Abstract
To discriminate the transport characteristics of residue-derived carbon (Cres) from soil native carbon (Csoil) in black soil with split nitrogen application, a 540-day incubation study was conducted with four treatments: Control (unamended soil), R (soil + residue), RN1 (soil + residue + one-time application of nitrogen fertilizer), and RN3 (soil + residue + three-time application of nitrogen fertilizer). The total soil organic carbon (TOC) of the incubated soil was separated into three fractions: light fraction (LF), occluded-particulate organic matter fraction (OPOM), and heavy fraction (HF). The results showed that the TOC content was significantly higher in the RN1 and RN3 (averaging 20.77 g/kg) than in the R (18.43 g/kg) and Control (19.03 g/kg) after 540 days. Nitrogen fertilization significantly increased the residual rate of HF−Cres by 11.75% (p < 0.05), and the RN3 treatment significantly increased the residual rate of OPOM−Cres by 18.84% (p < 0.05) and reduced the loss rate of LF−Csoil by 77.01% (p < 0.05) compared with the R treatment. The soil catalase activity declined continuously along with incubation and was higher in the RN3 treatment than in the RN1 treatment after 180 days. The correlation analysis showed that the LF−Csoil and −Cres, as well as the HF−Csoil and catalase activity, were the main contributors to the TOC. Conclusively, nitrogen application, especially split nitrogen application, could stimulate the ability of soil to retain exogenous carbon and preserve native carbon.
1. Introduction
The soil carbon pool has attracted lots of attention in recent years in the research on carbon cycling in soil ecosystems and global climate change []. The mechanisms of soil organic carbon (SOC) fixation and stabilization in agricultural soils are key focuses of soil carbon pool research [,]. Determining how to reduce the degradation of carbon in farmland soil and improve the stability of the soil carbon pool is very important in soil carbon pool management. With a high SOC content and high fertility, black soil is an important resource in agricultural production. However, with the increase in human activities, the mineralization and turnover of SOC in black soil have been accelerated, resulting in a decline in soil quality and the deterioration of the ecological environment [,,]. Therefore, it is of great significance to study the regulation of the carbon turnover process in black soil for protecting soil resources and maintaining ecological balance.
The addition of exogenous organic materials and/or inorganic fertilizers has a certain impact on the storage and stability of the SOC pool [,,,]. Exogenous organic materials can promote the mineralization of soil organic carbon, which is called the priming effect []. Studies have found that crop residue returning increased the proportion of residue-derived carbon in the light fraction (LF) and heavy fraction (HF) of soil and increased the carbon mineralization in the HF of soil, thus accelerating the carbon turnover of LF and HF []. Our previous study found that residue addition had a positive priming effect on the mineralization of HF−SOC and promoted carbon turnover in black soil, and the application of nitrogen fertilizer inhibited soil native carbon loss, especially in the LF of the soil []. However, most of the current experiments focusing on the effect of nitrogen fertilizer on soil carbon turnover involve a one-time addition of nitrogen fertilizer at the beginning, followed by continuously adding water to adjust soil moisture during the incubation process, so that nitrogen might possibly be leached out, thereby narrowing the gap between nitrogen-fertilized and non-nitrogen-fertilized treatments and covering up the priming effect of nitrogen fertilizer on soil carbon. Theoretically, if nitrogen fertilizer is split-applied instead of administered in a one-time application, the inhibitory effect of excessive nitrogen fertilizer on the degradation of exogenous organic matter could be avoided []. Meanwhile, the split application of nitrogen could continuously provide exogenous available nitrogen for soil microorganisms and reduce the utilization of soil native nitrogen resources by these microorganisms [,,], thus promoting the microbial degradation of exogenous organic material (e.g., crop residue). The carbon and nitrogen released during straw degradation could be used for the reproduction and metabolism of microorganisms, which would ultimately improve the fixation efficiency of exogenous carbon and improve the stability of the soil carbon pool. From the perspective of agricultural practice, split nitrogen application performs better than one-time nitrogen application in matching the nitrogen demand of crops and reducing nitrogen loss [,,]. However, the process of soil carbon transport after split nitrogen application remains unclear.
Therefore, the isotope 13C labeling was used in this study to compare the effects of a one-time application of nitrogen fertilizer and split nitrogen application on soil carbon transport in black soil through an incubation study, as well as to examine the transportation of crop residue-derived carbon and soil native carbon among different soil carbon pools associated with enzyme activities. This study can provide a theoretical basis for the optimized management of nitrogen fertilization under residue return and for the sustainability of soil fertility and carbon pool management.
2. Materials and Methods
2.1. Site Description and Soil Sampling
Soil samples were collected from farmland (43°30′23″ N, 124°48’33″ E) near the Middle Layer Black Soil Fertility Experimental Station in Gongzhuling County, Jilin Province, China. The sampling farmland is located in the cold dry winter–hot summer climate zone (based on the Koppen–Geiger Climate Classification), with annual precipitation of 450–600 mm and an annual average temperature of 4–5 °C. The sampling area has a farming history of maize–maize–soybean rotation for more than 15 years and has been cultivated using chisel ploughing with inorganic nitrogen, phosphorus, and potassium fertilizers. Composite soil sample, which was mixed by 20 soil cores with sampling depth of 0–20 cm and diameter of 3–4 cm, was collected before corn planting in June 2018. Plant residues and small stones were removed. The soil sample was air-dried and ground through a 2 mm sieve, then kept sealed in sealing bags at room temperature for the incubation study. The soil particle composition (sand 476 g/kg, clay 237 g/kg, silt 238 g/kg) was estimated using hydrometer method, the soil pH (6.89) was measured via electrode method at soil/water ratio of 1:2.5, and the SOC content (22.02 g/kg) was determined using carbon isotope analyzer (iTOC-CRDS, Picarro, Santa Clara, CA, USA).
