Effects of Nitrogen Fertilizer Spraying Time on Source–Sink Nitrogen Metabolism and Seed Oil Quality of Paeonia ostii ‘Fengdan’
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Materials and Design
2.3. Measurement Indicators and Methods
2.4. Data Analysis and Processing
3. Results
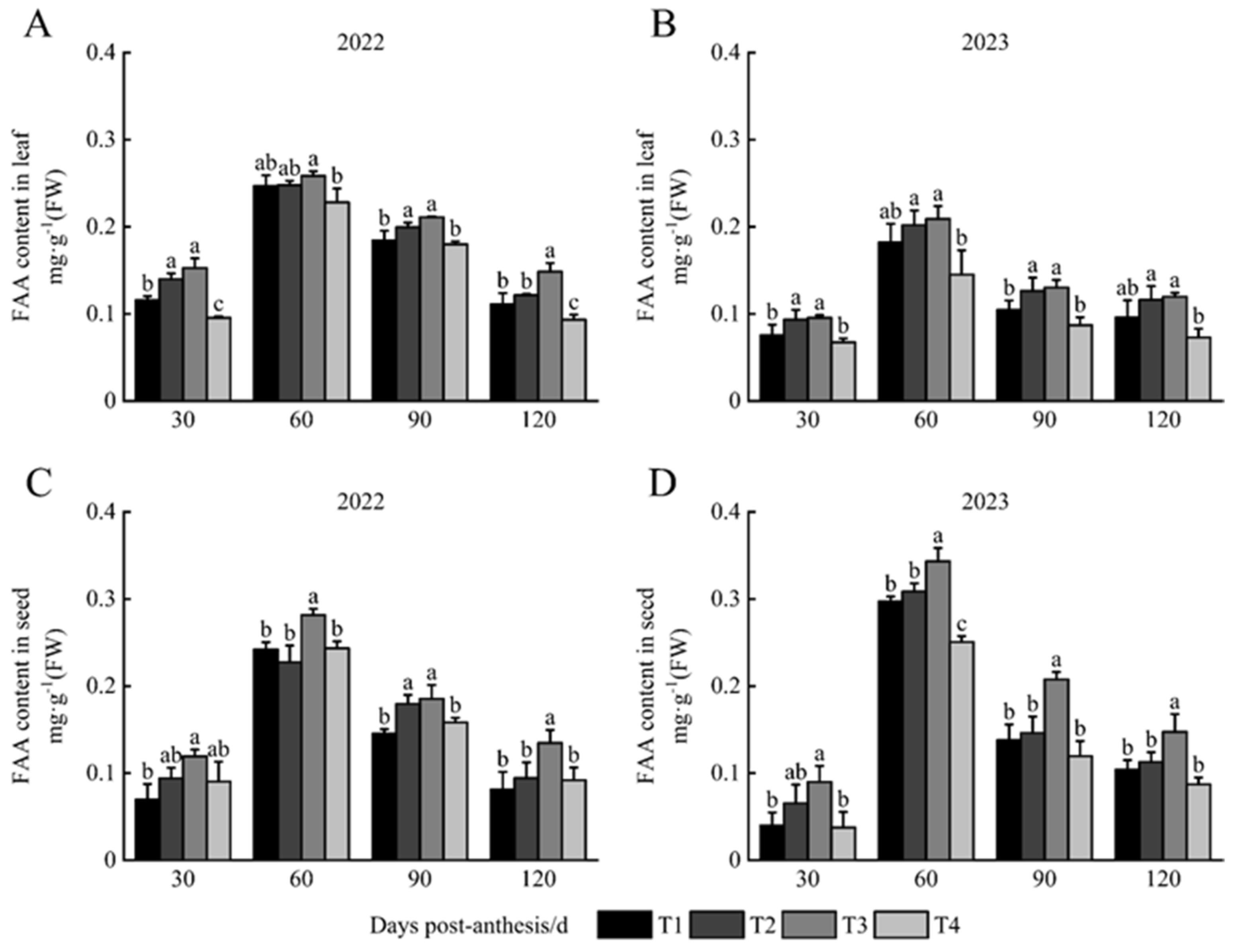
3.1. FAA Content in Leaves and Seeds
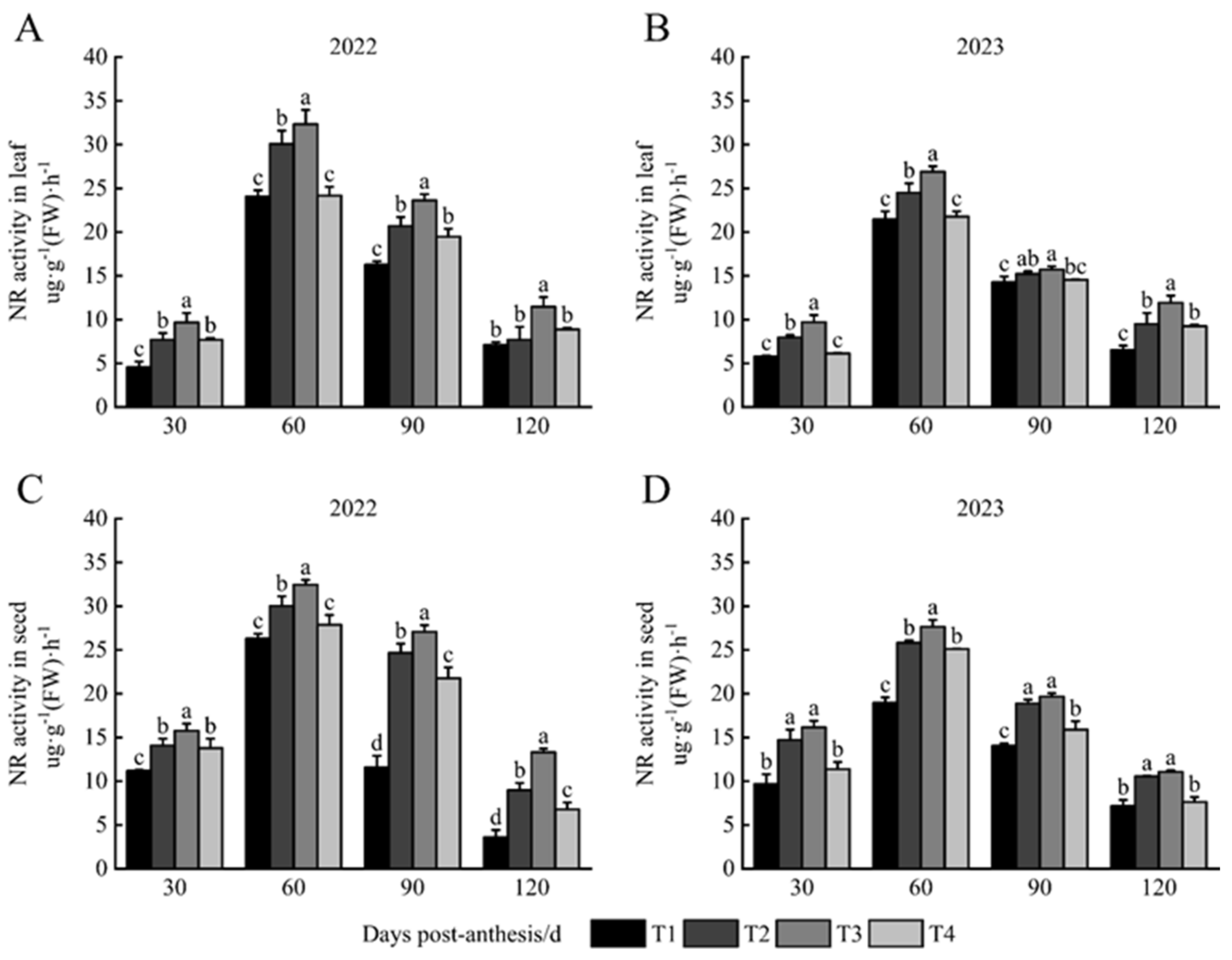
3.2. NR Activity in Leaves and Seeds
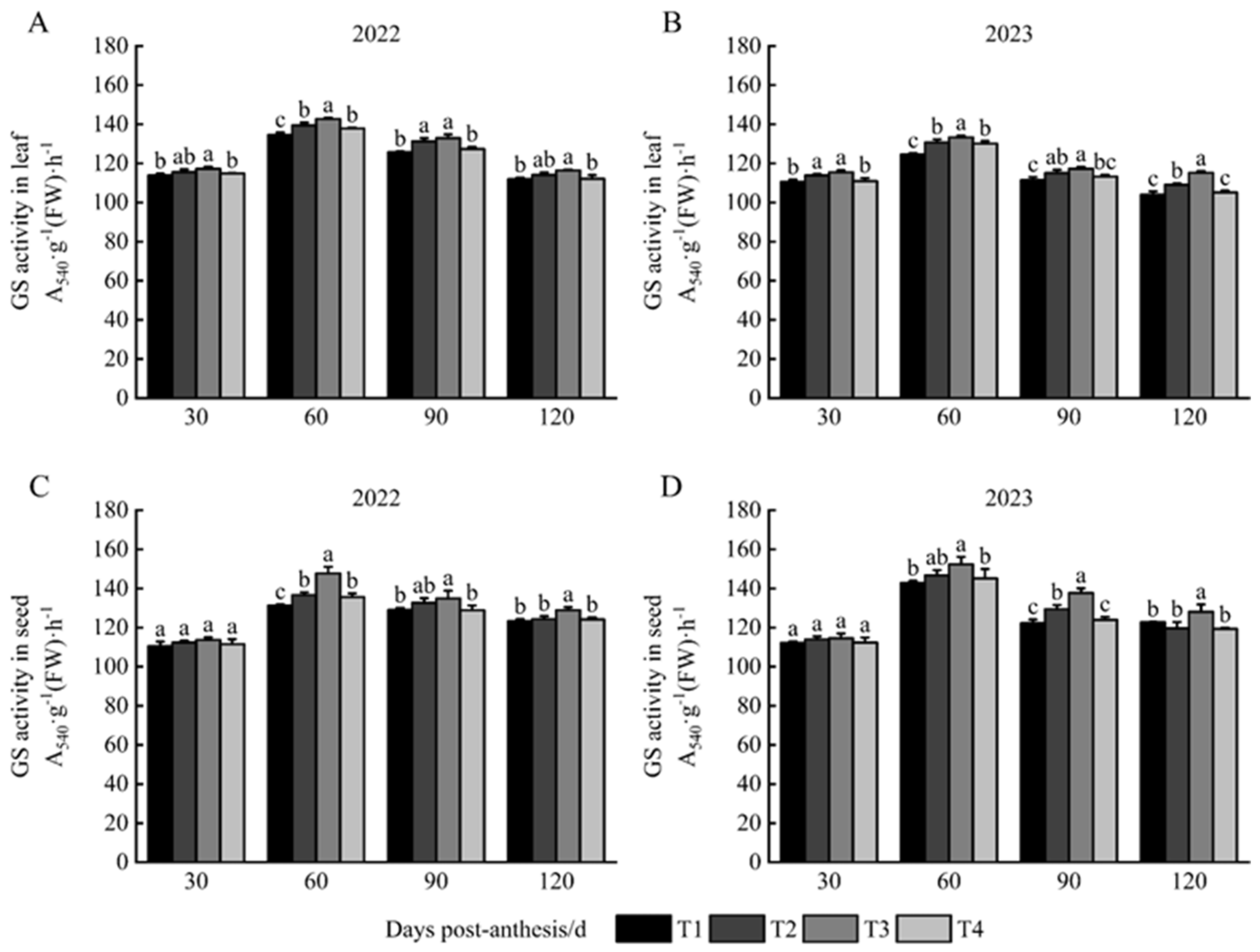
3.3. GS Activity in Leaves and Seeds
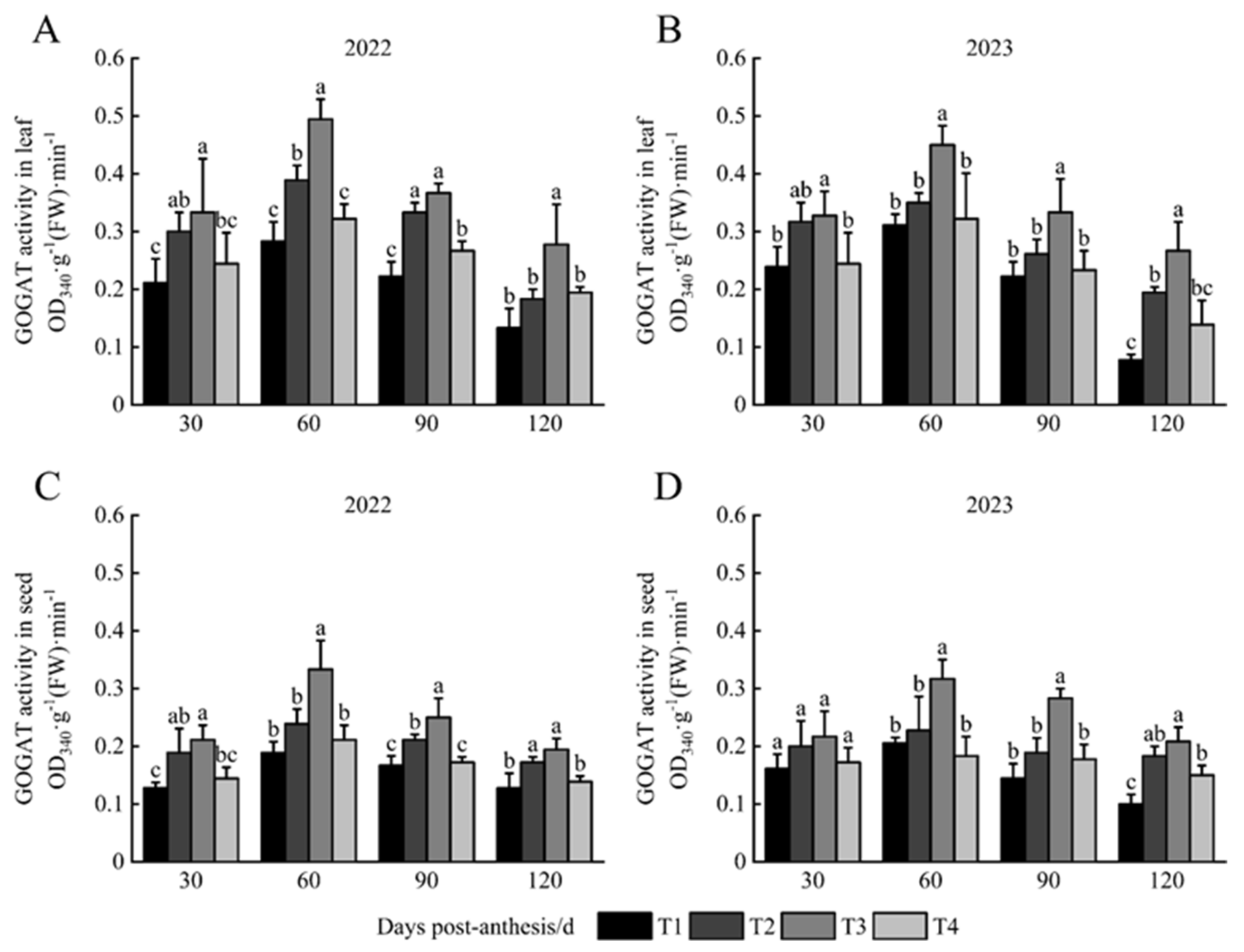
3.4. GOGAT Activity in Leaves and Seeds
3.5. Main Fatty Acid Composition
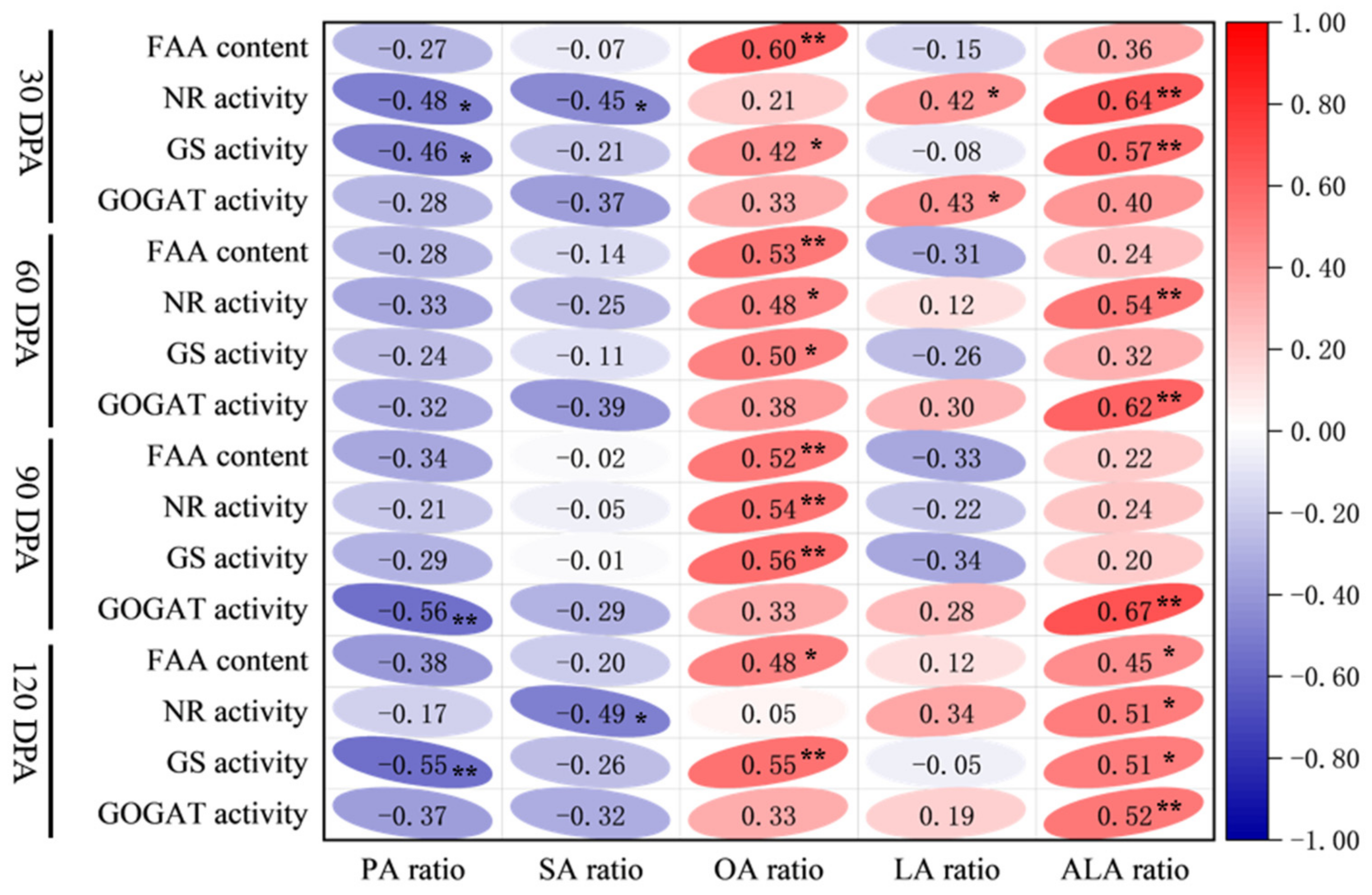
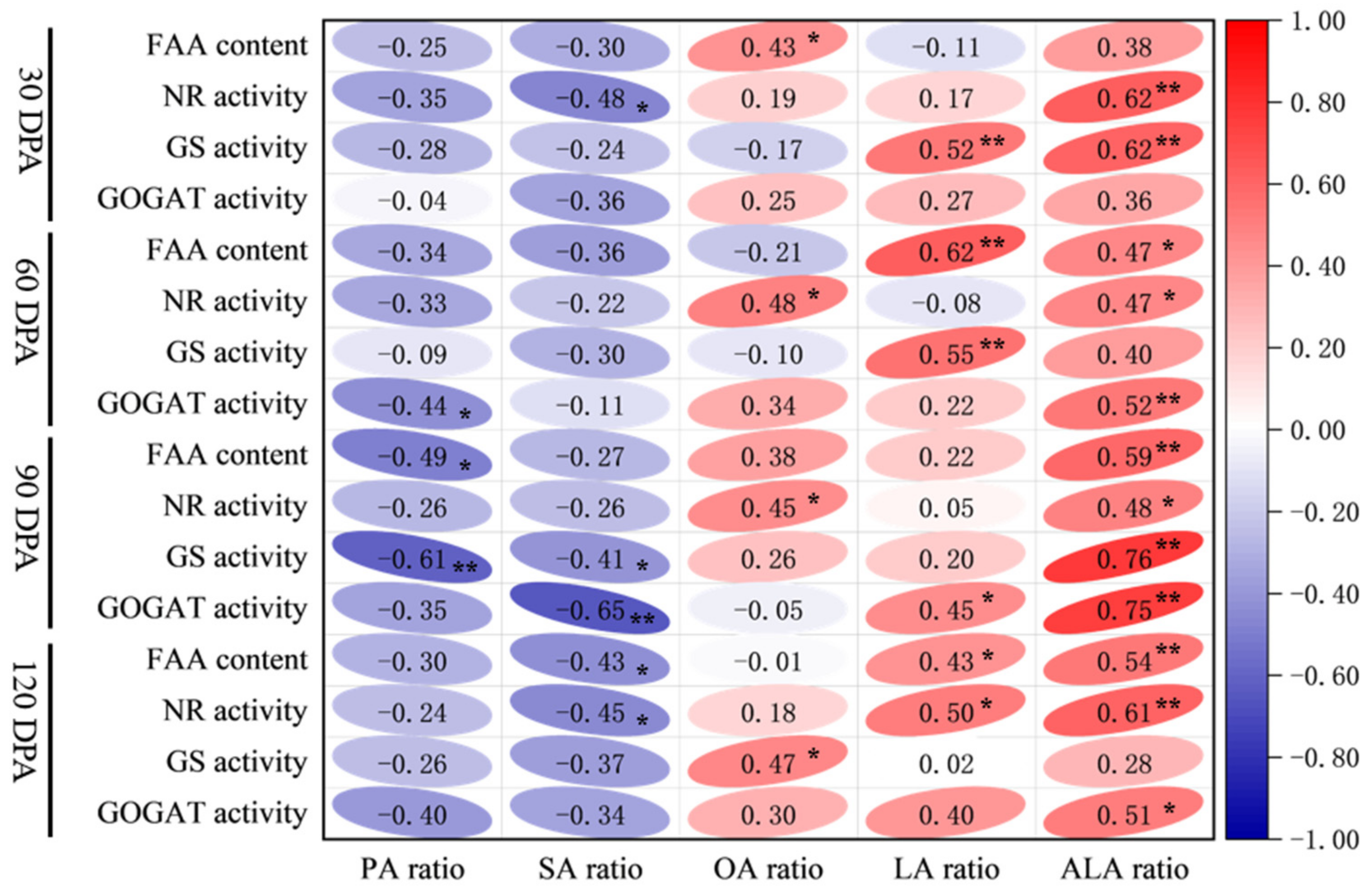
3.6. Relationship Between Nitrogen Metabolism Indicators and Fatty Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekiert, H.; Klimek-Szczykutowicz, M.; Szopa, A. Paeonia × suffruticosa (Moutan Peony)—A Review of the chemical composition, traditional and professional use in medicine, position in cosmetics industries, and biotechnological studies. Plants 2022, 11, 3379–3404. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C. Metabolism of alpha linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar]
