Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Herbal Extracts
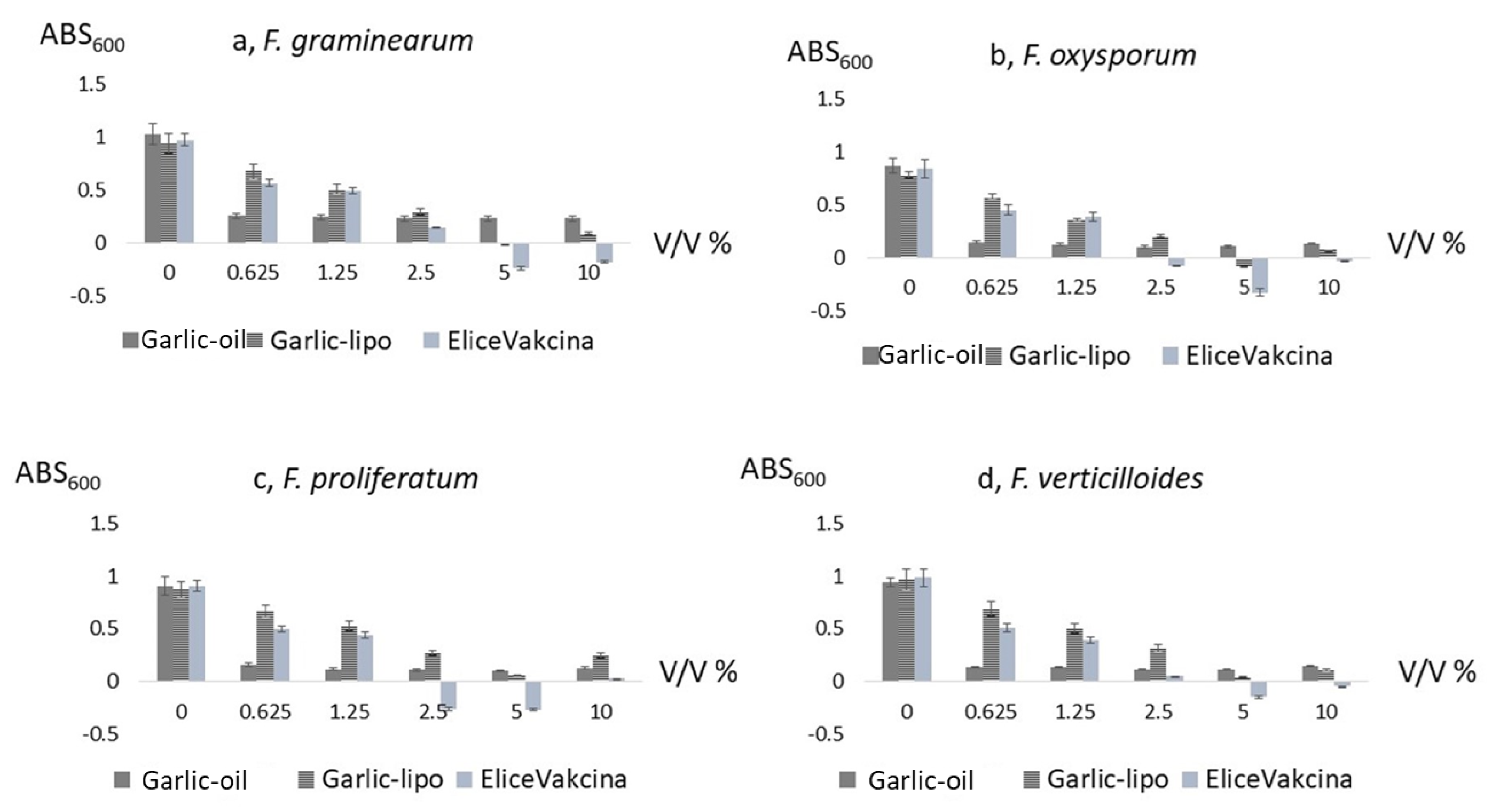
2.2. The Toxicity Test of Garlic-Containing PBs on Four Fusarium Species
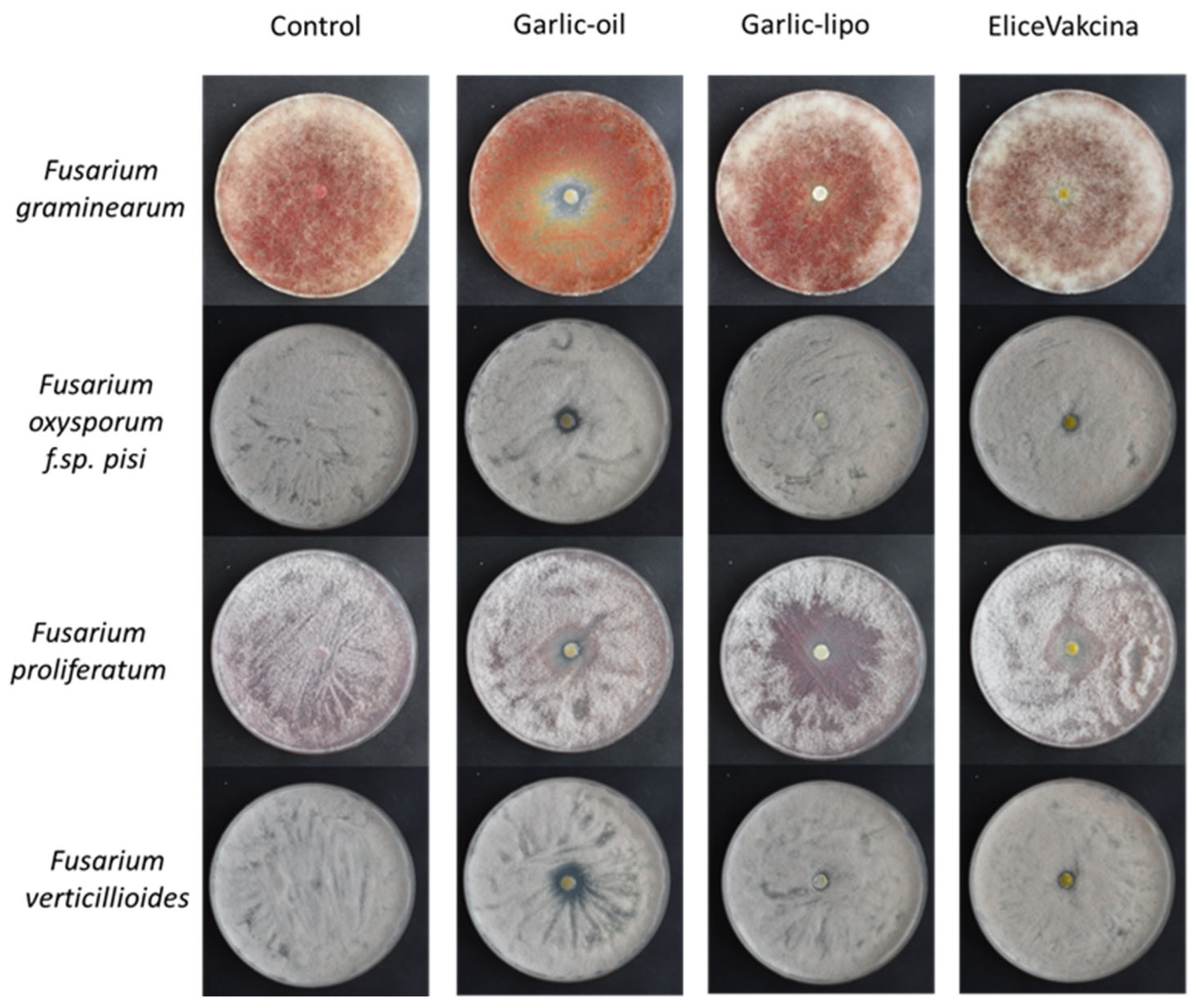
2.3. Antifungal Activity Test of Solid Agar Plate Assay
