Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Sequential Extractions of P Fractions
2.4. Determination of Soil Physicochemical Properties
2.5. Determinations of Alkaline Phosphatase (ALP) and Dehydrogenase (DHA) Activities
2.6. Determinations of the P Solubilization and P Mineralization Potentials
2.7. DNA Extraction, Real-Time Quantitative PCR, and Sequencing of phoD and gcd Genes
2.8. Statistical Analysis
3. Results
3.1. Soil Properties, Enzyme Activities, and P Fractions
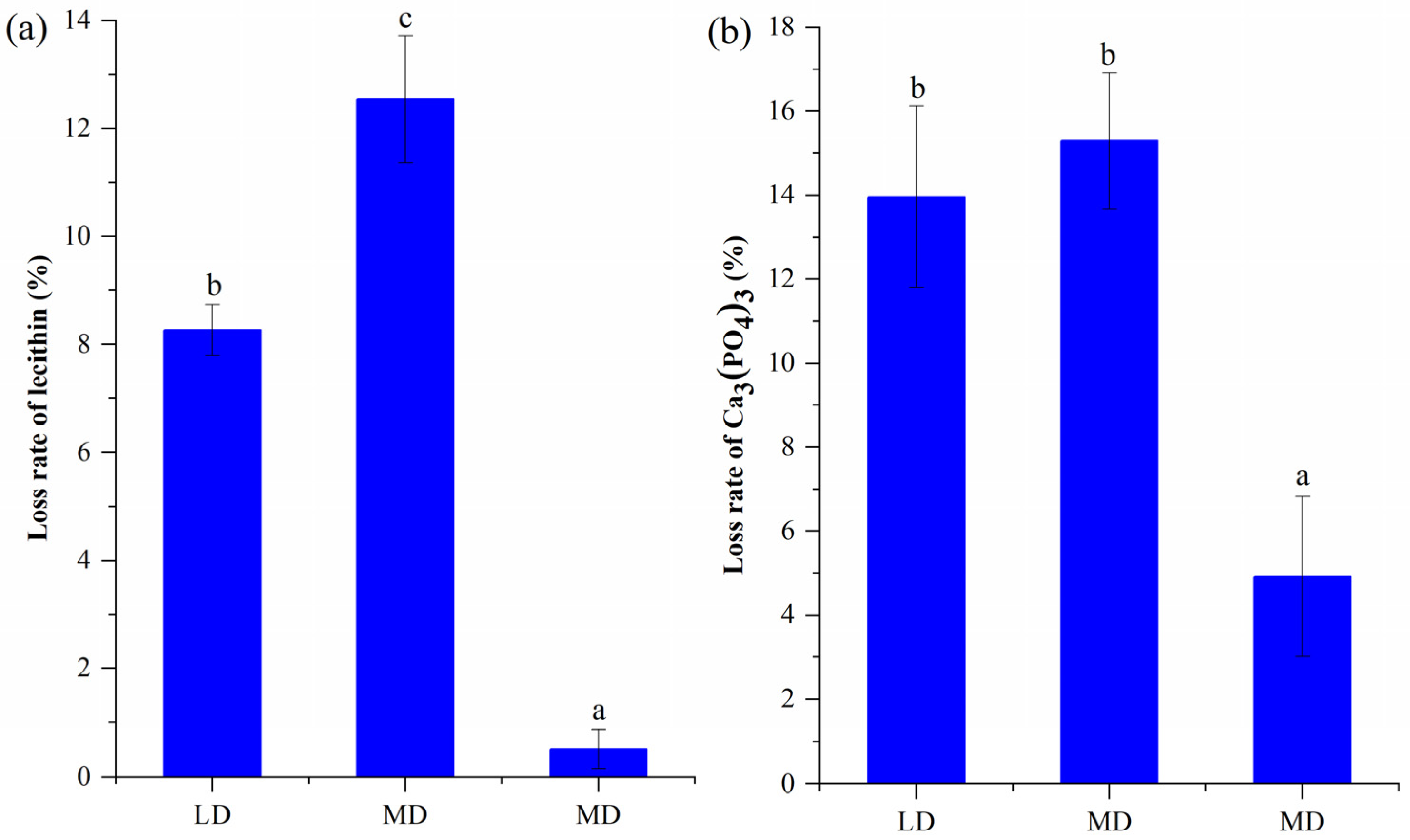
3.2. Potentials of Inorganic P Solubilization and Organic P Mineralization
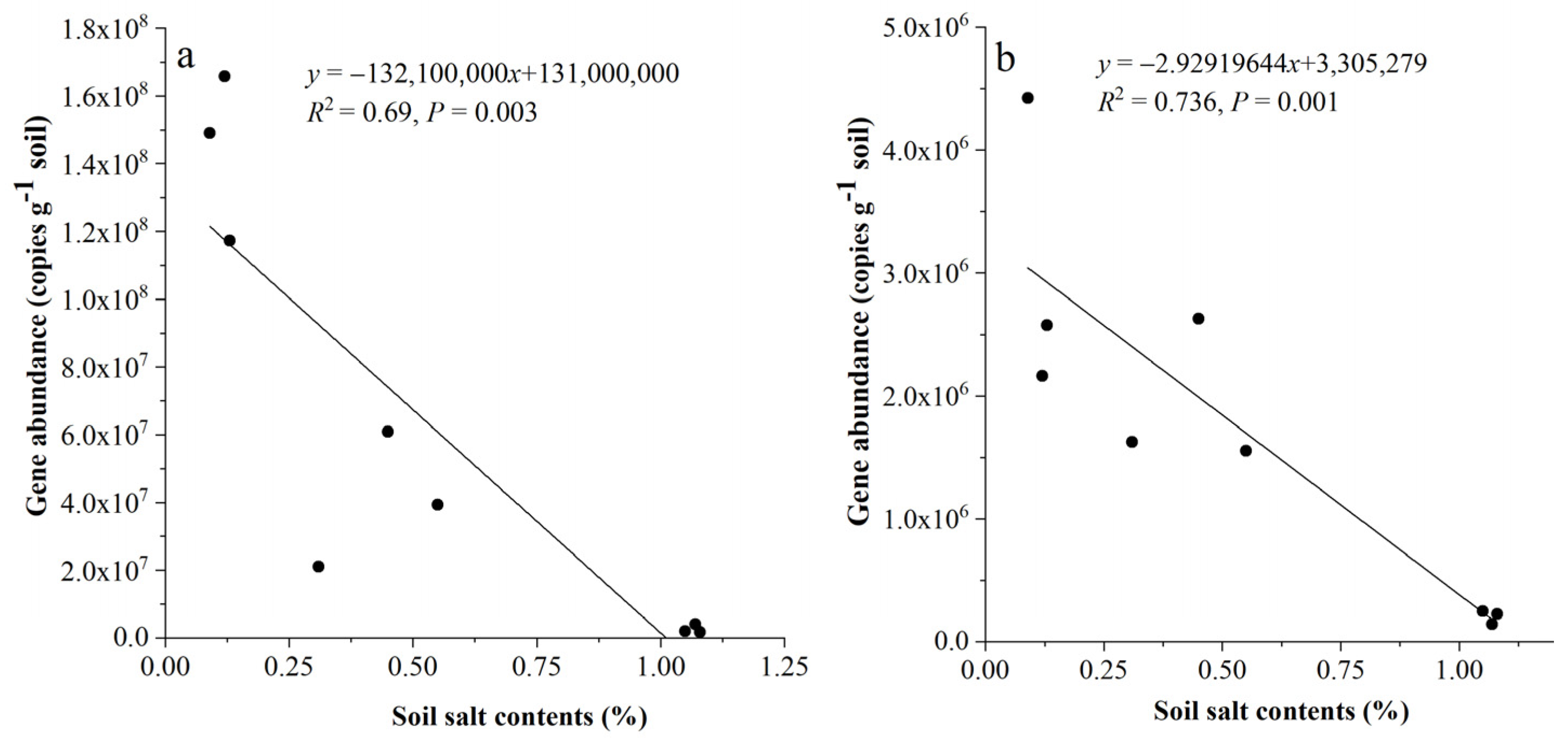
3.3. The Abundance of the phoD and gcd Genes
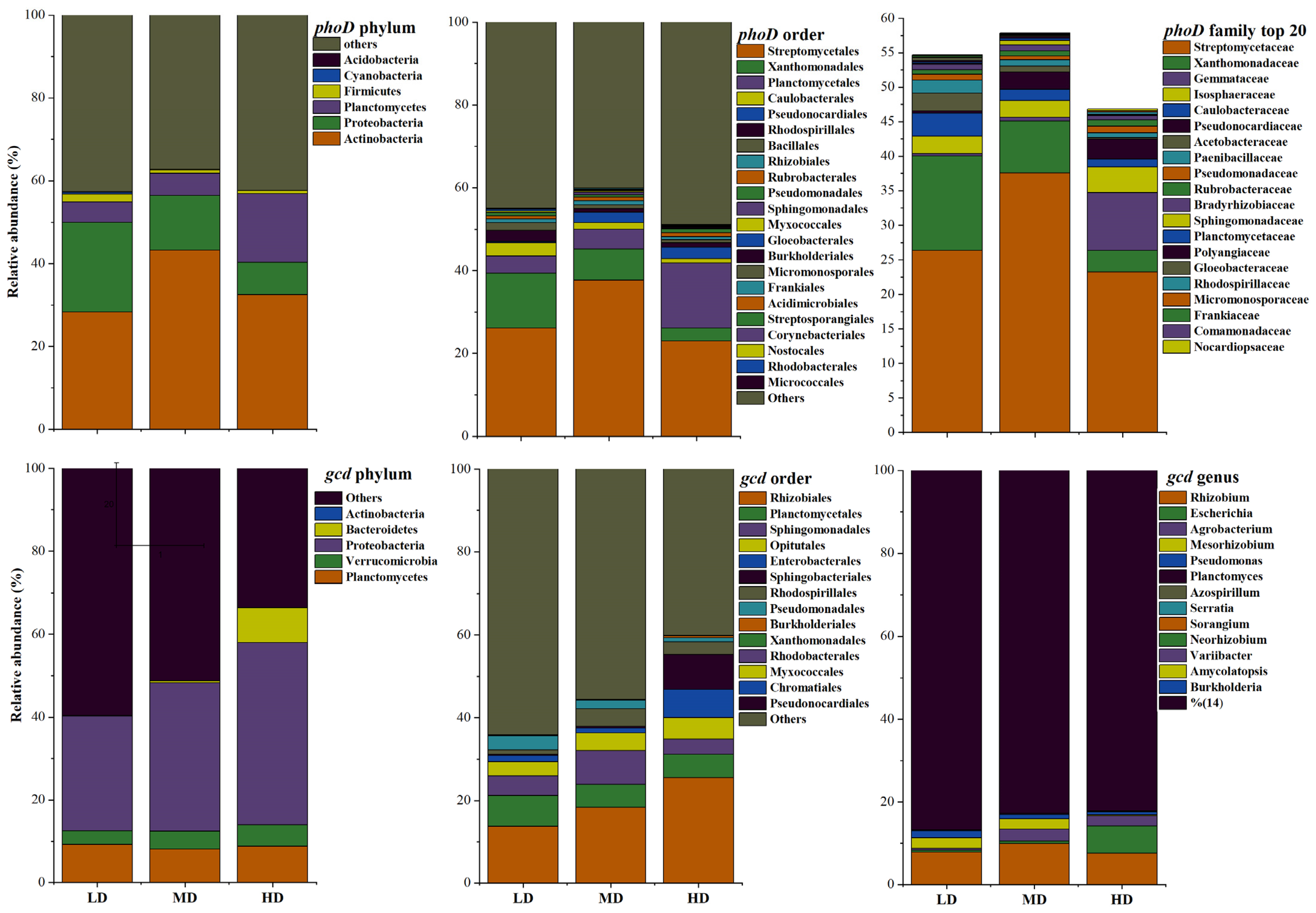
3.4. The Structures of phoD- and gcd-Harboring Microbial Communities
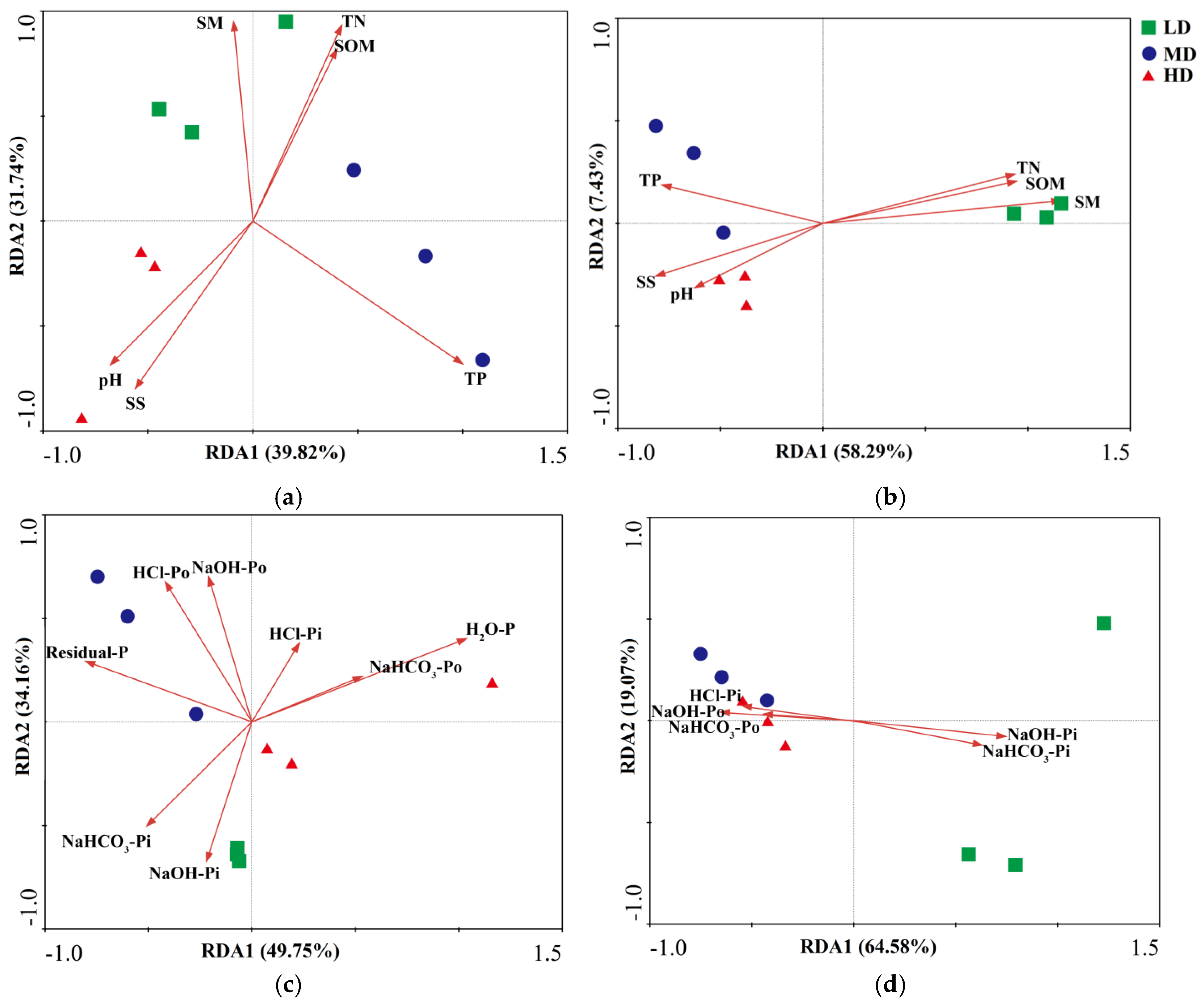
3.5. Dependence of phoD/gcd Community Structure on Soil Properties and P Fractions
4. Discussion
4.1. Responses of P Fractions and the P Solubilization and P Mineralization Potentials to Grassland Salinization
4.2. Responses of phoD- and gcd-Harboring Communities to Grassland Salinization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Waldrip, H.M.; He, Z.; Erich, M.S. Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphatase activity. Biol. Fert. Soils 2011, 47, 407–418. [Google Scholar] [CrossRef]
- Ye, D.H.; Li, T.X.; Yu, H.Y.; Chen, G.D.; Zheng, X.Z.; Zhang, Z.C.; Li, J.X. P accumulation of Polygonum hydropiper, soil P fractions and phosphatase activity as affected by swine manure. Appl. Soil Ecol. 2015, 86, 10–18. [Google Scholar] [CrossRef]
- Yan, X.; Wei, Z.; Hong, Q.; Lu, Z.; Wu, J. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Hu, Y.J.; Xia, Y.H.; Sun, Q.; Liu, K.P.; Chen, X.B.; Ge, T.D.; Zhu, B.L.; Zhu, Z.K.; Zhang, Z.H.; Su, Y.R. Effects of long-term fertilization on phoD-harboring bacterial community in karst soils. Sci. Total Environ. 2018, 628–629, 53–63. [Google Scholar] [CrossRef]
- Memiaghe, J.D.; Cambouris, A.N.; Ziadi, N.; Karam, A.; Perron, I. Spatial variability of soil phosphorus indices under two contrasting grassland fields in Eastern Canada. Agronomy 2021, 11, 24. [Google Scholar] [CrossRef]
