 a | 486 ± 8.8 b | 81.1 ± 1.30 c | 28.3 ± 9.41 a | 2.6 ± 0.11 b | 1418 ± 3.00 a | 105 ± 5.29 ab | 18.6 ± 0.50 a |
| 46 | 55.2 ± 1.37 ab | 8.8 ± 0.10 b | 470 ± 7.6 a | 79.2 ± 0.36 d | 37.0 ± 1.65 b | 2.8 ± 0.06 d | 587 ± 1.02 ab | 179 ± 9.17 a | 16.9 ± 0.61 a | |
| 74 | 43.2 ± 7.68 b | 8.4 ± 0.32 a | 459 ± 2.1 a | 78.0 ± 8.96 a | 37.0 ± 2.29 b | 3.4 ± 0.22 ab | 490 ± 9.41 a | 627 ± 1.11 a | 13.4 ± 0.80 ab | |
| 104 | 26.6 ± 0.86 c | 9.0 ± 0.00 b | 451 ± 11.5 a | 77.7 ± 1.98 a | 41.7 ± 9.41 b | 2.9 ± 0.04 b | 408 ± 5.03 a | 931 ± 9.85 ab | 15.5 ± 0.21 a |
| GHG | Unit | MIX1-MT | MIX2-MT | MIX1-ST | MIX2-ST |
|---|---|---|---|---|---|
| (a) Cumulative gas emissions | |||||
| CO2-C | mg C kg−1 FM | 34,275 ± 27.0 a | 31,024 ± 62.8 b | 28,769 ± 10.9 c | 31,219 ± 24.6 b |
| CH4-C | mg C kg−1 FM | 0.014 ± 0.00 c | 0.070 ± 0.00 b | 0.004 ± 0.00 d | 0.086 ± 0.00 a |
| N2O-N | mg N kg−1 FM | 168 ± 8.0 b | 7 ± 0.1 d | 231 ± 14.6 a | 36 ± 1.9 c |
| (b) Emission factor * | |||||
| CO2-C | (%) | 3.87 ± 0.04 a | 3.62 ± 0.03 b | 3.25 ± 0.01 c | 3.64 ± 0.03 b |
| CH4-C | (%) | (0.16 ± 0.01 c) 10−7 | (0.82 ± 0.02 b) 10−7 | (0.05 ± 0.02 d) 10−7 | 1.00 ± 0.03 a |
| N2O-N | (%) | 0.595 ± 0.035 b | 0.026 ± 0.001 d | 0.816 ± 0.025 a | 0.142 ± 0.003 c |
| GWP | Unit | MIX1-MT | MIX2-MT | MIX1-ST | MIX2-ST |
|---|---|---|---|---|---|
| CH4 | mg CO2 eq kg−1 FM | 0.38 ± 0.0 c | 1.90 ± 0.0 b | 0.12 ± 0.0 d | 2.32 ± 0.1 a |
| N2O | mg CO2 eq kg−1 FM | 45,815 ± 211 b | 1784 ± 15.7 d | 62,950 ± 386 a | 9875 ± 50.5 c |
| TOT | mg CO2 eq kg−1 FM | 45,816 ± 211 b | 1786 ± 15.7 d | 62,950 ± 386 a | 9877 ± 50.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assandri, D.; Giacomello, G.; Bianco, A.; Zara, G.; Budroni, M.; Pampuro, N. Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions. Agronomy 2025, 15, 771. https://doi.org/10.3390/agronomy15040771
Assandri D, Giacomello G, Bianco A, Zara G, Budroni M, Pampuro N. Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions. Agronomy. 2025; 15(4):771. https://doi.org/10.3390/agronomy15040771
Chicago/Turabian StyleAssandri, Davide, Ginevra Giacomello, Angela Bianco, Giacomo Zara, Marilena Budroni, and Niccolò Pampuro. 2025. "Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions" Agronomy 15, no. 4: 771. https://doi.org/10.3390/agronomy15040771
APA StyleAssandri, D., Giacomello, G., Bianco, A., Zara, G., Budroni, M., & Pampuro, N. (2025). Environmental Sustainability of Brewers’ Spent Grains Composting: Effect of Turning Strategies and Mixtures Composition on Greenhouse Gas Emissions. Agronomy, 15(4), 771. https://doi.org/10.3390/agronomy15040771