2.2. 13C-Labeling of Wheat Residue
After germination, wheat (Liaochun 10) was transferred to a growth chamber for sand culture with nutrients provided by Hoagland’s nutrient solution. After seedling, wheat plants were pulse-labeled weekly with 13CO2 (99% purity) gas for a total of 6 labeling rounds until wheat heading. For each labeling, about 150 mL 13CO2 (purity = 99%) was injected into a transparent polyester bag, which covered the whole plants, to increase 13CO2 concentration inside to 400 ul·L−1. Then, the bag was sealed for assimilation and removed after 20–25 min. The wheat residue (including above-ground and below-ground parts) was harvested after the wheat reached maturity. The spikes were removed and the residue was oven-dried at 40 °C and cut into 1–2 cm long pieces, then ground to pass a 1 mm sieve and stored at 4 °C. The carbon content (405.20 g/kg) and δ13C value (280.12‰, n = 3) in wheat residue were determined by carbon isotope analyzer (iTOC-CRDS, Picarro, Santa Clara, CA, USA). The nitrogen content (12.78 g/kg) in wheat residue was analyzed by Kjeldahl nitrogen determination method.
2.3. Incubation Set-Up
The incubation was established in 800 mL jars with a cylindrical flat-bottomed uncovered box containing 40 g soil. There was a small hole of 6 mm in diameter at the top of the jar for aeration. Four treatments were set up in this experiment: unamended (Control); residue amended (R); residue + one-time application of nitrogen fertilizer (RN1), with nitrogen fertilizer added on the 0th day of incubation; residue + three-time application of nitrogen fertilizer (RN3), with nitrogen fertilizer added on the 0th, 180th and 360th days of incubation. This study was a completely randomized block design with 3 replicates. The nitrogen fertilizer was urea (CO(NH2)2) in the form of solution. The total amount of nitrogen applied in RN1 and RN3 treatments was the same, 100 mg N·kg−1 soil (equivalent to 225 kg N·ha−1). The amount of wheat residue added was 7.626 g·kg−1 soil, equivalent to a field residue return rate of 17,160 kg·ha−1. After adding residue to the R, RN1 and RN3 treatments, all four treatments were pre-incubated at 50% soil water-holding capacity (WHC) at 4 °C for 2 days to allow soil moisture to diffuse evenly and to stimulate microbial activity. The soils were then warmed to room temperature and adjusted to 60% WHC using H2O or urea solution to produce four different treatments, respectively. This experiment lasted for 540 days. The jars containing incubated soil were kept aerated in the dark at 25 °C in an incubator with constant temperature and humidity, and the soil moisture was adjusted weekly to maintain at 60% WHC.
2.4. Sample Collection and Analysis
Soil samples were destructively harvested at the beginning, 180th day, 360th day and 540th day of incubation. A 20 g soil sample was weighed and separated into three fractions following the procedures described by Chen et al. (2022) []. Briefly, light fraction (LF), which was assumed to have little or no physical protection, was floated via density separation using sodium polytungstate (1.60 g·cm−3, Tianjin Kemiou, Tianjin, China), shaken at speed of 200 r·min−1 for 30 min, then centrifuged at 3000 r·min−1 for 15 min and collected on 0.20 μm polycarbonate membrane filters (0.2 μm × 50 mm, Ami Glass Instrument, Kunshan, China). The dirt was then shaken at speed of 300 r·min−1 for 40 min with 50 mL deionized water, so that the occluded particular organic matter (OPOM) was separated by using 53 μm poly-carbonate mesh. Finally, the heavy fraction (HF), which was assumed as relatively humified, mineral-associated and biochemically recalcitrant organic material, was collected by oven-drying the deposit, which passed through a 53 μm mesh. All the LF, OPOM and HF fractions were dried at 80 °C and ground using a ball mill (MM400, Retsch GmbH, Haan, Germany). The inorganic carbon was removed by hydrochloric acid fumigation; then, the SOC content and δ13C abundance of the unfractionated soil and its three fractions were determined by carbon isotope analyzer (iTOC-CRDS, Picarro, Santa Clara, CA, USA). The isotope values were expressed using δ-notation.
2.5. Calculations
The residue-derived carbon (Cres) and soil native carbon contents in each soil fraction were calculated based on the soil mass before fractionation:
where the SOC is soil organic carbon content of each fraction, g/kg; δ13Csmp is the δ13C value of soil samples treated with residue, ‰; δ13Cna is the δ13C value of soil samples with no residue amendment, ‰; δ13Cres is δ13C of wheat residue, ‰.
where the SOC0 and Cres0 are the SOC and Cres contents of each soil fraction at 0 day of incubation, respectively, g/kg; similarly, the SOC540 and Cres540 refer to the corresponding values at 540th day, g/kg; TOC is the total SOC content in the unfractionated soil prior to incubation, g/kg.
The content of Cres = SOC × (δ13Csmp − δ13Cna)/(δ13Cres − δ13Cna)
The loss rate (%) of Csoil = [(SOC0 − Cres0) − (SOC540 − Cres540)]/TOC × 100
The residual rate of Cres (%) is the percentage of Cres content in each soil fraction at 540th day to the Cres content in the unfractionated soil at the beginning of incubation.
2.6. Statistics
Microsoft Excel 2016 was used for preliminary data calculation. Data were checked in SPSS 21.0 by normal distribution test and homogeneity of variance test, and variance analysis and multiple comparisons were carried out. Pearson correlation analysis was performed by using R-4.3.2 software ‘corrplot’ package. Partial least squares path modeling (PLS-PM) was constructed by using the ‘plspm’ package. The effects of SOC of different fractions and enzyme activity on the TOC were evaluated, and the goodness of fit (GOF) of the model was used to evaluate the overall fitting degree of the model. The ‘rfPermute’ package was used to model the importance of random forests with model parameters of ntree 1000, nrep 299, and num.cores 2, in which the TOC was the predictive variable, and the Csoil, Cres, enzyme activity in different soil fractions were used as explanatory variables. The importance function was used to rank the importance of these explanatory variables in different fractions based on the permutation feature importance evaluation method. The experimental data were plotted by Origin 2021 software (OriginLab, Northampton, MA, USA).
