Population Parameters as Key Factors for Site-Specific Distribution of Invasive Weed Rhynchosia senna in Semiarid Temperate Agroecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Study Site and Plant Material
2.2. Demographic Parameter Estimation
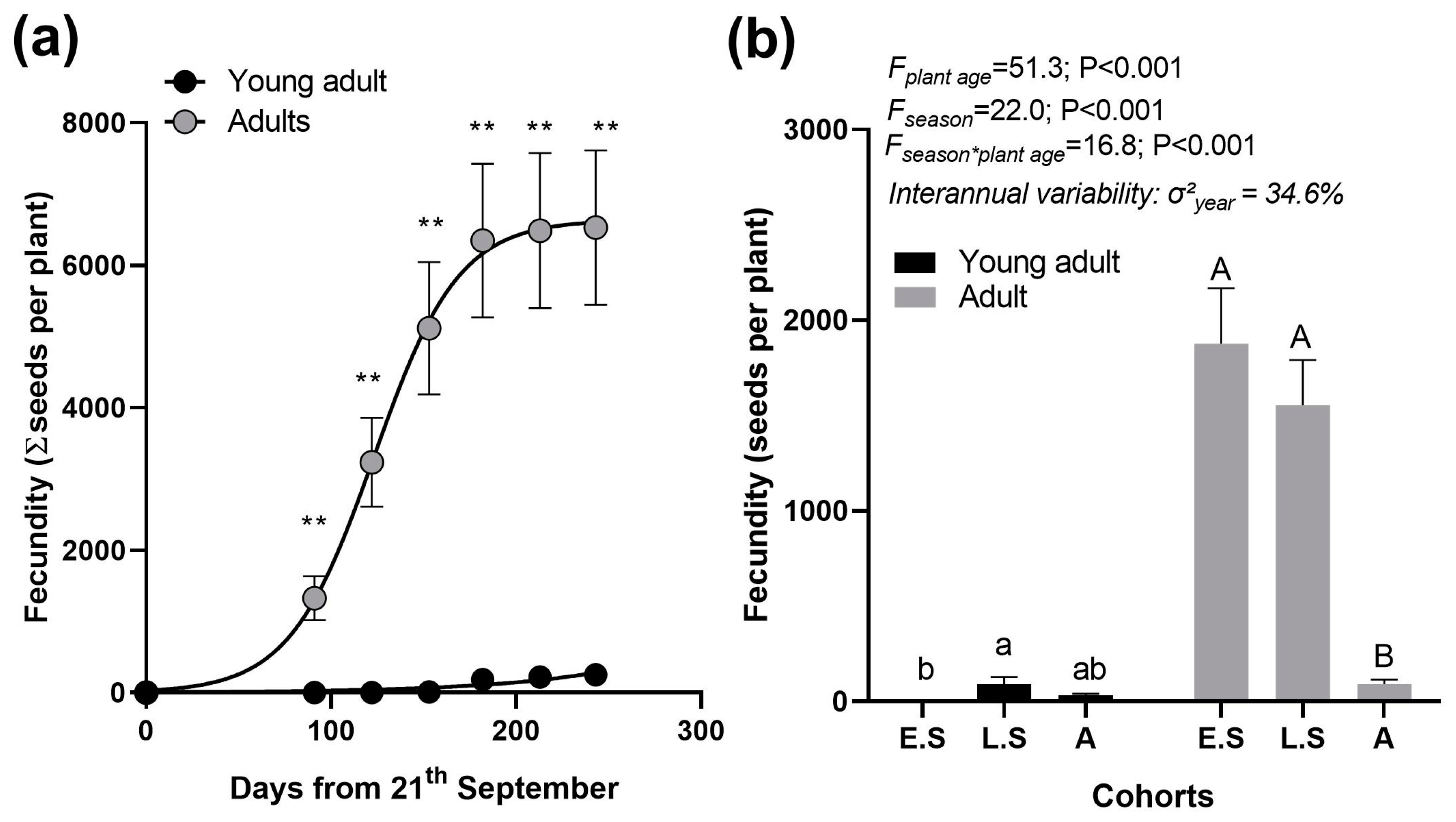
2.2.1. Experiment 1: Fecundity
2.2.2. Experiment 2: Pre-Dispersal Seed Predation
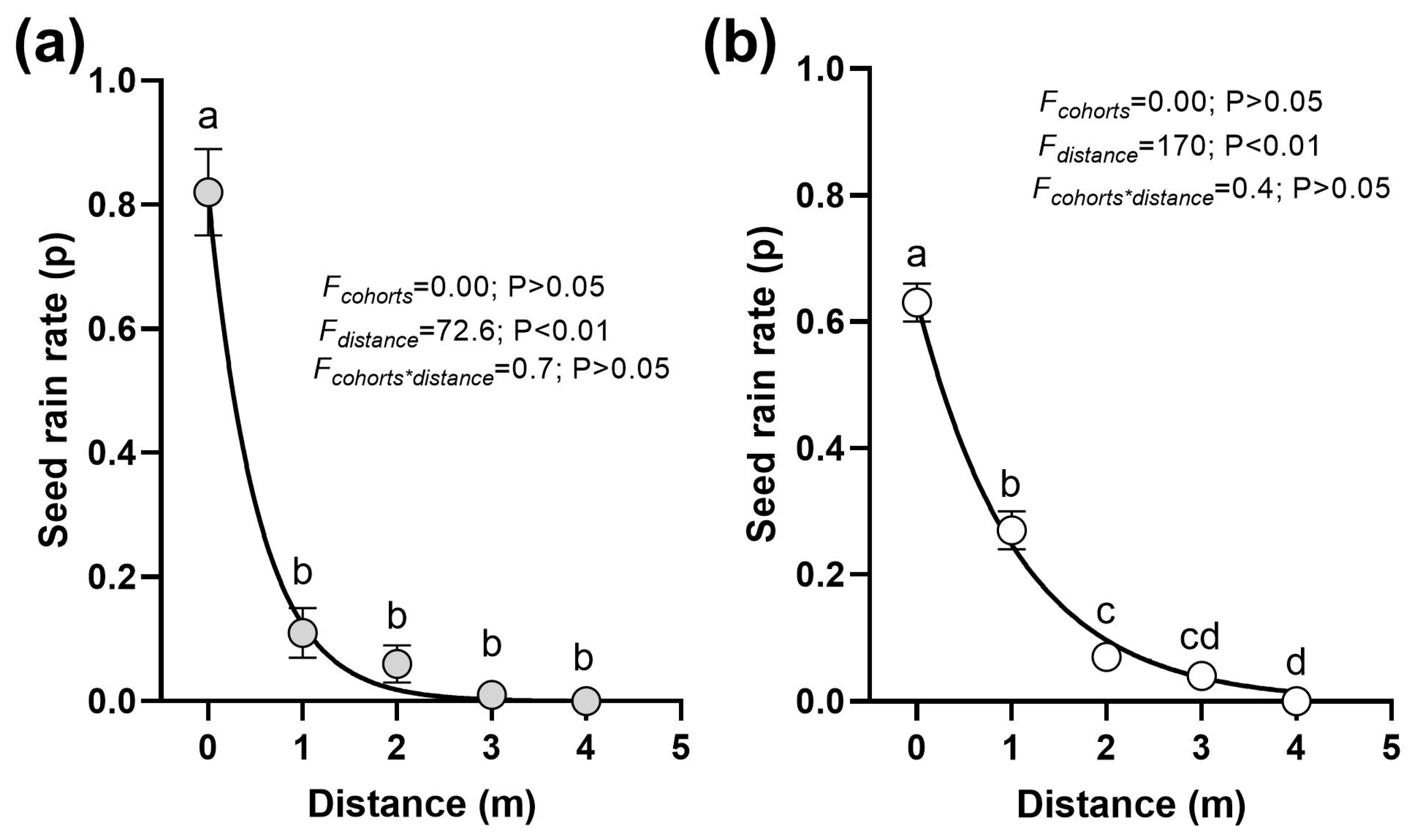
2.2.3. Experiment 3: Seed Rain Dispersal Distance
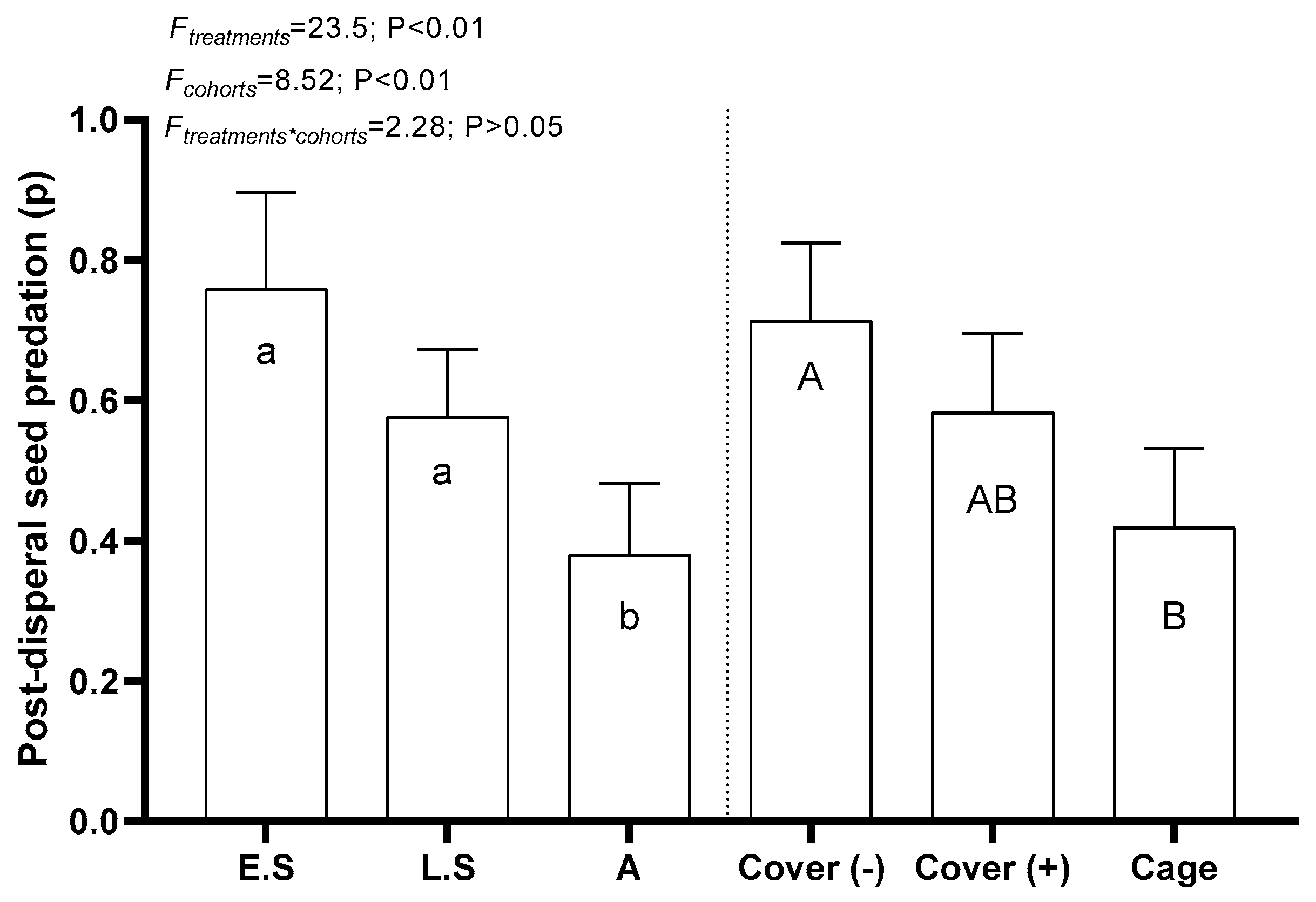
2.2.4. Experiment 4: Post-Dispersal Seed Predation
2.2.5. Experiment 5: Seed Bank Viability and Dormancy Loss
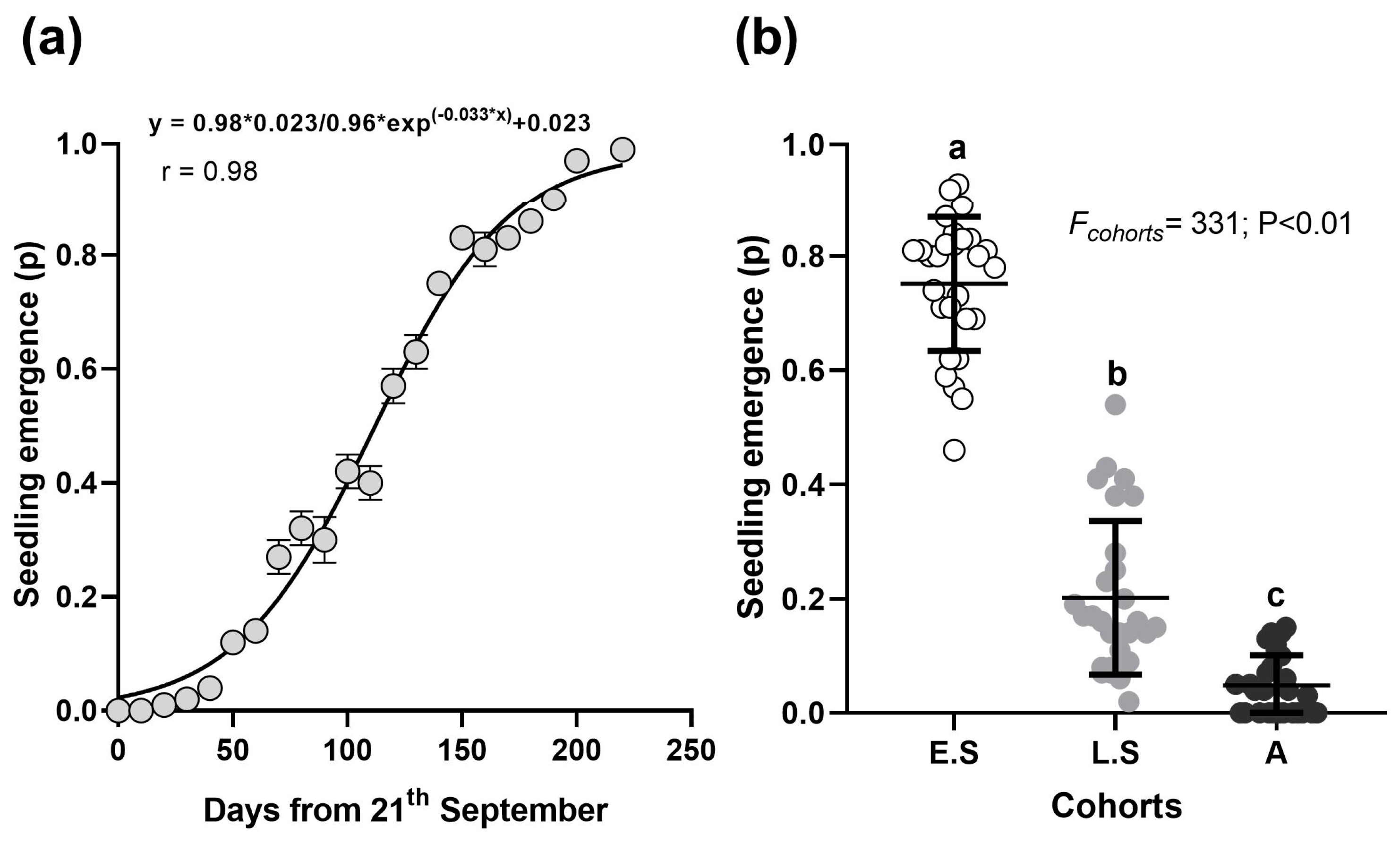
2.2.6. Experiment 6: Field Emergence
2.2.7. Experiment 7: Plant Survivorship in Summer and Winter
2.3. Statistical Analysis
3. Results
3.1. Fecundity
3.2. Pre-Dispersal Seed Predation
3.3. Seed Rain Dispersal Distance
3.4. Post-Dispersal Seed Predation
3.5. Seed Bank Behavior
3.6. Field Emergence Experiment
3.7. Survivorship During Summer and Winter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Exp. | Experiment |
| P1000 | Weight of 1000 seeds |