- Aleixo, S.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Campello, E.F.C.; Silva, E.C.; Schripsema, J. Can soil phosphorus availability in tropical forest systems be increased by nitrogen-fixing leguminous trees? Sci. Total Environ. 2020, 712, 136405. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.Q.; Zhu, Y.Y.; Guo, S.W.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phod- and phoc-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Nourbakhsh, F. Salinity and plant residue effects on soil available phosphorus. J. Plant Nutr. 2009, 32, 954–966. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.S.; Marschner, P. Soil pH is the main factor influencing growth and rhizosphere properties of wheat following different pre-crops. Plant Soil 2012, 360, 271–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.Y.; Han, X.S.; Jiang, S.Y. Soil nutrient and microbiological property recoveries via native shrub and semi-shrub plantations on moving sand dunes in Northeast China. Ecol. Eng. 2013, 53, 1–5. [Google Scholar] [CrossRef]
- Pan, C.C.; Liu, C.G.; Zhao, H.L.; Wang, Y. Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 2013, 55, 13–19. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar] [CrossRef]
- Zeng, Q.W.; Wu, X.Q.; Wen, X.Y. Effects of soluble phosphate on phosphate-solubilizing characteristics and expression of gcd gene in Pseudomonas frederiksbergensis JW-SD2. Curr. Microbiol. 2016, 72, 198–206. [Google Scholar] [CrossRef]
- Zaheer, R.; Morton, R.; Proudfoot, M.; Yakunin, A.; Finan, T.M. Genetic and biochemical properties of an alkaline phosphatase PhoX family protein found in many bacteria. Environ. Microbiol. 2009, 11, 1572–1587. [Google Scholar] [CrossRef]
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-um, S.; Waditee-Sirisattha, R.; Takabe, T. An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Appl. Environ. Microb. 2011, 77, 5178–5183. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, X.H.; Li, C.L.; Sun, Y.H.; Zhang, J.J.; Gao, Q.; Wang, L.C. Forms of phosphorus in several zonal soils of China. Spectrosc. Spectr. Anal. 2019, 39, 3210–3216. [Google Scholar]