3. Results
3.1. Effects of Different Treatments on Total Soil Organic Carbon
The total soil organic carbon (TOC) includes soil native carbon (Csoil) and residue-derived carbon (Cres) in the unfractionated soil. Figure 1 shows that, along with incubation, the TOC content decreased continuously in the Control and R treatments, from 22.53 g/kg to 18.73 g/kg on average, and the values tended to be stable after 360 days and 180 days in the RN1 (20.52 g/kg) and RN3 (20.92 g/kg) treatments, respectively. Residue amendment increased the TOC content (averaging 23.51 g/kg) compared to the Control (22.02 g/kg) at the beginning of incubation. After 180 days, the RN1 treatment showed a higher TOC content (21.94 g/kg) than the RN3 treatment (20.86 g/kg). After 540 days, the TOC contents showed no significant difference between the RN1 and RN3 treatments, but both were significantly higher (averaging 20.77 g/kg) than that of the R treatment (18.43 g/kg).
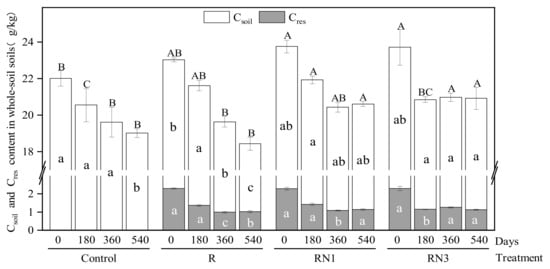
Figure 1.
Changes of the TOC, Csoil, Cres contents in unfractionated soil in different treatments. Different lowercase letters indicate significant differences of the residue-derived carbon (Cres) or soil native carbon (Csoil) among different treatments at the same time (p < 0.05); different uppercase letters indicate significant differences of the total soil organic carbon (TOC = Cres + Csoil) among different treatments at the same time (p < 0.05).
The changing patterns over time of the Cres and Csoil contents in different treatments were basically consistent with the TOC contents, except that the Cres content tended to be stable after 360 days of incubation in the R treatment (Figure 1). From the perspective of differences among treatments, the Cres contents ranked as R < RN1 < RN3 on the 360th day, and the Csoil content in the RN3 treatment was slightly higher than in the RN1 treatment (p = 0.095) after 360 days. However, the Cres and Csoil contents of the RN1 and RN3 treatments (averaging 1.13 g/kg, 19.64 g/kg, respectively) were significantly higher than those of the R treatment (1.03 g/kg, 17.41 g/kg) (p < 0.05) on the 540th day.
3.2. Dynamics of Cres and Csoil Distribution in Soil Fractions
Along with incubation, the LF−Cres content decreased continuously, and the OPOM− and HF−Cres contents increased to varying degrees (Figure 2). On the 180th day, the LF−Cres content was significantly higher in the RN1 treatment than in the R and RN3 treatments, the OPOM−Cres content was significantly higher in the RN1 and RN3 treatments than that in the R treatment, and the HF−Cres content was significantly lower in the RN3 treatment than in the R and RN1 treatments. After 360 days, the HF−Cres contents in the residue-added treatments ranked as RN3 > RN1 > R. On the 540th day, the OPOM− and HF−Cres contents were significantly higher in the RN3 treatment than that in the R treatment.
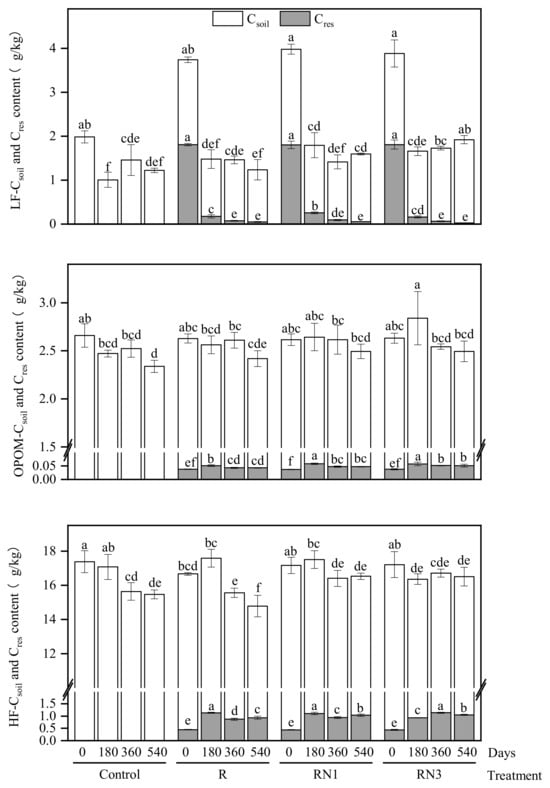
Figure 2.
Dynamic distribution of soil native carbon (Csoil) and residue-derived carbon (Cres) in different fractions. Different lowercase letters at the top of each column indicated significant differences of the Csoil or Cres contents in each soil fraction among different incubation time (0, 180, 360, 540 days) and different treatments (p < 0.05).
In terms of Csoil, the LF−Csoil content decreased from 2.04 g/kg to 1.34 g/kg during the first 180 days of incubation, and the change was reduced after 180 days (averaging 1.42 g/kg). The OPOM−Csoil content remained steady during the first 360 days and significantly decreased from 2.61 g/kg to 2.40 g/kg after that. The HF−Csoil content stabilized at 15.20 g/kg after 360 days. Meanwhile, after 180 days of incubation, the LF−Csoil content was significantly higher in the RN1 and RN3 treatments than that in the Control, and the OPOM− and HF−Csoil contents were significantly higher in the RN3 treatment than that in the Control and R treatments. The LF− and HF−Csoil contents were significantly higher in the RN1 and RN3 treatments than that in the R treatment, and no significant difference was observed among treatments for the OPOM−Csoil content by the end of incubation.
Overall, the Cres contents gradually decreased in LF and increased in OPOM and HF after 180 days, while the Csoil contents of LF, OPOM and HF decreased significantly after 180 days, 540 days and 360 days, respectively (Table 1). Comparing with the Control and R treatments, the RN3 treatment relatively increased the LF−Csoil content (p < 0.05). The HF−Csoil content in the R treatment was significantly lower than in the Control and RN1 treatments (p < 0.05) (Table 1).

Table 1.
The contents of soil native carbon (Csoil) and residue-derived carbon (Cres) influenced by incubation time and different treatments.