| RS | Rynchosia senna var. senna |
| Non-PY | Nonphysical dormancy seeds |
| PY | Physical dormancy |
References
- Cabrera, D.C.; Chaila, S.; Sobrero, M.T. Phytosociological survey of sugarcane crop weeds in different agroecological areas in Tucumán Province, Argentina. Planta Daninha 2019, 37, 31–38. [Google Scholar] [CrossRef]
- Pannell, D.J.; Stewart, V.; Bennet, A.; Monjardino, M.; Schmidt, C.; Powles, S.B. RIM: A bioeconomic model for integrated weed management of Lolium rigidum in Western Australia. Agric. Syst. 2004, 79, 305–325. [Google Scholar] [CrossRef]
- Parsons, D.J.; Benjamin, L.R.; Clarke, J.; Ginsburg, D.; Mayes, A.; Milne, A.E.; Wilkinson, D.J. Weed manager—A model-based decision support system for weed management in arable crops. Comput. Electron. Agric. 2009, 65, 155–167. [Google Scholar] [CrossRef]
- Lodovichi, M.V.; Blanco, A.M.; Chantre, G.R.; Bandoni, J.A.; Sabbatini, M.R.; Vigna, M.; López, R.; Gigón, R. Operational planning of herbicide-based weed management. Agric. Syst. 2013, 121, 117–129. [Google Scholar] [CrossRef]