- Acuña, J.J.; Durán, P.; Lagos, L.M.; Ogram, A.; Mora, M.L.; Jorquera, M.A. Bacterial alkaline phosphomonoesterase in the rhizospheres of plants grown in Chilean extreme environments. Biol. Fert. Soils 2016, 52, 763–773. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fert. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Bergkemper, F.; Kublik, S.; Lang, F.; Krüger, J.; Vestergaard, G.; Schloter, M.; Schulz, S. Novel oligonucleotide primers reveal a high diversity of microbes which drive phosphorous turnover in Soil. J. Microbiol. Methods 2016, 125, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. phoD alkaline phosphatase gene diversity in Soil. Appl. Environ. Microb. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, N.; Chen, Z.; Tian, J.; Sun, N.; Xu, M.; Chen, L.J. Response of soil phoD phosphatase gene to long-term combined applications of chemical fertilizers and organic materials. Appl. Soil Ecol. 2017, 119, 197–204. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.Q.; Gong, Z.L.; Xu, W.L.; Mou, Z.S. Gcd gene diversity of quinoprotein glucose dehydrogenase in the sediment of Sancha Lake and its response to the environment. J. Environ. Res. Public Health 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Randall, K.; Brennan, F.; Clipson, N.; Creamer, R.; Griffiths, B.; Storey, S.; Doyle, E. Soil bacterial community structure and functional responses across a long-term mineral phosphorus (Pi) fertilisation gradient differ in grazed and cut grasslands. Appl. Soil Ecol. 2019, 138, 134–143. [Google Scholar] [CrossRef]
- Zheng, B.X.; Zhang, D.P.; Wang, Y.; Hao, X.L.; Wadaan, M.A.M.; Hozzein, W.N.; Penuelas, J.; Zhu, Y.G.; Yang, X.R. Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Sci. Rep. 2019, 9, 25. [Google Scholar] [CrossRef]
- Wan, W.J.; Li, X.; Han, S.; Wang, L.; Luo, X.S.; Chen, W.L.; Huang, Q.Y. Soil aggregate fractionation and phosphorus fraction driven by long-term fertilization regimes affect the abundance and composition of P-cycling related bacteria. Soil Tillage Res. 2020, 196, 104475. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Guo, L.; Wu, Q.; Cui, Z. Soil properties, bacterial community composition, and metabolic diversity responses to soil salinization of a semiarid grassland in northeast Chin. J. Soil Water Conserv. 2015, 70, 110–120. [Google Scholar] [CrossRef]
- Cao, C.Y.; Tao, S.; Cui, Z.B.; Zhang, Y. Response of soil properties and microbial communities to increasing salinization in the meadow grassland of Northeast China. Microb. Ecol. 2021, 82, 722–735. [Google Scholar] [CrossRef]