- Wang, H.; Wei, S.; He, Y. Characterization of agronomic and seed oil features for different cultivars of tree peony. Plants 2023, 12, 3112–3127. [Google Scholar] [CrossRef]
- Regni, L.; Proietti, P. Effects of nitrogen foliar fertilization on the vegetative and productive performance of the olive tree and on oil quality. Agriculture 2019, 9, 252–259. [Google Scholar] [CrossRef]
- Li, G.; Guo, X.; Sun, W.; Hou, L.; Wang, G.; Tian, R.; Wang, X.; Qu, C.; Zhao, C. Nitrogen application in pod zone improves yield and quality of two peanut cultivars by modulating nitrogen accumulation and metabolism. BMC Plant Biol. 2024, 24, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Z.; Zhang, Y.; Shi, Y. Optimized nitrogen fertilizer application strategies under supplementary irrigation improved winter wheat (Triticum aestivum L.) yield and seed protein yield. PeerJ 2021, 9, e11467. [Google Scholar] [CrossRef]
- Zapletalová, A.; Ducsay, L.; Varga, L.; Sitkey, J.; Javoreková, S.; Hozlár, P. Influence of nitrogen nutrition on fatty acids in oilseed rape (Brassica napus L.). Plants 2021, 11, 44–52. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, D.; Wang, Z.; Chen, Y.; Yang, Z.; Wu, C.; Yang, H.; Chen, L. Nitrogen application modifies the seed and oil yields and fatty acid composition of Nicotiana tabacum. HortScience 2020, 55, 1898–1902. [Google Scholar] [CrossRef]