- Wallinga, J.; Groeneveld, R.M.W.; Lotz, L.A.P. Measures that describe weed spatial patterns at different levels of resolution and their applications for patch spraying of weeds. Weed Res. 1998, 38, 351–359. [Google Scholar] [CrossRef]
- Deen, W.; Cousens, R.; Warringa, J.; Bastiaans, L.; Carberry, P.; Rebel, K.; Riha, S.; Murphy, C.; Benjamin, L.R.; Cloughley, C.; et al. An evaluation of four crop: Weed competition models using a common data set. Weed Res. 2003, 43, 116–129. [Google Scholar] [CrossRef]
- Forcella, F.; Arnold, R.L.B.; Sanchez, R.; Ghersa, C.M. Modeling seedling emergence. Field Crops Res. 2000, 67, 123–139. [Google Scholar] [CrossRef]
- González-Andújar, J.L.; Fernández-Quintanilla, C. Modelling the population dynamics of annual ryegrass (Lolium rigidum) under various weed management systems. Crop Prot. 2004, 23, 723–729. [Google Scholar] [CrossRef]
- Colbach, N.; Dürr, C.; Roger-Estrade, J.; Caneill, J. How to model the effects of farming practices on weed emergence. Weed Res. 2005, 45, 2–17. [Google Scholar] [CrossRef]
- Gardarin, A.; Dürr, C.; Colbach, N. Modeling the dynamics and emergence of a multispecies weed seed bank with species traits. Ecol. Model. 2012, 240, 123–138. [Google Scholar] [CrossRef]