- Naheed, G.; Shahbaz, M.; Latif, A.; Rha, E.S. Alleviation of the adverse effects of salt stress on rice (Oryza sativa L.) by phosphorus applied through rooting medium: Growth and gas exchange characteristics. Pak. J. Bot. 2007, 39, 729–737. [Google Scholar]
- Wilson, B.J.; Servais, S.; Charles, S.P.; Mazzei, V.; Gaiser, E.E.; Kominoski, J.S.; Richards, J.H.; Troxler, T.G. Phosphorus alleviation of salinity stress: Effects of saltwater intrusion on an Everglades freshwater peat marsh. Ecology 2019, 100, e02672. [Google Scholar] [CrossRef]
- Waldrip-Dail, H.; He, Z.; Erich, S.M.; Honeycutt, W.C. Soil phosphorus dynamics in response to poultry manure amendment. Soil Sci. 2009, 174, 195–201. [Google Scholar] [CrossRef]
- Institute of Soil Science, Chinese Academy of Sciences (ISSCAS). Methods on Soil Microorganism Study; Science Press: Beijing, China, 1985; pp. 260–275. [Google Scholar]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, 2nd ed.; Part 2; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 539–577. [Google Scholar]
- Zheng, H.Y.; Zhang, D.S. Methods on Dynamics of Soil Biochemistry Study; Science Press: Beijing, China, 1982; pp. 167–169. [Google Scholar]
- Tunesi, S.; Poggi, V.; Gessa, C. Phosphate adsorption and precipitation in calcareous soils: The role of calcium ions in solution and carbonate minerals. Nutr. Cycl. Agroecosyst. 1999, 53, 219–227. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S. Degradation processes and nutrient constraints in sodic soils. Land. Degrad. Dev. 2002, 13, 275–294. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.Y.; Bao, W.K.; Tian, G.L. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–66. [Google Scholar] [CrossRef]
- Zeng, Q.; Fan, Y.; Yuan, X.; Zhou, J.; Lin, H.; Xie, H.; Cui, J.; Wu, Y.; Chen, Y. Differential factors determine the response of soil P fractions to N deposition in wet and dry seasons in a subtropical Moso bamboo forest. Plant Soil 2022, 498, 161–179. [Google Scholar] [CrossRef]
- Yang, J.J.; Wu, X.F.; Ruan, H.; Song, Y.Q.; Xu, M.; Wang, S.N.; Wang, D.L.; Wu, D.H. How does grassland degradation affect soil enzyme activity and microbial nutrient limitation in saline-alkaline meadow? Land. Degrad. Dev. 2023, 34, 5863–5875. [Google Scholar] [CrossRef]