3.3. Effects of Different Treatments on the Loss Rate of Csoil and Residual Rate of Cres
Table 2 shows that the Csoil loss rate ranked as HF > LF > OPOM by the end of incubation, and different treatments showed no significant effect on the Csoil loss rate of OPOM and HF. The LF−Csoil loss rate in the RN3 treatment (0.77%) was significantly lower than that in other treatments (averaging 3.35%) (p < 0.05). For the residual rate of Cres, the HF accounted for much higher proportions than the LF and OPOM. In addition, the residual rate of Cres was affected by nitrogen fertilization and its frequency. Specifically, the residual rate of LF−Cres in the RN3 treatment was lower (0.79%) than that in the R and RN1 treatments (1.56–1.77%), and the rates of both HF−Cres in the RN1, RN3 treatments and OPOM−Cres in the RN3 treatments were significantly higher than those in the R treatment (p < 0.05). As a whole, nitrogen fertilization reduced the Csoil loss rate by 43.27% on average compared to the Control and R treatments, and the lowest value was observed in the RN3 treatment, while the Cres residue rates were significantly higher in the RN1 and RN3 treatments than in the R treatment.

Table 2.
The loss rate of soil native carbon (Csoil) and the residual rate of residue-derived carbon (Cres) in soil fractions by the end of incubation.
3.4. Changes of Soil Invertase and Catalase Activities Under Different Treatments
At the beginning of incubation, the R treatment reduced soil invertase activity by 5.38% compared to the Control, whereas the RN1 and RN3 treatments increased the invertase activity by 6.93% on average (Figure 3). Then, the invertase activity showed no significant change or difference among treatments throughout the incubation process. For the catalase activity, it decreased along with incubation in all treatments, and the RN1 treatment showed the highest decrease (by 39.48%) compared with other treatments (Figure 3). By the end of incubation, the catalase activity of the RN1 treatment (3.67 mg/g) was significantly lower than that of other treatments (averaging 4.22 mg/g) (p < 0.05). From the perspective of nitrogen application methods, the catalase activity of the RN3 treatment (averaging 4.85 mg/g) was continuously higher than that of the RN1 treatment (averaging 4.34 mg/g) after 180 days of incubation.
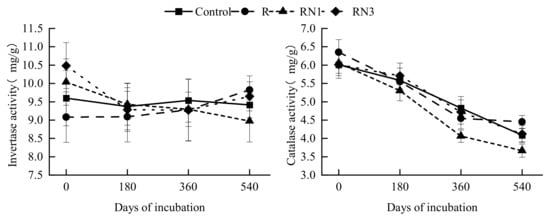
Figure 3.
Dynamic change of soil invertase and catalase activities in different treatments.
3.5. Relationships Among the TOC Content, Cres and Csoil Contents in Different SOC Fractions, and Enzyme Activity
Correlation analysis (Figure 4) showed that the TOC contents were significantly positively correlated with the LF−Cres, LF−Csoil, HF−Csoil and catalase activity (p < 0.001) and were negatively correlated with the residual rates of Cres and the loss rates of Csoil in the three SOC fractions (LF, OPOM, HF) (p < 0.05). In addition, significant positive correlations were observed between the LF−Cres and both the LF−Csoil and catalase activity (p < 0.001). The residual rates of Cres and the loss rates of Csoil in LF, OPOM and HF were all positively correlated with each other (p < 0.001), while they were all negatively correlated with the OPOM−Csoil, HF−Csoil contents and catalase activity (p < 0.05). In addition, catalase activity was positively correlated with the OPOM− and HF−Csoil contents (p < 0.001).
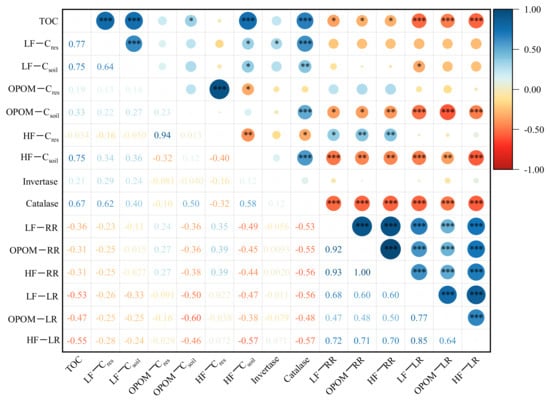
Figure 4.
Correlations among contents of total soil organic carbon (TOC), residue-derived carbon (Cres) and soil native carbon (Csoil) in different SOC fractions and enzyme activity. * means p < 0.05, ** means p < 0.01, *** means p < 0.001. TOC: total soil organic carbon; LF−Cres, OPOM−Cres, HF−Cres: the contents of residue-derived carbon in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; LF−Csoil, OPOM−Csoil, HF−Csoil: soil native carbon contents in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; invertase: invertase activity; catalase: catalase activity; LF−RR, OPOM−RR, HF−RR: the residual rate of residue-derived carbon in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; LF−LR, OPOM−LR, HF−LR: the loss rate of soil native carbon in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively.
The PLS−PM model (Figure 5) explained 88.4% of the variances of the TOC, 32.2% for LF, 9.9% for the OPOM and 58.4% for the HF. The goodness of fit of the model was 0.578. The LF− and OPOM−SOC contents had a significant direct positive effect on the TOC, with path coefficients of 0.607 and 0.362, respectively; the HF−SOC had a significant negative effect on the TOC, with a path coefficient of −0.525. Additionally, the enzyme activity had little direct effect on the TOC (path coefficient = −0.075) and could indirectly affect the TOC through the LF and HF.
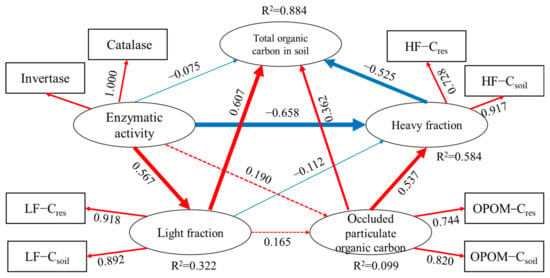
Figure 5.
Partial least squares path model. The number on the PLS−PM path represents the path coefficient, and the arrow thickness is proportional to the path coefficient. The red arrow indicates positive coefficient, the blue arrow indicates negative coefficient, the real line indicates significant impact, and the dotted line indicates insignificant impact. LF−Cres, OPOM−Cres, HF−Cres: the contents of residue-derived carbon in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; LF−Csoil, OPOM−Csoil, HF−Csoil: soil native carbon contents in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; invertase: invertase activity; catalase: catalase activity.