- Renzi, J.P.; Chantre, G.R.; González-Andujar, J.L.; Cantamutto, M.A. Development and validation of a simulation model for hairy vetch (Vicia villosa Roth) self-regeneration under different crop rotations. Field Crop Res. 2019, 235, 79–86. [Google Scholar] [CrossRef]
- Demaría, M.R.; Aguado Suárez, I.; Steinaker, D.F. Reemplazo y fragmentación de pastizales pampeanos semiáridos en San Luis, Argentina. Ecol. Austral 2008, 18, 55–70. [Google Scholar]
- Viglizzo, E.F.; Frank, F.C.; Carreño, L.V.; Jobbágy, E.G.; Pereyra, H.; Clatt, J.; Pincén, D.; Ricard, M.F. Ecological and environmental footprint of 50 years of agricultural expansion in Argentina. Glob. Change Biol. 2011, 17, 959–973. [Google Scholar] [CrossRef]
- Rauber, R.B.; Demaría, M.R.; Jobbágy, E.G.; Arroyo, D.N.; Poggio, S.L. Weed Communities in Semiarid Rainfed Croplands of Central Argentina: Comparison between Corn (Zea mays) and Soybean (Glycine max) Crops. Weed Sci. 2018, 66, 368–378. [Google Scholar] [CrossRef]