2.4. Inoculation of Pea Seeds with F. oxysporum f. sp. pisi
2.5. Nanoliposome Formulated EliceVakcina Treatments of Pea Cultures in Field
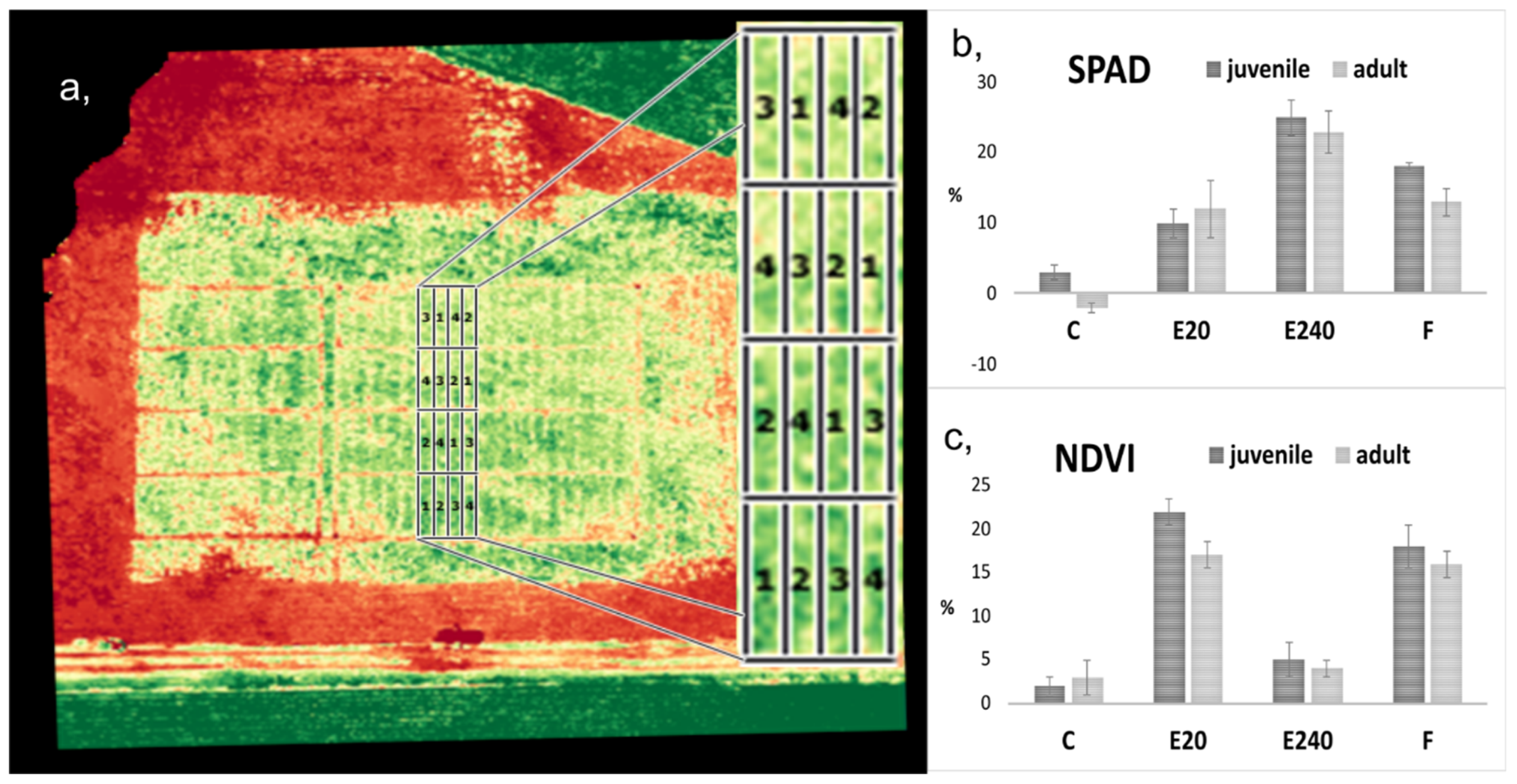
2.6. Measurements of NDVI and SPAD in EliceVakcina-Treated Field Plants