- Liu, W.; Yin, D.; Zhang, T.; Hou, X.; Qiao, Q.; Song, P. Major fatty acid compositions and antioxidant activity of cultivated Paeonia ostii under different nitrogen fertilizer application. Chem. Biodivers. 2020, 17, e2000617. [Google Scholar] [CrossRef]
- Hasani, M.; Zamani, M.; Savaghebi, G.; Sofla, H. Effect of foliar and soil application of urea on leaf nutrients concentrations, yield and fruit quality of pomegranate. J. Plant Nutr. 2016, 39, 749–755. [Google Scholar] [CrossRef]
- Ishfaq, M.; Kiran, A.; Rehman, H.U.; Farooq, M.; Ijaz, N.H.; Nadeem, F.; Azeem, I.; Li, X.X.; Wakeel, A. Foliar nutrition: Potential and challenges under multifaceted agriculture. Environ. Exp. Bot. 2022, 200, 104909. [Google Scholar]
- Cavero, J.; Faci, J.M.; Martínez-Cob, A. Relevance of sprinkler irrigation time of the day on alfalfa forage production. Agric. Water Manag. 2016, 178, 304–313. [Google Scholar]
- Farouk, A.S.; Abdelghany, A.M.; Shehab, A.A.; Alwakel, S.E.; Makled, K.M.; Naif, E.; Ren, H.; Lamlom, S.F. Optimizing wheat productivity through integrated management of irrigation, nutrition, and organic amendments. BMC Plant Biol. 2024, 24, 548–562. [Google Scholar] [CrossRef]