- Bianco, C.A.; Kraus, T.A. El género Rhynchosia en el sur de la provincia de Córdoba. Rev. Fac. Agron. UNLPm 1995, 8, 29–39. [Google Scholar]
- Bezerra, L.M.P.A.; Oliveira, A.C.S.; Santos, J.S.; Vargas, W.; Candido, E.S.; Monteiro, T.C.; Vatanparast, M.; Fortuna-Perez, A.P.A. New species of Rhynchosia (Leguminosae, Papilionoideae) from Bahia State, Brazil. Phytotaxa 2019, 406, 84–90. [Google Scholar] [CrossRef]
- Solomon Raju, A.J.; Venkata Ramana, K. Pollination ecology of Rhynchosia cana (Willd.) DC. (Fabaceae), an erect sub-shrub, in peninsular India. J. Threat. Taxa 2017, 9, 10757–10770. [Google Scholar] [CrossRef]
- Solomon Raju, A.J.; Venkata Ramana, K. Pollination Ecology of Rhynchosia minima (L.) DC. (Fabaceae) in the Southern Eastern Ghats, Andhra Pradesh, India. Ecol. Balk. 2019, 11, 108–126. [Google Scholar]
- Ríos, A.; García, M.A.; Belgeri, A.; Caulin, M.P.; Mailhos, V.; San Ramón, G. Comunidad Florística Asociadas a los Sistemas de Siembra Directa en Uruguay. Seminario Internacional “Viabilidad del Glifosato en Sistemas Productivos Sustentables”; Instituto Nacional de Investigación Agropecuaria: Montevideo, Uruguay, 2008.