The random forest model was used to analyze the importance of the Csoil and Cres contents in three soil fractions (LF, OPOM, HF) and enzyme activity on the TOC content. The model used the mean square error increase rate (Inc MSE%) as the variable importance measurement indicator; the higher the value, the more important it is. Figure 6 exhibits that among the factors affecting the TOC content, the LF−Cres showed the highest value (23.48%) (p < 0.01), followed by the HF−Csoil (21.01%) and LF−Csoil (19.35%) (p < 0.01). The OPOM−Csoil showed little effect on the TOC content. For the enzyme activities, the catalase accounted for 16.86% (p < 0.05) and invertase showed no significant effect.
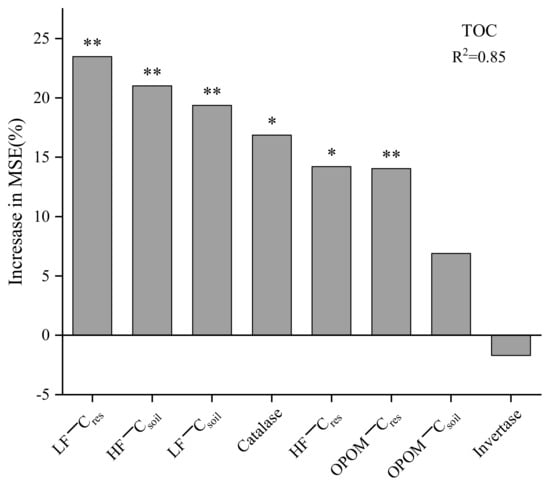
Figure 6.
Random forest importance model. * means p < 0.05, ** means p < 0.01. TOC: total soil organic carbon; LF−Cres, OPOM−Cres, HF−Cres: the contents of residue-derived carbon in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; LF−Csoil, OPOM−Csoil, HF−Csoil: soil native carbon contents in light fraction, occluded-particulate organic matter fraction and heavy fraction, respectively; Invertase: invertase activity; Catalase: catalase activity.
4. Discussion
4.1. Changes of the TOC Contents After Nitrogen Application
The change in SOC content is a long and complex process. Our study found that the TOC content decreased along with incubation in different treatments, which agreed with the perspective that soil incubation is a process of carbon loss []. The carbon efflux by soil mineralization and carbon influx via residue humification together determined the change in SOC content. When the soil lacked available nitrogen, K-strategy microorganisms would show an advantage in competition and they could achieve nitrogen from soil organic matter to alleviate nitrogen deficiency and promote the microbial decomposition of soil native carbon [,]. And the SOC influx from residue could not compensate for the decomposition of soil native carbon [], ultimately leading to a decrease in the TOC content []. Soil microorganisms play an important role in the transformation and fixation of soil organic carbon. Future studies should integrate microbiological methods to further explore the role of microorganisms in the soil carbon turnover under split nitrogen application, so as to understand the dynamic changes in soil carbon more comprehensively. In this study, the nitrogen fertilization slowed down the loss of SOC on a certain time scale (Figure 1). It might be due to the fact that the application of nitrogen fertilizer combined with residue addition provided relatively sufficient nitrogen resources for the incubated soil, then weakened the positive priming effect caused by residue input, and reduced the decomposition of soil native carbon by microorganisms. This study further verified that the application of nitrogen fertilizer on the basis of residue addition could reduce the loss of soil native carbon [].
By comparing different nitrogen application methods, our study identified that one-time application increased the TOC content compared to three-time application in the short term (up to 180 days), while in the long term (after 360 days), the SOC content became similar regardless of nitrogen application methods (Figure 1). On the one hand, it might be due to the fact that in the case of exogenous carbon input, one-time nitrogen application provided sufficient nitrogen during the early stage of soil incubation. Consequently, soil microorganisms preferentially decomposed readily available organic matter [,], which avoided the decomposition of nitrogen-containing organic matter (protein, amino sugar, etc.) by soil microorganisms []. On the other hand, one-time nitrogen application might promote the decomposition, transformation and fixation of residue-derived carbon [] in the soil in the short term and finally form soil particulate organic carbon [] or microorganism-synthesized organic carbon [], thereby increasing the TOC content. In addition, the C/N ratio of wheat residue is usually higher than the optimum value of 25:1 required for microbial degradation. In the event of no sufficient external nitrogen fertilization, some soil-derived nitrogen will be snatched for residue decomposition in the early stage of incubation, and the microbial activity and metabolism would be weak [], resulting in the relatively low SOC content under three-time application of nitrogen.
4.2. The Loss Pattern of Soil Native Carbon (Csoil) in the SOC Fractions Under Different Treatments
We found that the Csoil contents in the LF, OPOM and HF decreased significantly after 180, 540 and 360 days of incubation, respectively (Figure 2), indicating that the LF was more sensitive to exogenous substances (residue and nitrogen fertilizer) than OPOM and HF, which is consistent with the reports by Zhang et al. []. This should be mainly attributed to the differences in the composition, stability, and conversion rate of different SOC fractions. Specifically, as the active organic carbon pool, the LF was mainly composed of incompletely decomposed plant residues and microbial residues, which could be easily decomposed and utilized by microorganisms, and was more sensitive and responsive to exogenous substances. The OPOM was physically protected by soil aggregates, and the HF was mainly a combination of humus and soil minerals with high stability [,]. Compared with non-nitrogen-fertilized treatments, nitrogen fertilization, especially split application, relatively increased the Csoil contents in LF, OPOM and HF over 0–180 days of incubation. This is because soil microorganisms could obtain abundant nitrogen sources from fertilizer without having to compete for available nitrogen in the soil. The utilizations of carbon and nitrogen sources in the soil by microorganisms were both reduced since soil typically maintains a relatively stable C/N ratio. Therefore, the continuous application of nitrogen sources was conducive to the stability of the soil carbon pool and, thus, helped to maintain stable Csoil in this study. In the long run (540 days), both one-time and three-time nitrogen applications inhibited the Csoil loss from LF and HF compared with the only residue-added treatment, which further supported the hypothesis that microorganisms preferentially use exogenous nitrogen and induce less soil native organic carbon mineralization []. In addition, the addition of urea rapidly increased the concentration of NH4+, causing ammonium toxicity to soil microorganisms [,], which was not conducive to the mineralization and decomposition of soil organic matter.