- Perroni, Y.; García-Oliva, F.; Tapia-Torres, Y.; Souza, V. Relationship between soil P fractions and microbial biomass in an oligotrophic grassland-desert scrub system. Ecol. Res. 2014, 29, 463–472. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. Biological and geochemical controls on phosphorus fractions in semiarid soils. Biogeochemistry 2001, 52, 155–172. [Google Scholar] [CrossRef]
- Khan, K.S.; Joergensen, R.G. Relationships between P fractions and the microbial biomass in soils under different land use management. Geoderma 2012, 173–174, 274–281. [Google Scholar] [CrossRef]
- Stahr, S.; Graf-Rosenfellner, M.; Klysubun, W.; Mikutta, R.; Prietzel, J.; Lang, F. Phosphorus speciation and C:N:P stoichiometry of functional organic matter fractions in temperate forest soils. Plant Soil 2018, 427, 53–69. [Google Scholar] [CrossRef]
- Rose, T.J.; Hardiputra, B.; Rengel, Z. Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 2010, 326, 159–170. [Google Scholar] [CrossRef]
- Khan, A.; Jin, X.; Yang, X.Y.; Guo, S.L.; Zhang, S.L. Phosphorus fractions affected by land use changes in soil profile on the loess Soil. J. Soil Sci. Plant Nutr. 2021, 21, 722–732. [Google Scholar] [CrossRef]
- Wang, C.Q.; Xue, L.; Jiao, R.Z. Soil phosphorus fractions, phosphatase activity, and the abundance of phoC and phoD genes vary with planting density in subtropical Chinese fir plantations. Soil Tillage Res. 2021, 209, 104946. [Google Scholar] [CrossRef]
- Fu, D.G.; Wu, X.N.; Duan, C.Q.; Chadwick, D.R.; Jones, D.L. Response of soil phosphorus fractions and fluxes to different vegetation restoration types in a subtropical mountain ecosystem. Catena 2020, 193, 104663. [Google Scholar] [CrossRef]
- Zhu, M.N.; Guo, Y.F.; Cao, X.D.; Yang, Y.B.; Du, Q.; Lou, J.; Wang, W.J.; Wu, Y.; Wang, H.M. Shelterbelt-farmland differences in P fractions interacted with soil alkalization, geoclimatic conditions, and soil fungi in Northeast China Plain. J. Soils Sediments 2023, 23, 3937–3957. [Google Scholar] [CrossRef]
- Heng, T.; Hermansen, C.; de Jonge, L.W.; Chen, J.; Yang, L.L.; Zhao, L.; He, X.L. Differential responses of soil nutrients to edaphic properties and microbial attributes following reclamation of abandoned salinized farmland. Agric. Ecosyst. Environ. 2023, 347, 108373. [Google Scholar] [CrossRef]
- Kristin, M.R.; Noah, F.; Daniel, V.M.; Johannes, R. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar]