- Martínez-Bernal, A.; Duno-de Stefano, R.; Lorena-Can, L. Los géneros Cajanus y Rhynchosia (Leguminosae, Papilionoideae, Phaseoleae, Cajaninae) en la península de Yucatán, México. Rev. Mex. Biodivers. 2011, 82, 1098–1107. [Google Scholar]
- Ali, H.H.; Tanveer, A.; Nadeem, M.A.; Asghar, H.N. Methods to break seed dormancy of Rhynchosia capitata, a summer annual weed. Chil. J. Agric. Res. 2012, 71, 483–487. [Google Scholar] [CrossRef]
- Morales-Menéndez, A.M.; Martinez Ramirez, R.; Ruíz Traba, J.; Rodriguez, A.F. Distribución de malezas en el área cañera del complejo agroindustrial de derivados de la caña de azúcar (CADCA) “Pedro Camejo” del estado Apure, Venezuela. In Proceedings of the XXII Congreso Latinoamericano de Malezas (ALAM)—I Congreso Argentino de Malezas (ASACIM), Buenos Aires, Argentina, 9–10 September 2015. [Google Scholar]
- Milano, C. Leguminosas Herbáceas Nativas: Una Alternativa Para la Restauración de Pastizales y Suelos Degradados en el Sudoeste Bonaerense. Master’s Thesis, Universidad Nacional del Sur. Bahía Blanca, Buenos Aires, Argentina, 2018. [Google Scholar]
- Quintana, M.; Reinoso, O.J.; Renzi, J.P. Seed colour dimorphism in Rynchosia senna does not influence physical dormancy and predation levels. In Proceedings of the III Reunión Argentina de Biología de Semillas, Buenos Aires, Argentina, 2–25 August 2023. [Google Scholar]
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Gatsis, T.; Zacharis, K.; Galanis, M. Field bindweed (Convolvulus arvensis L.) and redroot pigweed (Amaranthus retroflexus L.) control in potato by pre-or post-emergence applied flumioxazin and sulfosulfuron. Chil. J. Agric. Res. 2013, 73, 24–30. [Google Scholar] [CrossRef]
- Hettinger, K.; Miller, Z.; Hubbel, K.; Seipel, T. Crop rotation and cultivation effects on Convolvulus arvensis population dynamics in small grain organic cropping systems. Front. Agron. 2023, 5, 1177461. [Google Scholar] [CrossRef]
- Naylor, R.E. Weed population dynamics. In Encyclopedia of Applied Plant Science; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1485–1494. [Google Scholar] [CrossRef]
- Freckleton, R.P.; Watkinson, A.R. How does temporal variability affect predictions of weed population numbers? J. Appl. Ecol. 1998, 35, 340–344. [Google Scholar] [CrossRef]
- Somerville, G.J.; Sønderskov, M.; Mathiassen, S.K.; Metcalfe, H. Spatial modelling of within-field weed populations; a review. Agronomy 2020, 10, 1044. [Google Scholar] [CrossRef]
- Jurado-Expósito, M.; López-Granados, F.; García-Torres, L.; García-Ferrer, A.; de la Orden, M.S.; Atenciano, S. Multi-species weed spatial variability and site-specific management maps in cultivated sunflower. Weed Sci. 2003, 51, 319–328. [Google Scholar] [CrossRef]
- González-Andújar, J.L.; Saavedra, M. Spatial distribution of annual grass weed populations in winter cereals. Crop Prot. 2003, 22, 629–633. [Google Scholar] [CrossRef]
- Renzi, J.P.; Chantre, G.R.; Cantamutto, M.A. Development of a thermal-time model for combinational dormancy release of hairy vetch (Vicia villosa ssp. villosa). Crop Pasture Sci. 2014, 65, 470–478. [Google Scholar] [CrossRef]
- Renzi, J.P.; Chantre, G.R.; Cantamutto, M.A. Self-regeneration of hairy vetch (Vicia villosa Roth) as affected by seedling density and soil tillage method in a semi-arid agroecosystem. Grass Forage Sci. 2017, 72, 524–533. [Google Scholar] [CrossRef]
- Molinari, F.A.; Blanco, A.M.; Fré, F.R.N.; Juan, V.F.; Chantre, G.R. A Weed population dynamics model for integrated weed-management decision-making support: Euphorbia davidii subils in soybean crops as a simulation study. Agronomy 2022, 12, 2369. [Google Scholar] [CrossRef]