In terms of the total loss rate of Csoil, it ranked as HF > LF > OPOM (Table 2). It is related to the high carbon content in the HF; for example, the HF−Csoil content accounted for 78.93% of the TOC content in the studied black soil. At the same time, the phenomena also indicated that although the HF is defined as mineral-bound organic carbon with strong stability [,], it has always been in dynamic change states and is the main source of soil carbon loss. This study also found that residue addition increased the Csoil loss of HF (Table 2), which might be because the residue was released into the HF under the action of microorganisms, then formed new soil aggregates with residue-derived carbon as the cementation nucleus [,], thereby releasing carbon from the original soil aggregates and promoting the loss of native carbon from HF, i.e., the priming effect. Overall, the application of nitrogen fertilizer on the basis of residue incorporation inhibited the loss of native soil carbon in each SOC fraction to a certain extent, with the inhibitory effect being more pronounced when nitrogen was split applied.
4.3. The Retention Characteristics of Residue-Derived Carbon (Cres) in Soil Fractions Under Different Treatments
The newly added crop residue usually mainly exists in the form of undecomposed residue in the LF of soil, and a few exist in the OPOM and HF. With the progress of incubation, the residue could be decomposed and transformed under the action of microorganisms, then gradually transferred into the OPOM and HF []. Since the residue-derived carbon could be divided into easily degradable non-structural carbon (soluble sugar and starch, accounted for about 20%) and recalcitrant structural carbon (cellulose and lignin, about 80%) [], in the early stage of incubation, easily degradable organic matter in the residue was rapidly decomposed and transformed. Then, the residue entered into the slow decomposition stage, and, thus, the contents of residue-derived carbon in each SOC fraction were basically stable in the later stage of incubation. This study also exhibited that the application of nitrogen fertilizer on the basis of residue addition significantly increased the content of Cres in the OPOM on the 180th day of incubation, and the one-time nitrogen application increased the Cres content in the HF compared with the split nitrogen application. It revealed that nitrogen application might lead to the rapid decomposition of residue and release of easily degradable carbon sources, thereby increasing the Cres content in the OPOM in the short term. After an adequate amount of nitrogen application, soil microorganisms rapidly utilized nitrogen to decompose and transform the LF−Cres, thereby enhancing its transport and transformation processes. In the short term, one-time nitrogen application provided a larger amount of nitrogen in the soil than split nitrogen application, thus promoting the conversion of Cres more significantly. This was manifested as a relative increase in the Cres in HF. Specifically, one-time nitrogen application could lead to the release of almost all nitrogen in a short period, resulting in a rapid microbial reaction and an efficient use of available nitrogen, which promoted the decomposition of the residue-derived carbon and increased the Cres content in HF. Although the split application of nitrogen was not as rapid as the one-time application, it supplied nitrogen more durably and supported the growth and metabolism of microorganisms, stimulating the decomposition of residue at different stages, thus improving the carbon stability in the soil.
By the end of the incubation, nitrogen application, especially the split nitrogen application, significantly increased the contents and residual rates of Cres in the OPOM and HF, indicating that split nitrogen application could provide more sustainable nutritional support and allow microorganisms to convert external carbon into more recalcitrant organic matter, thereby enhancing the organic carbon storage capacity of the soil. This means that split nitrogen application could promote the fixation of external carbon and be conducive to improving soil carbon stability.
4.4. The Variation Characteristics of Soil Enzyme Activity
Soil enzymes could promote the circulation of nutrients by catalyzing biochemical reactions in the soil and play an important role in the agroecosystem. Our study found that under different treatments, the soil invertase activity did not change significantly, which was consistent with the results of Hu et al. [], since it was speculated that soil moisture was the key factor causing this phenomenon. Gong et al. [] found that there was a significant positive correlation between soil invertase activity and soil moisture content, and linear regression analysis also showed that soil invertase activity was mainly affected by soil moisture. Our incubation study was carried out in an incubator with ventilation, darkness, constant temperature, and constant humidity. The soil moisture was adjusted and maintained at a fixed level. Therefore, there was no significant change in invertase activity along with incubation under different treatments. For soil catalase activity, the addition of wheat residue resulted in a rapid increase after pre-incubation of 2 days at 4 °C, while as the incubation progressed, the catalase activity decreased in all treatments, with the fastest decrease observed in the one-time nitrogen application treatment. This might be because the residue contained a large amount of lignin and cellulose, and the catalase could mainly be involved in the decomposition of lignin, which accelerated the release of cellulose wrapped by lignin and provided sufficient substrates for the enzyme. In terms of nitrogen application methods, the soil catalase activity in the split nitrogen application treatment continued to be higher than that in one-time nitrogen application after 180 days of incubation. This might be due to the continuous and abundant supply of nitrogen provided by the split application of nitrogen fertilizer for soil fungi, which optimized the living environment of microorganisms [], then promoted fungal metabolic activities and stimulated the fungal secretion of catalase to be involved in carbon and nitrogen cycles, resulting in an increase in catalase activity in the soil [].
4.5. The Contribution of SOC Fractions and Enzyme Activities on the Stability of Soil Carbon Pool
Through correlation analysis, the PLS−PM structure model and random forest importance model, it could be found that there was a significant positive correlation between the LF−SOC and TOC, and the LF−SOC showed a significant positive effect on the TOC (Figure 4). It reflected that the content of SOC in active fractions determined the storage of soil carbon pool, and the LF−SOC could be used to predict the stability of the TOC in the short term, which is consistent with the research conducted by Ma et al. []. It might be due to the high carbon content in the light fraction, despite the small proportion in the whole soil []. This study also found that the HF−Csoil was significantly positively correlated with and contributed greatly to the TOC; nevertheless, through the PLS−PM structural equation, it was found that the HF-SOC content had a significant negative effect on the TOC. This may be because soil clay particles are the main component of the HF and an important carrier of SOC []. However, the adsorption sites for organic carbon on clay particles would be easily saturated since the Csoil content in the HF was already high, which led to the reduced adsorption capacity for exogenous organic carbon []. Therefore, the amount of residue carbon that could enter the HF was limited, which suggested that it was relatively difficult to increase the TOC by transferring external carbon into the HF. Furthermore, the invertase and catalase could mainly drive the decomposition and transformation of soil organic matter []. This study found that the catalase activity was significantly positively correlated with and had a significant positive effect on the LF-SOC content (Figure 4), possibly because the catalase accelerated the oxidation of aromatic compounds and oxidized simple aromatic compounds in the soil to quinones, then reacted with proteins and sugars in the soil to generate complex organic matter. This would be beneficial to the retention of soil active organic matter and could provide carbon sources and nutrients for microorganisms, thereby promoting the accumulation of active organic carbon in the soil [].