- Zhang, K.P.; Cui, X.Q.; Yue, P.; Li, K.H.; Liu, X.J.; Tripathi, B.M.; Chu, H.Y. Salinity is a key determinant for soil microbial communities in a desert ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Ma, K.Y.; Huang, Y.; Fu, Q.; Qiu, Y.B.; Yao, Z.Y. Significant response of microbial community to increased salinity across wetland ecosystems. Geoderma 2022, 415, 115778. [Google Scholar] [CrossRef]
- Park, H.J.; Chae, N.; Sul, W.J.; Lee, B.Y.; Lee, Y.K.; Kim, D. Temporal changes in soil bacterial diversity and humic substances degradation in subarctic Tundra Soil. Microb. Ecol. 2015, 69, 668–675. [Google Scholar]
- Ding, Y.; Ye, Q.; Liu, M.; Shi, Z.; Liang, Y. Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid. Sci. Total Environ. 2020, 763, 142937. [Google Scholar] [CrossRef]
- Yang, J.J.; Li, W.J.; Teng, D.X.; Yang, X.D.; Zhang, Y.J.; Li, Y. Metagenomic insights into microbial community structure, function, and salt adaptation in saline soils of arid land, China. Microorganisms 2022, 10, 2183. [Google Scholar] [CrossRef]
- Guan, T.W.; Lin, Y.J.; Ou, M.Y.; Chen, K.B. Isolation and diversity of sediment bacteria in the hypersaline aiding lake, China. PLoS ONE 2020, 15, e0236006. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Liu, Y.; Xie, S.; Xing, Y.; Dao, J.; Wei, B.; Peng, Y.; Duan, W.; Wang, Z. Response of sugarcane rhizosphere bacterial community to drought stress. Front. Microbiol. 2021, 12, 716196. [Google Scholar] [CrossRef]
- Kim, Y.; Liesack, W. Differential assemblage of functional units in paddy soil microbiomes. PLoS ONE 2015, 10, e0122221. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Yadav, V.; Deshmukh, Y.; Das, P.; Singh, R.P.; Bano, N.; Kumar, M.; Shukla, A.K.; Krishna, A.; Khare, P. Decoding the link between bacterial diversity and enzymatic activities of soil from Cymbopogon flexuosus growing dryland. Appl. Soil Ecol. 2021, 168, 10415. [Google Scholar] [CrossRef]
- Bainard, L.D.; Hamel, C.; Gan, Y.T. Edaphic properties override the influence of crops on the composition of the soil bacterial community in a semiarid agroecosystem. Appl. Soil Ecol. 2016, 105, 160–168. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, J.L.; Jiang, L.M.; Lv, G.H.; Hu, D.; Wu, D.Y.; Yang, X.D. Rhizosphere effect and water constraint jointly determined the roles of microorganism in soil phosphorus cycling in arid desert regions. Catena 2023, 222, 106809. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yuan, J.; Tang, Z.H.; Wang, J.D.; Zhang, Y.C. Long-term organic fertilization strengthens the soil phosphorus cycle and phosphorus availability by regulating the pqqC- and phoD-harboring bacterial communities. Microb. Ecol. 2023, 86, 2716–2732. [Google Scholar] [CrossRef]
- Zhang, L.C.; Ren, G.; Chu, G.X. Land reclamation increased labile and moderately labile P fractions and strengthened co-occurrence network of gcd community in calcareous soils. Land. Degrad. Dev. 2023, 34, 5542–5555. [Google Scholar] [CrossRef]
- Fan, T.N.; Luo, M.; Tan, J.; Hu, D.H.; Chen, X.; Huang, J.F.; Li, S.H.; Guo, P.P.; Chen, J. Incorporating biotic phosphorus-acquisition strategies into soil phosphorus transformation under long-term salinization in a tidal wetland. Catena 2023, 231, 107274. [Google Scholar] [CrossRef]

| Index | LD | MD | HD | ANOVA in Response to Salt Content | |||
|---|---|---|---|---|---|---|---|
| Regression Equation | R2 | F | p | ||||
| SOM (%) | 4.50 ± 0.626 | 3.17 ± 0.527 | 2.16 ± 0.380 | y = −2.2362x + 4.4821 | 0.7292 | 18.847 | 0.003 |
| TN (%) | 0.18 ± 0.016 | 0.15 ± 0.008 | 0.11 ± 0.001 | y = −0.0665x + 0.1824 | 0.8209 | 32.09 | 0.001 |
| TP (%) | 0.042 ± 0.013 | 0.027 ± 0.004 | 0.060 ± 00.04 | ns | - | - | - |
| TK (%) | 2.15 ± 0.041 | 2.30 ± 0.062 | 2.41 ± 0.038 | y = 0.2589x + 2.1443 | 0.8675 | 45.826 | <0.001 |
| NH4-N (mg·kg−1) | 2.51 ± 0.31 | 2.48 ± 0.19 | 1.51 ± 0.26 | y = −1.053x + 2.7316 | 0.6865 | 15.328 | 0.00 |
| NO3-N (mg·kg−1) | 1.49 ± 0.68 | 0.39 ± 0.06 | 0.17 ± 0.04 | y = −1.2345x + 1.3457 | 0.5585 | 8.854 | 0.021 |
| AK (mg·kg−1) | 88.2 ± 0.28 | 194 ± 13.5 | 137 ± 31.6 | ns | - | - | - |
| pH | 7.82 ± 0.001 | 7.93 ± 0.05 | 8.38 ± 0.16 | y = 0.601x + 7.7206 | 0.8816 | 52.144 | <0.001 |
| EC (µs·cm−1) | 186 ± 17.2 | 270 ± 8.0 | 2400 ± 631 | y = 2407.4x − 345.12 | 0.8153 | 30.902 | 0.001 |
| SM (%) | 0.30 ± 0.02 | 0.07 ± 0.01 | 0.03 ± 0.001 | y = −0.2448x + 0.2636 | 0.6763 | 14.626 | 0.007 |
| BD (g·cm−3) | 1.54 ± 0.06 | 1.63 ± 0.04 | 1.76 ± 0.06 | y = 0.2196x + 1.5262 | 0.821 | 32.194 | 0.001 |
| ALP (mg g−1 h−1) | 0.797 ± 0.267 | 0.443 ± 0.797 | 0.388 ± 0.352 | y = 1.598x + 0.332 | 0.719 | 17.948 | 0.004 |