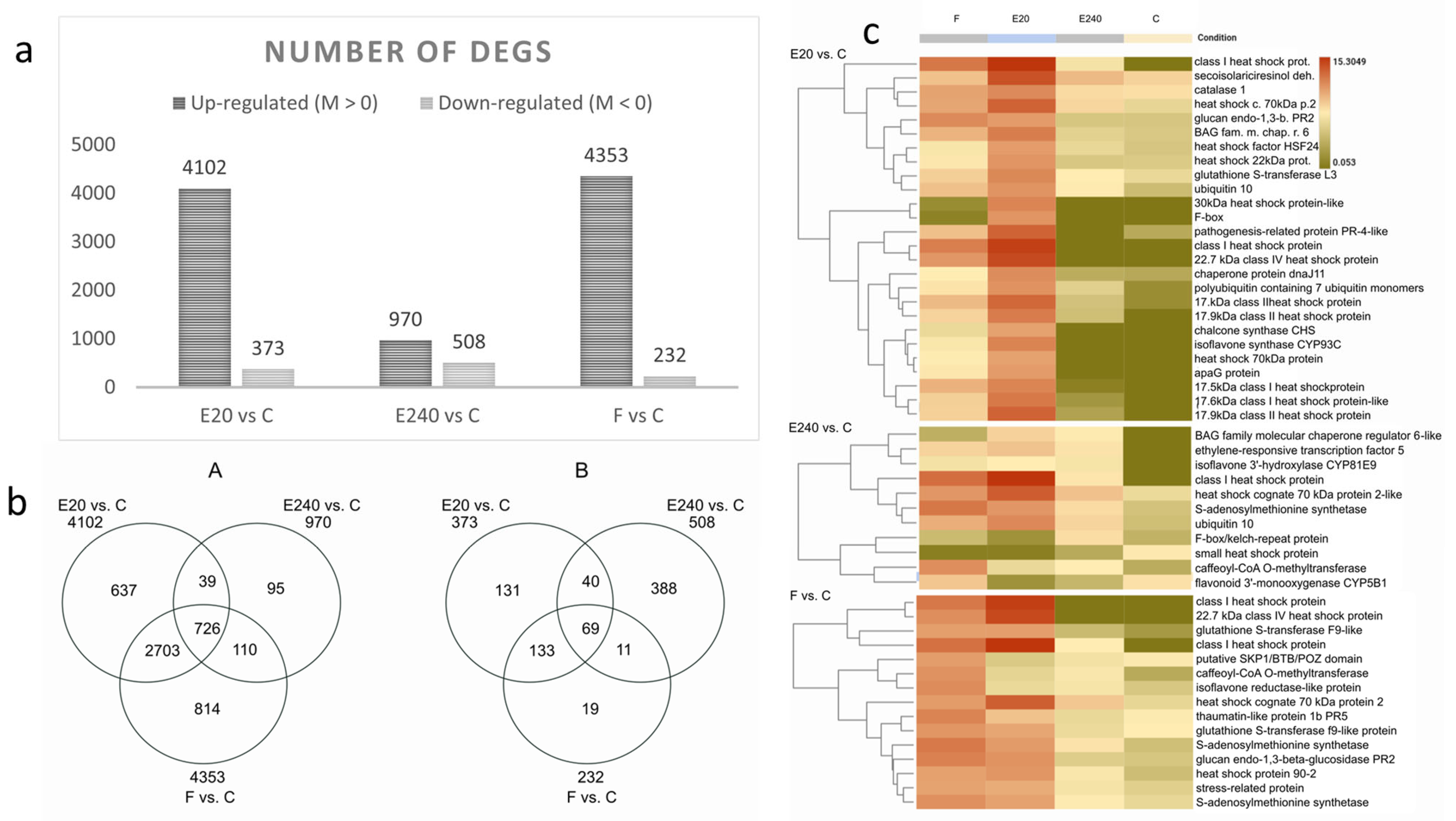
2.7. RNA-Sequencing and Differential Gene Expression Analysis of Treated Field Pea Plants
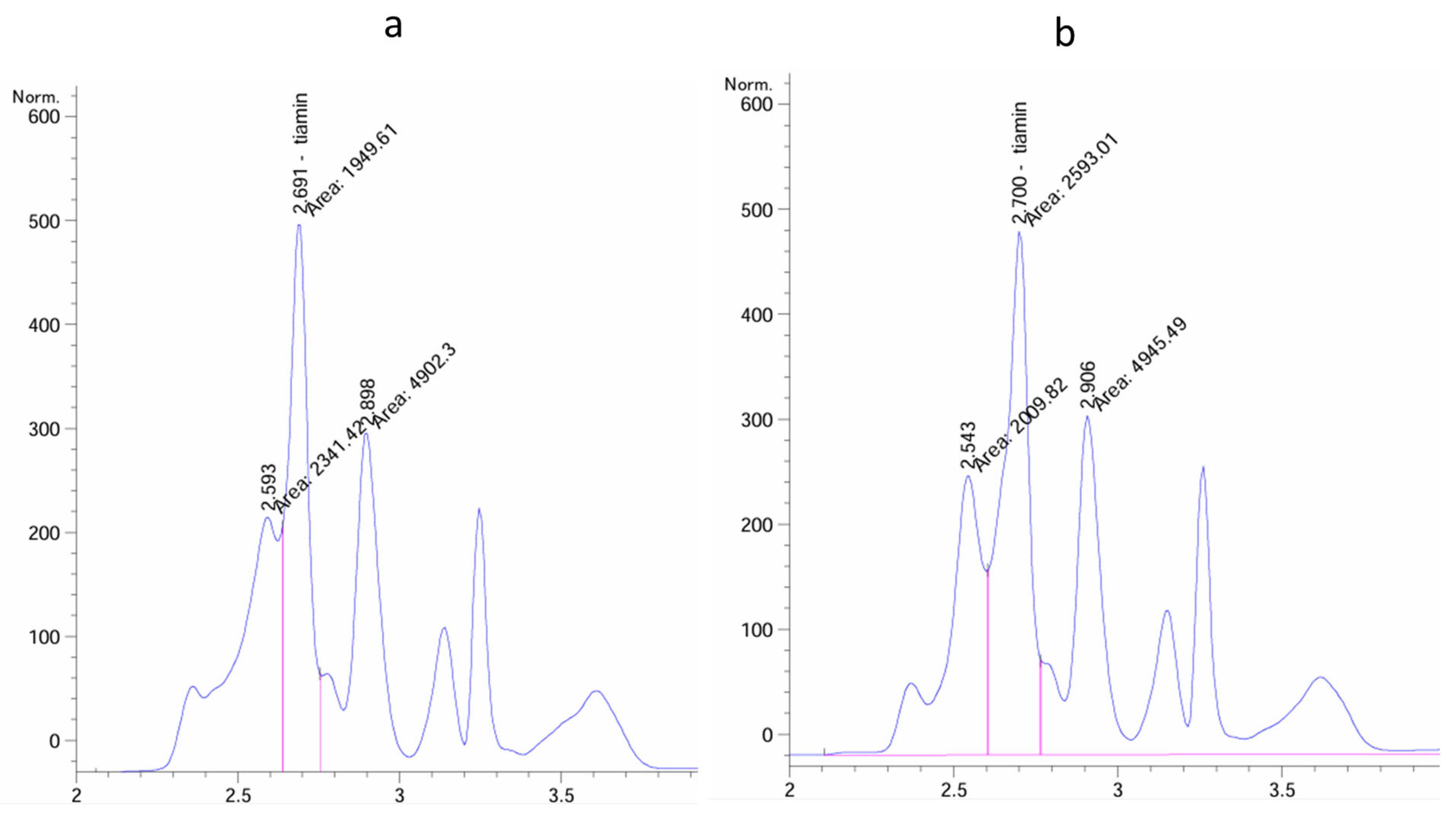
2.8. Measurements of ABA and Thiamine Content
3. Results