The change in the TOC resulted from the dynamic equilibrium between the fixation of Cres and the loss of Csoil. Through correlation analysis, it was found that the loss rate of Csoil and residual rate of Cres were both significantly negatively correlated with the TOC, OPOM− and HF−SOC, indicating that the higher the SOC content in the OPOM and HF, the higher the overall stability of soil carbon pool, and the less soil carbon to be lost. On the contrary, if the content of soil stable carbon was low and the proportion of labile carbon was high, the loss rate of soil carbon and the residual rate of external carbon may both increase. In this case, the addition of exogenous organic materials will be particularly important as it could determine whether the soil functions as a carbon source or sink in the ecosystem. Our results also showed that the more exogenous carbon entered the soil, the greater the loss of native carbon; this is precisely a reflection of the turnover and renewal of soil carbon pools.
As seen from the above analysis, this study revealed the effect of split nitrogen application on the transport of residue-derived carbon individual carbon pools in black soil. However, further studies on the microbial mechanisms of the priming effect are needed in the future, which will contribute to a more comprehensive understanding of soil carbon cycle mechanisms and provide scientific support for carbon management in agricultural ecosystems.
5. Conclusions
During the incubation, the LF−Cres was gradually decomposed and transferred into the OPOM and HF, and the transfer became slower after 180 days. The sensitivity of Csoil in SOC fractions to exogenous materials ranked as LF > HF > OPOM according to the time interval, after which their Csoil began to be lost following the addition of wheat residue. Nitrogen application, especially split nitrogen application, could reduce the loss of Csoil in LF and increase the residual rate Cres in OPOM and HF, thereby enhancing exogenous carbon fixation and native carbon protection in black soil, ultimately stabilizing the soil carbon pool.
Author Contributions
Conceptualization, X.C., S.Z. and X.X.; methodology, X.C., S.J., J.G., X.W., M.X. and X.X.; investigation, X.C., S.J., J.G. and D.G.; data curation, X.C., S.Z., S.J., S.D. and X.X.; writing—original draft preparation, X.C. and S.Z.; writing—review and editing, X.C., S.Z., S.J., J.G., S.D., X.W., M.X., D.G. and X.X.; visualization, X.C. and S.Z.; supervision, X.X. and M.X.; resources, X.C. and M.X.; funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (41601309), Henan Provincial Joint Fund for Science and Technology Research and Development Program (242103810032), Student Research Training Program of Henan University of Science and Technology (2024437).
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Junaid, N.M.; Li, G.; Mudassir, N.M.; Zulfiqar, F.; Siddique, K.H.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration–An interplay between soil microbial community and soil organic matterdynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, J.; Dong, W.; Cui, S. The Spatiotemporal Characteristics and Driving Factors of Soil Degradation in the Black Soil Region of Northeast China. Agronomy 2024, 14, 2870. [Google Scholar] [CrossRef]
- Sulaeman, Y.; Maftuah, E.; Wulanningtyas, H.S. Assessment of the Susceptibility of Tropical Black Soil Formed in Various Parent Materials to Degradation under Different Land Uses. Mosc. Univ. Soil Sci. Bull. 2024, 79, 674–683. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, J.; Zhu, Y.; Kuang, E.; Han, J.; Shi, Y.; Chi, F.; Wei, D.; Liu, J. Transformation and sequestration of total organic carbon in black soil under different fertilization regimes with straw carbon inputs. Agriculture 2024, 14, 887. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef]
- Mendoza, O.; De Neve, S.; Deroo, H.; Li, H.; Sleutel, S. Do interactions between application rate and native soil organic matter content determine the degradation of exogenous organic carbon? Soil. Biol. Biochem. 2022, 164, 108473. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Zhang, Q. Effects of Long-Term Plastic Film Mulching and Straw Incorporation on Soil Organic Carbon Turnover. Master’s Thesis, Lanzhou University, Lanzhou, China, 2019. [Google Scholar]
- Chen, X.; Wu, S.; Kou, T.; Xue, P.; Tan, X.; Guo, D. Transport of straw-derived carbon in black soil and cinnamon soil and its response to nitrogen fertilization. Acta Pedol. Sin. 2022, 59, 1248–1257. [Google Scholar] [CrossRef]
- Sajjad, M.; Hussain, K.; Wajid, S.A.; Saqib, Z.A. The Impact of Split Nitrogen Fertilizer Applications on the Productivity and Nitrogen Use Efficiency of Rice. Nitrogen 2025, 6, 1. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 8227–8234. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, X.; Duan, J.; Pi, Y.; Lin, S. Effects of nitrogen reduction combined with biochar application on organic carbon mineralization and enzyme activity in paddy field. J. Soil Water Conserv. 2021, 35, 369–374. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, C.; McDowell, N.G.; Johnson, D.J.; Wang, M.; Luo, Y.; Zhou, X.; Huang, Z. Linking microbial community composition to C loss rates during wood decomposition. Soil Biol. Biochem. 2017, 104, 108–116. [Google Scholar] [CrossRef]
- Jahan, A.; Islam, A.; Sarkar, I.U.; Iqbal, M.; Ahmed, N.; Islam, R. Nitrogen response of two high yielding rice varieties as influenced by nitrogen levels and growing seasons. Geol. Ecol. Landsc. 2020, 6, 24–31. [Google Scholar] [CrossRef]