- Molinari, F.A.; Blanco, A.M.; Vigna, M.R.; Chantre, G.R. A Simulation Model as the Core for Integrated Weed Management Decision Support Systems: The Case of Avena fatua-Winter Wheat in the Semiarid Pampean Region of Argentina. In Decision Support Systems for Weed Management; Chantre, G., González-Andújar, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 311–333. [Google Scholar] [CrossRef]
- Benvenuti, S.; Mazzoncini, M. “Active” Weed Seed Bank: Soil Texture and Seed Weight as Key Factors of Burial-Depth Inhibition. Agronomy 2021, 11, 210. [Google Scholar] [CrossRef]
- Ali, H.H.; Tanveer, A.; Nadeem, M.A.; Asghar, H.N.; Javaid, M.M. Germination Ecology of Rhynchosia capitata: An emerging summer weed in Asia. Planta Daninha 2013, 31, 249–257. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Berger, J.D.; Shrestha, D.; Ludwig, C. Reproductive strategies in mediterranean legumes: Trade-offs between phenology, seed size and vigor within and between wild and domesticated Lupinus species collected along aridity gradients. Front. Plant Sci. 2017, 8, 548. [Google Scholar] [CrossRef]
- Gardarin, A.; Dürr, C.; Mannino, M.R.; Busset, H.; Colbach, N. Seed mortality in the soil is related to seed coat thickness. Seed Sci. Res. 2010, 20, 243–256. [Google Scholar] [CrossRef]
- Woods, T.M.; Hartnett, D.C.; Ferguson, C.J. High propagule production and reproductive fitness homeostasis contribute to the invasiveness of Lespedeza cuneata (Fabaceae). Biol. Invasions 2009, 11, 1913–1927. [Google Scholar] [CrossRef]
- Renzi, J.P.; Brus, J.; Pirintsos, S.; Erdős, L.; Duchoslav, M.; Smýkal, P. Release of Medicago truncatula Gaertn. and Pisum sativum subsp. elatius (M. Bieb.) Asch. et Graebn. Seed dormancy tested in soil conditions. Agronomy 2020, 10, 1026. [Google Scholar] [CrossRef]
- Jermy, T.; Szentesi, Á. Evolutionary aspects of host plant specialization a study on bruchids (Coleoptera: Bruchidae). Oikos 2003, 101, 196–204. [Google Scholar] [CrossRef]
- Han, Y.J.; Baskin, J.M.; Tan, D.Y.; Baskin, C.C.; Wu, M.Y. Effects of predispersal insect seed predation on the early life history stages of a rare cold sand-desert legume. Sci. Rep. 2018, 8, 3240. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Villagra, P.E. Prosopis flexuosa DC. (Fabaceae, Mimosoideae). Kurtziana 2010, 35, 47–61. [Google Scholar]
- Velez, S.; Chacoff, N.P.; Campos, C.M. Pre-dispersal seed loss in two Prosopis species (Fabacea: Mimosoidea) from the Monte Desert, Argentina. Ecol. Austral 2018, 28, 325–479. [Google Scholar] [CrossRef]
- Raghu, S.; Wiltshire, C.; Dhileepan, K. Intensity of pre-dispersal seed predation in the invasive legume Leucaena leucocephala is limited by the duration of pod retention. Austral Ecol. 2005, 30, 310–318. [Google Scholar] [CrossRef]
- Van Klinken, R.D.; White, A.J. The role of pre-and post-dispersal seed predation in determining total seed loss. Basic Appl. Ecol. 2014, 15, 581–589. [Google Scholar] [CrossRef]
- Heredia Pinos, M.R. Predación Post-Dispersiva de Semillas de Malezas en un Agroecosistema Pampeano. Master’s Thesis, Facultad de Cs. Agrarias, Universidad Nacional de Rosario, Córdoba, Spain, 2017. [Google Scholar]
- Ooi, K.J.; Auld, T.D.; Denham, A.J. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: Implications for seed bank persistence under climate change. Plant Soil 2012, 353, 289–303. [Google Scholar] [CrossRef]