| DHA (mg TPF·kg−1soil·24 h−1) | 12.94 ± 0.77 | 11.84 ± 0.70 | 8.53 ± 1.00 | y = −4.599x + 13.582 | 0.850 | 22.753 | <0.001 |
| Index | LD | MD | HD | ANOVA in Response to Salt Content | ||
|---|---|---|---|---|---|---|
| R2 | F | p | ||||
| H2O-P | 0.74 ± 0.08 | 1.77 ± 0.24 | 11.55 ± 9.19 | 0.495 | 6.854 | 0.035 |
| NaHCO3-Pi | 12.10 ± 0.71 | 8.89 ± 1.09 | 6.88 ± 0.40 | 0.838 | 36.207 | 0.001 |
| NaHCO3-Po | 53.90 ± 9.70 | 67.11 ± 18.33 | 83.12 ± 21.67 | 0.474 | 6.317 | 0.040 |
| NaOH-Pi | 23.01 ± 1.82 | 9.48 ± 0.43 | 6.83 ± 0.77 | 0.693 | 15.811 | 0.005 |
| NaOH-Po | 42.99 ± 26.33 | 96.52 ± 3.12 | 77.17 ± 6.77 | 0.405 | 1.372 | 0.080 |
| HCl-Pi | 11.08 ± 0.88 | 20.32 ± 0.51 | 28.87 ± 5.48 | 0.835 | 35.377 | 0.001 |
| HCl-Po | 88.93 ± 19.20 | 135.68 ± 0.41 | 75.13 ± 30.40 | 0.071 | 0.538 | 0.087 |
| Residual-P | 261.33 ± 29.84 | 291.00 ± 42.14 | 245.67 ± 37.23 | 0.078 | 0.596 | 0.465 |
| H2O-P + NaHCO3-Pi | 12.84 ± 0.76 | 10.66 ± 1.02 | 18.43 ± 8.81 | 0.227 | 2.053 | 0.555 |
| Genes | Sites | Chao1 | Observed Species | Shannon–Wiener | Simpson |
|---|---|---|---|---|---|
| phoD | LD | 1635.3 ± 96.6 | 1246.1 ± 46.8 | 7.33 ± 0.11 | 0.98 ± 0.001 |
| MD | 1823.5 ± 175.1 | 1404.2 ± 119.3 | 7.50 ± 0.61 | 0.96 ± 0.030 | |
| HD | 1331.0 ± 772.9 | 1119.83 ± 644.0 | 7.20 ± 2.02 | 0.96 ± 0.050 | |
| gcd | LD | 1497.2 ± 86.3 | 1267.7 ± 136.2 | 8.39 ± 0.16 | 0.99 ± 0.001 |
| MD | 1287.8 ± 393.5 | 1042.2 ± 342.0 | 7.67 ± 0.34 | 0.99 ± 0.001 | |
| HD | 1082.4 ± 165.0 | 785.5 ± 114.8 | 6.82 ± 0.42 | 0.98 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cui, Z.; Cao, C. Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China. Agronomy 2025, 15, 960. https://doi.org/10.3390/agronomy15040960
Zhang Y, Cui Z, Cao C. Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China. Agronomy. 2025; 15(4):960. https://doi.org/10.3390/agronomy15040960
Chicago/Turabian StyleZhang, Ying, Zhenbo Cui, and Chengyou Cao. 2025. "Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China" Agronomy 15, no. 4: 960. https://doi.org/10.3390/agronomy15040960
APA StyleZhang, Y., Cui, Z., & Cao, C. (2025). Effects of Secondary Salinization on Soil Phosphorus Fractions and Microbial Communities Related to Phosphorus Transformation in a Meadow Grassland, Northeast China. Agronomy, 15(4), 960. https://doi.org/10.3390/agronomy15040960