- Waseem, M.; Kaleel, I.; Mallikarjuna; Patil, R. Comparsion of micro sprinkler irrigation and surface irrigation methodson growth and yield for groundnut under Raichur region. Agric. Update 2017, 12, 2031–2035. [Google Scholar] [CrossRef]
- Artacho, P.; Bonomelli, C. Changes in fine-root production, phenology and spatial distribution in response to N application in irrigated sweet cherry trees. Tree Physiol. 2016, 36, 601–617. [Google Scholar] [CrossRef]
- Li, H. Modern Plant Physiology, 3rd ed.; Higher Education Press: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Cheng, X.; Liang, Y.; Zhang, A.; Wang, P.; He, S.; Zhang, K.; Wang, J.; Fang, Y.; Sun, X. Using foliar nitrogen application during veraison to improve the flavor components of grape and wine. J. Sci. Food Agric. 2021, 101, 1288–1300. [Google Scholar] [CrossRef]
- Pannico, A.; Modarelli, G.; Stazi, S.; Giaccone, M.; Romano, R.; Rouphael, Y.; Cirillo, C. Foliar nutrition influences yield, nut quality and kernel composition in hazelnut cv mortarella. Plants 2023, 12, 2219–2233. [Google Scholar] [CrossRef]
- Liu, M.; Tang, D.; Shi, Y.; Ma, L.; Zhang, Q.; Ruan, J. Foliar N application on tea plant at its dormancy stage increases the N concentration of mature leaves and improves the quality and yield of spring tea. Front. Plant Sci. 2021, 12, 753086. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, J.; Yang, Y.; Sun, F.; Yang, Z. Effect of water and nitrogen coupling regulation on the growth, physiology, yield, and quality attributes and comprehensive evaluation of wolfberry (Lycium barbarum L.). Front. Plant Sci. 2023, 14, 1130109. [Google Scholar] [CrossRef]
- Song, Y.; Dong, M.; Chen, F.; Hu, Y.; Zhu, Y.; Gu, J.; Chen, P.; Xie, Y.; Yuan, C.; Qiao, Z.; et al. Effects of nitrogen fertilizer reduction combined with foliar fertilizer application on the physiological characteristics and yield of high-quality japonica rice. Int. J. Plant Prod. 2024, 18, 239–254. [Google Scholar] [CrossRef]
- Guo, D.; Wang, R.; Chen, C.; Yin, B.; Ding, Z.; Wang, X.; Zhao, M.; Zhou, B. Nitrogen supply mitigates heat stress on photosynthesis of Maize (Zea mays L.) during early seed filling by improving nitrogen assimilation. J. Agron. Crop Sci. 2024, 210, e12750. [Google Scholar] [CrossRef]