- Baskin, J.M.; Nan, X.; Baskin, C.C. A comparative study of seed dormancy and germination in an annual and a perennial species of Senna (Fabaceae). Seed Sci. Res. 1998, 8, 501–512. [Google Scholar] [CrossRef]
- Renzi, J.P.; Quintana, M.; Bruna, M.; Reinoso, O. Environmental drivers of seed persistence and seedling trait variation in two Neltuma species (Fabaceae). Seed Sci. Res. 2024, 34, 186–193. [Google Scholar] [CrossRef]
- Tao, Q.; Chen, D.; Bai, M.; Zhang, Y.; Zhang, R.; Chen, X.; Sun, X.; Niu, T.; Nie, Y.; Zhong, S.; et al. Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions. Plants 2023, 12, 1876. [Google Scholar] [CrossRef]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental control of dormancy in weed seed banks in soil. Field Crops Res. 2000, 67, 105–122. [Google Scholar] [CrossRef]
- Jurado, E.; Westboy, M. Germination biology of selected central Australian plants. Aust. J. Ecol. 1992, 17, 341–348. [Google Scholar] [CrossRef]
- Bélanger, G.; Castonguay, Y.; Bertrand, A.; Dhont, C.; Rochette, P.; Couture, L.; Drapeau, R.; Mongrain, R.; Chalifour, F.P.; Michaud, R. Winter damage to perennial forage crops in eastern Canada: Causes, mitigation, and prediction. Can. J. Plant Sci. 2006, 86, 33–47. [Google Scholar] [CrossRef]
- Pufal, G.; Klein, A.M. Post-dispersal seed predation of three grassland species in a plant diversity experiment. J. Plant Ecol. 2013, 6, 468–479. [Google Scholar] [CrossRef]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Kumar, L.; Solhjouy-fard, S.; Tehrany, M.S.; Shabani, F.; Kalantar, B.; Esmaeili, A. Invasive weed species’ threats to global biodiversity: Future scenarios of changes in the number of invasive species in a changing climate. Ecol. Indic. 2020, 116, 106436. [Google Scholar] [CrossRef]
- Parker, T.A.; Lo, S.; Gepts, P. Pod shattering in grain legumes: Emerging genetic and environment-related patterns. Plant Cell 2021, 33, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Rani, S.; Shafi, S.; Zaffar, A.; Riyaz, I.; Wani, M.A.; Zargar, S.M.; Vara Prasad, P.V.; Sofi, P.A. Pod physical traits significantly implicate shattering response of pods in beans (Phaseolus vulgaris L.). Biol. Plant. 2024, 68, 107–116. [Google Scholar] [CrossRef]

| Plant Survival | Cohorts | p-Value | σ2year (%) | ||
|---|---|---|---|---|---|
| E.S | L.S | A | |||
| summer period | 0.82 | 0.64 | 0.84 | ns | 14.5 |
| winter period | 0.98 a | 0.74 b | 0.32 c | ** | |
| p-value | * | ns | ** | ||
| Cohort*periods | ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana, M.; Chantre, G.R.; Reinoso, O.; Renzi, J.P. Population Parameters as Key Factors for Site-Specific Distribution of Invasive Weed Rhynchosia senna in Semiarid Temperate Agroecosystems. Agronomy 2025, 15, 858. https://doi.org/10.3390/agronomy15040858
Quintana M, Chantre GR, Reinoso O, Renzi JP. Population Parameters as Key Factors for Site-Specific Distribution of Invasive Weed Rhynchosia senna in Semiarid Temperate Agroecosystems. Agronomy. 2025; 15(4):858. https://doi.org/10.3390/agronomy15040858
Chicago/Turabian StyleQuintana, Matías, Guillermo R. Chantre, Omar Reinoso, and Juan P. Renzi. 2025. "Population Parameters as Key Factors for Site-Specific Distribution of Invasive Weed Rhynchosia senna in Semiarid Temperate Agroecosystems" Agronomy 15, no. 4: 858. https://doi.org/10.3390/agronomy15040858
APA StyleQuintana, M., Chantre, G. R., Reinoso, O., & Renzi, J. P. (2025). Population Parameters as Key Factors for Site-Specific Distribution of Invasive Weed Rhynchosia senna in Semiarid Temperate Agroecosystems. Agronomy, 15(4), 858. https://doi.org/10.3390/agronomy15040858