- Liang, K.; Zhong, X.; Huang, N.; Lampayan, R.M.; Liu, Y.; Pan, J.; Peng, B.; Hu, X.; Fu, Y. Nitrogen losses and greenhouse gas emissions under different N and water management in a subtropical double-season rice cropping system. Sci. Total Environ. 2017, 609, 46–57. [Google Scholar] [CrossRef]
- Maltese, E.N.; Carciochi, D.W.; Caviglia, P.O.; Rozas, H.R.S.; García, M.; Lapaz, A.O.; Ciampitti, I.A.; Calvo, N.I.R. Assessing the effect of split and additional late N fertilisation on N economy of maize. Field Crops Res. 2024, 308, 109279. [Google Scholar] [CrossRef]
- Wang, H.; Hu, G.; Xu, W.; Boutton, T.W.; Zhuge, Y.; Bai, E. Effects of nitrogen addition on soil organic carbon mineralization after maize stalk addition. Eur. J. Soil Biol. 2018, 89, 33–38. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Luo, Y.; Liu, S.; Xu, X.; Tong, C.; Shibistova, O.; Guggenberger, G.; Wu, J. Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Sun, Z.-A.; Zhang, X.; Hu, Z.-J.; Wang, K.-Y.; Chen, Q.; Meng, F.-Q. How different ratios of straw incorporation to nitrogen fertilization influence endogenous and exogenous carbon release from agricultural soils. Environ. Sci. 2021, 42, 459–466. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, Y.; Deng, X.; Qi, F.; Bi, X.; Liu, Y. Effects of controlled-release nitrogen fertilizer on decomposition of maize straw and organic carbon fractions in fluvo-aquic soil. J. Soil Water Conserv. 2020, 34, 292–298. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Lin, H.; Ntacyabukura, T.; Wang, Y.; Zhu, B. Effects of different long-term crop straw management practices on ammonia volatilization from subtropical calcareous agricultural soil. Atmos. Ocean. Sci. Lett. 2020, 13, 232–239. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Li, W.; Qiao, Y.Q.; Chen, H.; Du, S.Z.; Zhao, Z.; Cao, C.F. Soil organic matter composition and carbon pool management Index in Shajiang Black Soil as Affected by Straw Incorporation Coupled With Fertilization. J. Ecol. Rural Environ. 2014, 30, 475–480. [Google Scholar] [CrossRef]
- Zhou, M.; Gao, H.; Liu, S.; Li, H.; Liu, F.; Jiang, G.; Zhao, Y. Effects of Combined Application of Straw and Nitrogen Fertilizer on Microbial Activity and Aggregate Distribution in Fluvo Aquic Soil. J. Soil Water Conserv. 2022, 36, 340–345. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.; He, Q.; Wu, Y.; Xie, D. Effect of tillage systems on light fraction carbon in a purple paddy soil. Acta Ecol. Sin. 2012, 32, 4379–4387. [Google Scholar] [CrossRef]
- Dong, J.; Wang, W.; Zhao, D.; Zhang, C.; Fang, J.; Wang, L.; Zhang, Q.; Liu, J. A novel organic carbon accumulation mechanism in croplands in the Yellow River Delta, China. Sci. Total Environ. 2022, 806, 150629. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, J.; Ji, S.; Huang, M.; Quan, Q.; Liu, J. Effects of Contemporary Land Use Types and Conversions from Wetland to Paddy Field or Dry Land on Soil Organic Carbon Fractions. Sustainability 2020, 12, 2094. [Google Scholar] [CrossRef]
- Treseder, K.-K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, W. Review of researches on soil aggregate and soil organic carbon. Chin. J. Eco-Agric. 2011, 19, 447–455. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Wu, X.; Liang, G.; Gao, C.; Li, J.; Jin, D.; Wang, B.; Zhang, M.; Zheng, F.; et al. The dominant microorganisms vary with aggregates sizes in promoting soil carbon accumulation under straw application. Arch. Agron. Soil. Sci. 2021, 69, 1–17. [Google Scholar] [CrossRef]
- Fang, Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Change Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef]
- Hu, J.; Tao, R.; Chu, G. Partial replacement of inorganic N with cattle manure and combining use of biochemical inhibitors inhibit organic carbon conversion in soil. Plant Nutr. Fertil. Sci. 2020, 26, 19–31. [Google Scholar] [CrossRef]
- Gong, S.; Liu, X.; Zhang, Z.; Ma, X.; Kong, Y. Effect of different nitrogen application measures on soil enzyme activities and nitrogen turnover in winter wheat cropland. Ecol. Environ. Sci. 2020, 29, 2215–2222. [Google Scholar] [CrossRef]
- Borase, D.-N.; Nath, C.-P.; Hazra, K.-K.; Senthilkumar, M.; Singh, S.; Praharaj, C.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Wang, Y.; Yao, Q.; Yang, K. Effects of slow-release nitrogen and urea combined application on soil physicochemical properties and fungal community under total straw returning condition. Environ. Res. 2024, 252, 118758. [Google Scholar] [CrossRef]
- Ma, S.; Li, Z.; Wang, B.; Liu, R.; Ge, R.; Wang, G. Changes in soil active organic carbon under different management types of bamboo stands. Acta Ecol. Sin. 2012, 32, 2603–2611. [Google Scholar] [CrossRef][Green Version]
- Yagüe, M.R.; Domingo-Olivé, F.; Bosch-Serra, À.D.; Poch, R.M.; Boixadera, J. Dairy Cattle Manure Effects on Soil Quality: Porosity, Earthworms, Aggregates and Soil Organic Carbon Fractions. Land. Degrad. Dev. 2016, 27, 1753–1762. [Google Scholar] [CrossRef]
- Huntington, T.G. Carbon sequestration in an aggrading forest ecosystem in the Southern USA. Soil Sci. Soc. Am. J. 1995, 59, 1459–1467. [Google Scholar] [CrossRef]
- Islam, R.; Singh, B.; Dijkstra, F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Zhu, C.; Long, Q.; Dong, S.; Shi, K.; Jiang, G.; Li, X.; Zhang, C.; Liu, F.; Shen, F.; Liu, S. Effects of rotary and deep tillage modes on soil microbial biomass carbon and nitrogen and enzyme activities in fluvo-aquic soil under wheat-maize rotation system. Plant Nutr. Fertil. Sci. 2020, 26, 51–63. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil. Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).