- Lyu, X.; Liu, Y.; Li, N.; Ku, L.; Hou, Y.; Wen, X. Foliar applications of various nitrogen (N) forms to winter wheat affect seed protein accumulation and quality via N metabolism and remobilization. Crop J. 2022, 10, 1165–1177. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.; Zhou, X.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Yu, H.; Gong, Q.; Zhang, B.; Liu, G.; Xiao, Y.; Peng, F. Effects of valine and urea on carbon and nitrogen accumulation and lignin content in peach trees. Plants 2023, 12, 1596–1610. [Google Scholar] [CrossRef]
- Luo, Z.; Kong, X.; Dai, J.; Dong, H. Soil plus foliar nitrogen application increases cotton growth and salinity tolerance. J. Plant Nutr. 2015, 38, 443–455. [Google Scholar] [CrossRef]
- Noor, H.; Wang, Q.; Islam, M.A.; Sun, M.; Lin, W.; Ren, A.X.; Feng, Y.; Yu, S.B.; Fida, N.; Dong, S.F.; et al. Effects of sowing methods and nitrogen rates on photosynthetic characteristics, yield, and quality of winter wheat. Photosynthetica 2021, 59, 277–285. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Sweeney, E.; Mastinu, A. Nitrogen and phosphorus addition to soil improves seed yield, foliar stomatal conductance, and the photosynthetic response of rapeseed (Brassica napus L.). Agriculture 2021, 11, 483–492. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wu, W. Understanding fatty acid composition and lipid profile of rapeseed oil in response to nitrogen management strategies. Food Res. Int. 2023, 165, 112565. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Yin, L.; Wang, S.; Deng, X. Alteration in lipid metabolism is involved in nitrogen deficiency response in wheat seedlings. Plant Physiol. Biochem. 2024, 214, 108883. [Google Scholar] [CrossRef]
| Year | Treatment | Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | α-Linolenic Acid |
|---|---|---|---|---|---|---|
| 2022 | T1 | 6.87 ± 0.18 a | 3.00 ± 0.29 a | 25.46 ± 0.18 b | 25.28 ± 0.43 a | 35.77 ± 0.91 b |
| T2 | 6.77 ± 0.36 a | 2.75 ± 0.14 a | 26.03 ± 0.43 ab | 25.99 ± 0.87 a | 37.23 ± 0.80 a | |
| T3 | 6.74 ± 0.18 a | 2.62 ± 0.22 a | 26.17 ± 0.09 a | 26.04 ± 0.74 a | 37.50 ± 0.25 a | |
| T4 | 6.80 ± 0.06 a | 2.81 ± 0.07 a | 25.54 ± 0.45 ab | 25.18 ± 0.17 a | 35.57 ± 0.36 b | |
| 2023 | T1 | 7.12 ± 0.49 a | 3.03 ± 0.35 a | 25.06 ± 0.79 a | 26.09 ± 0.24 a | 35.43 ± 0.16 b |
| T2 | 6.81 ± 0.64 a | 2.41 ± 0.27 a | 24.96 ± 1.60 a | 26.70 ± 0.71 a | 37.31 ± 1.31 ab | |
| T3 | 6.35 ± 0.86 a | 2.32 ± 0.93 a | 25.22 ± 1.16 a | 27.05 ± 1.36 a | 38.16 ± 1.84 a | |