3.1. Garlic Containing PBs May Inhibit Growth of Fusarium Species
3.2. Antifungal Activity Test of Solid Agar Plate Assay
3.3. Inoculation of Pea Seeds with Fusarium oxysporum f. sp. pisi
3.4. Increased NDVI and SPAD on Field Pea Plants Treated with EliceVakcina and Fitokondi
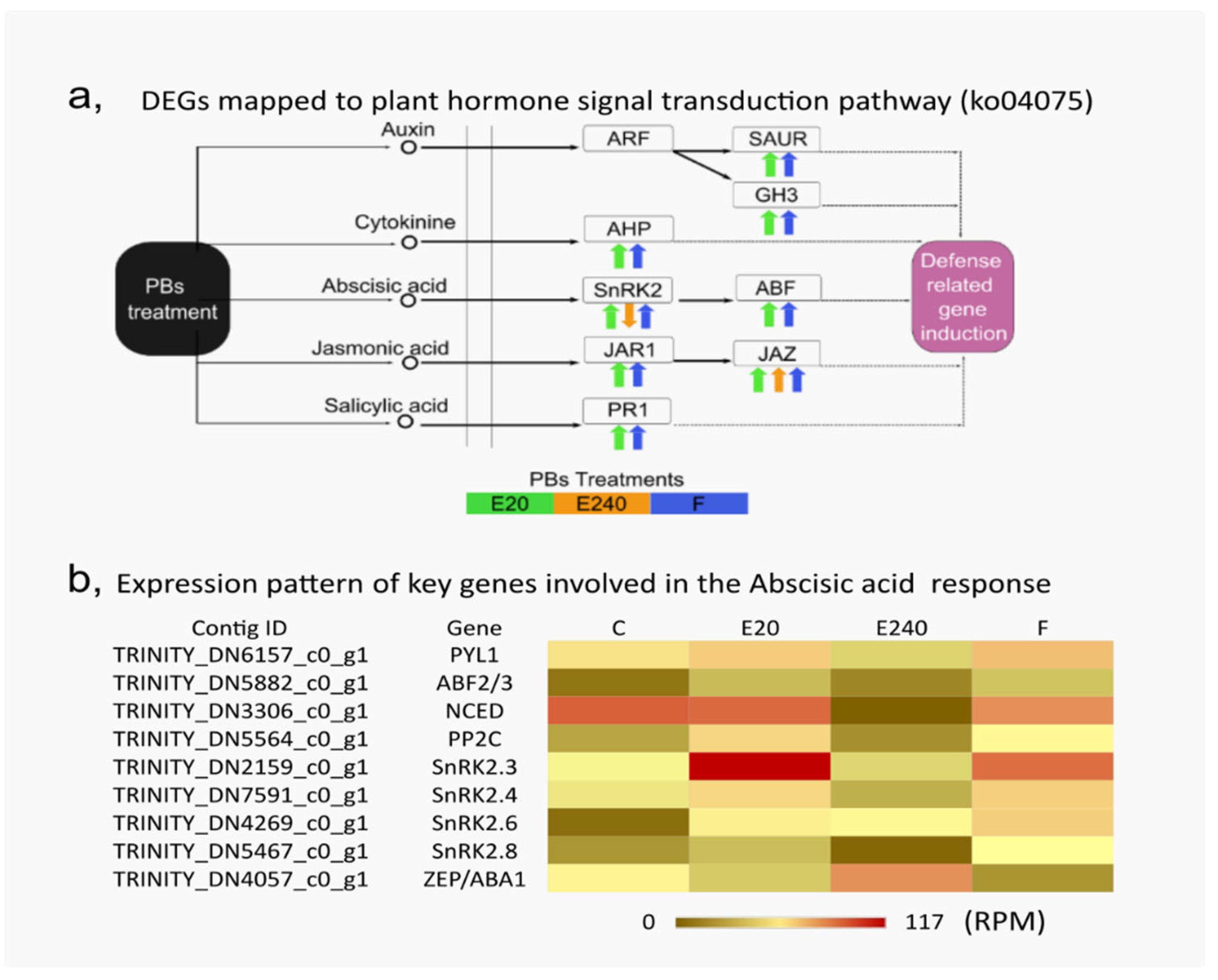
3.5. RNA-Seq Analysis of Field Pea Plants Treated by the Nanoliposome-Formulated EliceVakcina and Fitokondi
3.6. Differential Gene Expression Analysis Revealed the Upregulation of Stress Response