| T4 | 7.42 ± 0.18 a | 2.82 ± 0.07 a | 24.74 ± 0.27 a | 26.25 ± 0.18 a | 35.65 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Liu, X.; Ma, X.; Zhang, Y.; Wang, D.; Zuo, D.; Song, C.; Hou, X. Effects of Nitrogen Fertilizer Spraying Time on Source–Sink Nitrogen Metabolism and Seed Oil Quality of Paeonia ostii ‘Fengdan’. Agronomy 2025, 15, 892. https://doi.org/10.3390/agronomy15040892
Zhang N, Liu X, Ma X, Zhang Y, Wang D, Zuo D, Song C, Hou X. Effects of Nitrogen Fertilizer Spraying Time on Source–Sink Nitrogen Metabolism and Seed Oil Quality of Paeonia ostii ‘Fengdan’. Agronomy. 2025; 15(4):892. https://doi.org/10.3390/agronomy15040892
Chicago/Turabian StyleZhang, Nannan, Xingqiao Liu, Xiaolei Ma, Yabing Zhang, Duoduo Wang, Dingding Zuo, Chengwei Song, and Xiaogai Hou. 2025. "Effects of Nitrogen Fertilizer Spraying Time on Source–Sink Nitrogen Metabolism and Seed Oil Quality of Paeonia ostii ‘Fengdan’" Agronomy 15, no. 4: 892. https://doi.org/10.3390/agronomy15040892
APA StyleZhang, N., Liu, X., Ma, X., Zhang, Y., Wang, D., Zuo, D., Song, C., & Hou, X. (2025). Effects of Nitrogen Fertilizer Spraying Time on Source–Sink Nitrogen Metabolism and Seed Oil Quality of Paeonia ostii ‘Fengdan’. Agronomy, 15(4), 892. https://doi.org/10.3390/agronomy15040892