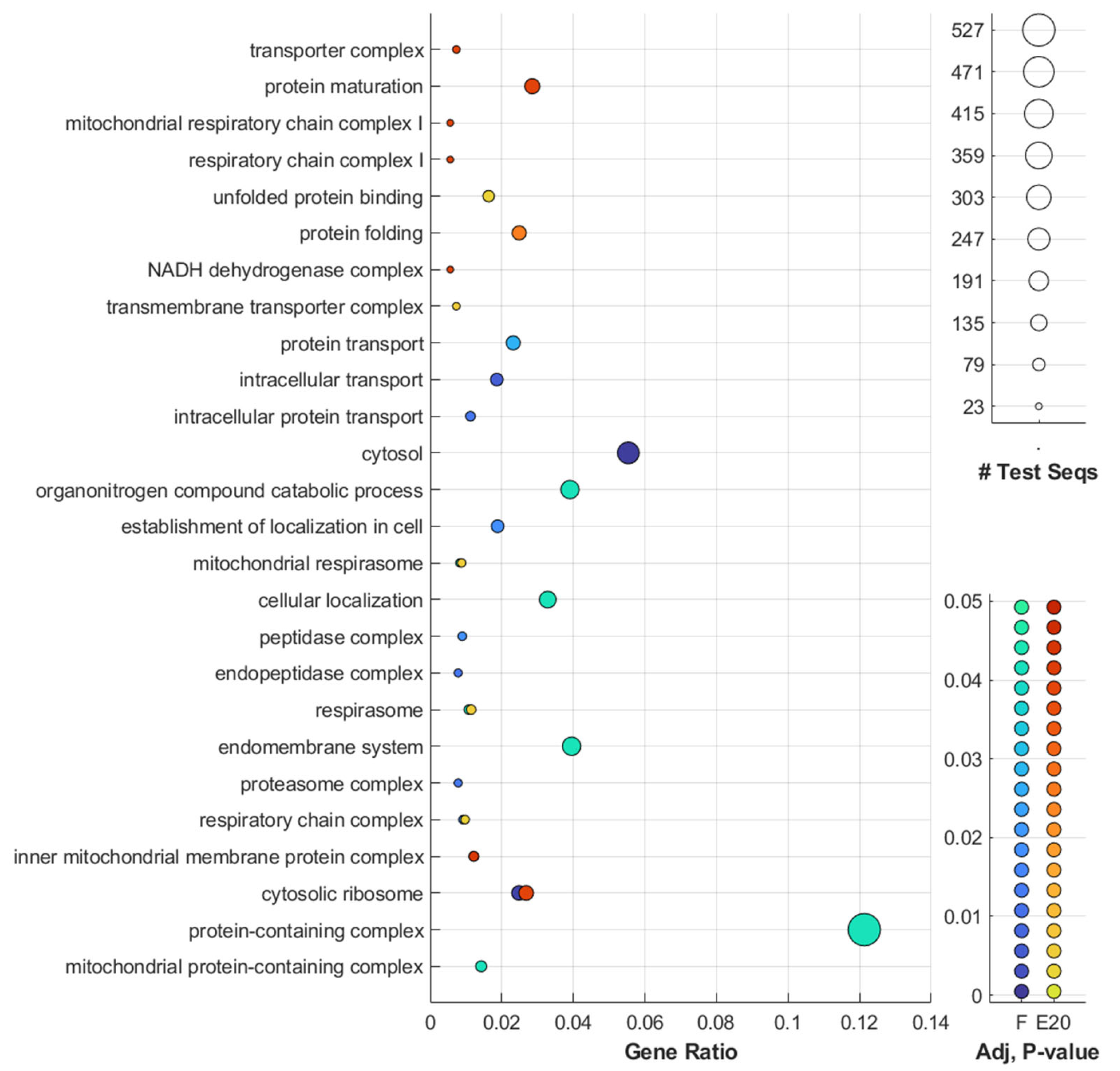
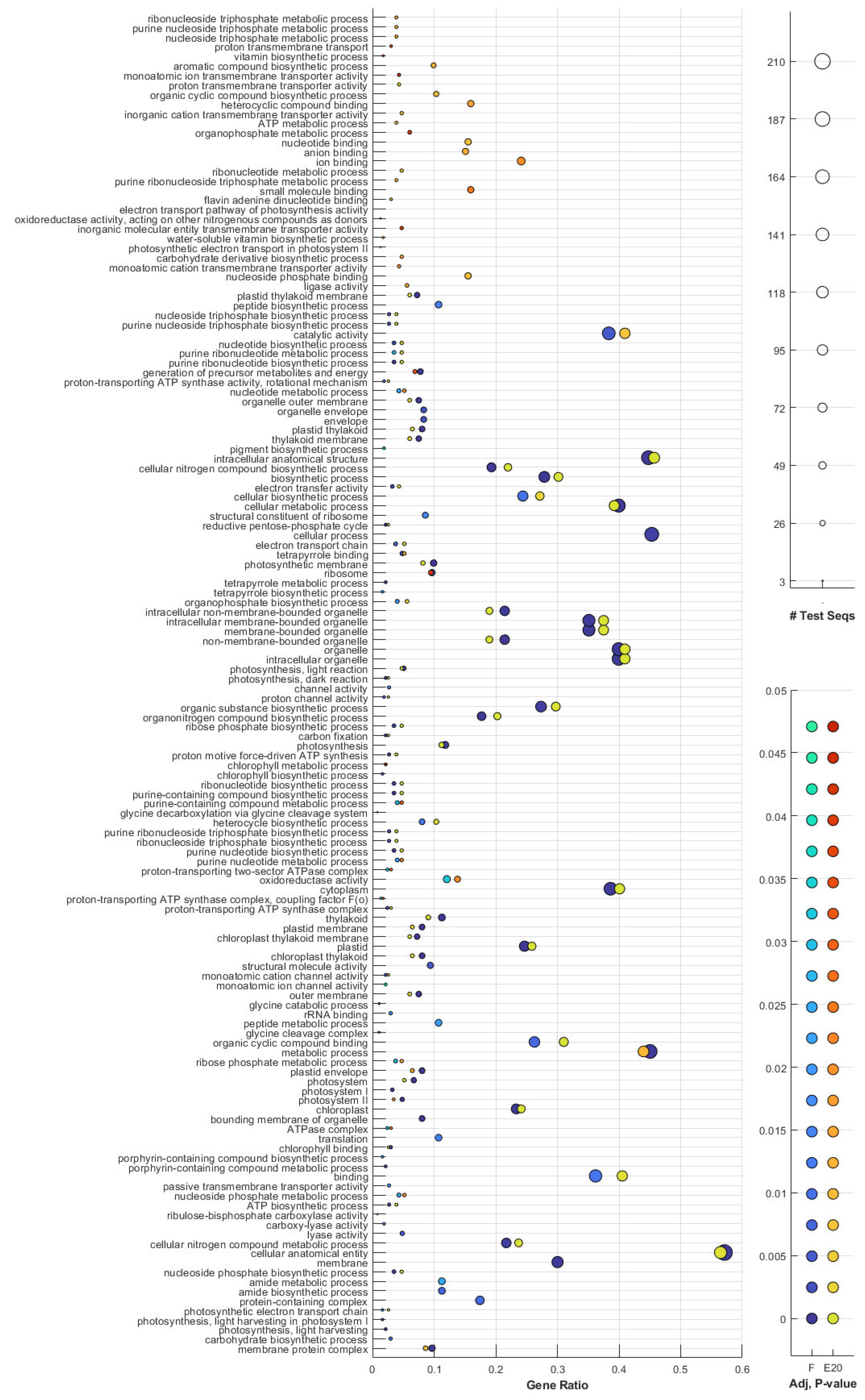
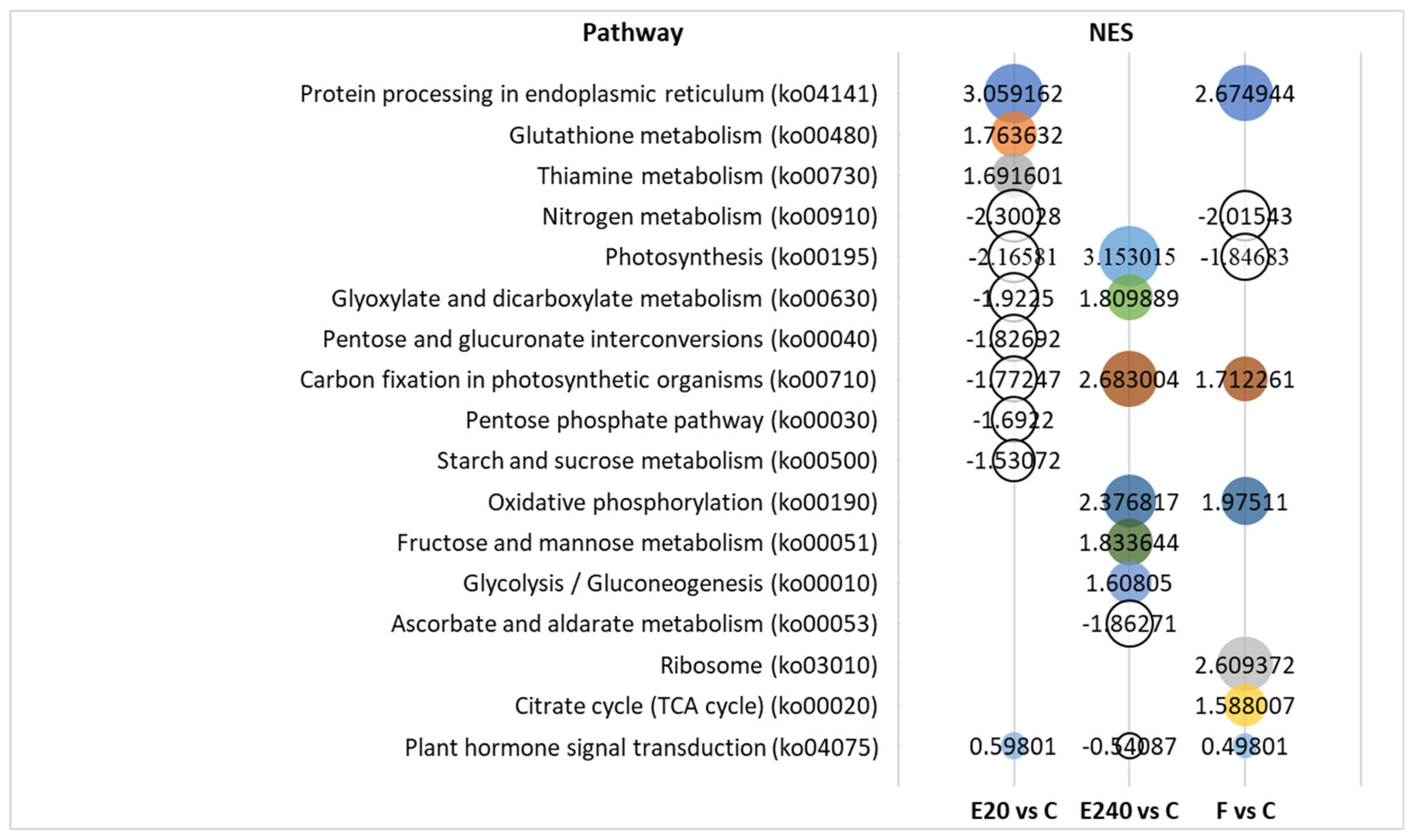
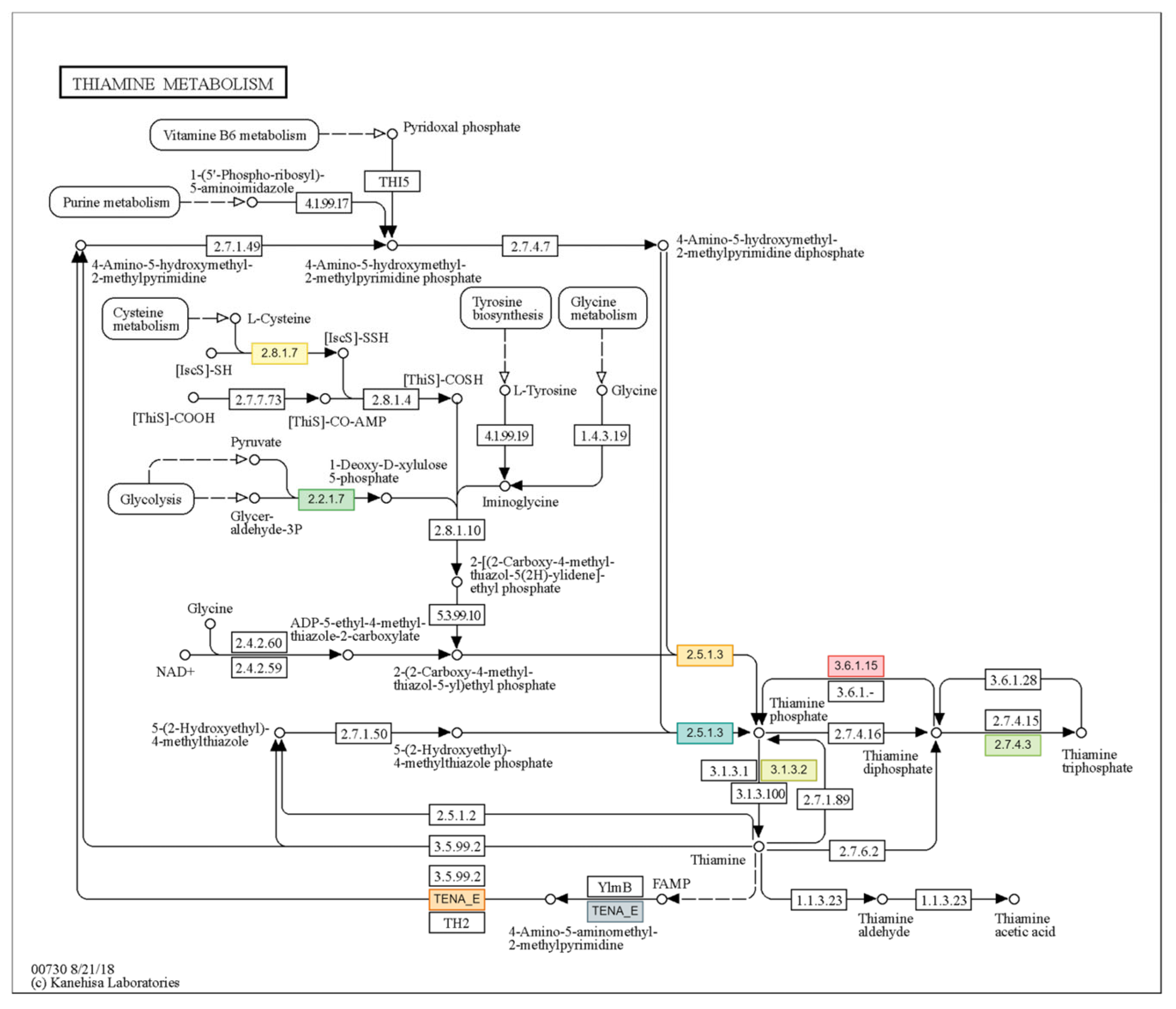
3.7. GO and Pathway Analysis Indicated Dose Dependency in Photosynthesis, Glutathione and Thiamine Metabolism
3.8. Strong Phytohormone Response to the Nanoliposome Formulated Garlic-Containing Elicevakcina
3.9. Increased Thiamine Content in Field Pea Plants Treated with Nanoformulated EliceVakcina
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ACP | Acid phosphatase |
| BH | Benjamini-Hochberg |
| C | Control, non-treated leaf |
| CCOAOMT | Caffeoyl-CoA O-methyltransferase |
| CHS | Chalcone synthase |
| CLSI | Clinical and Laboratory Standards Institute |
| CYP81E9 | Isoflavone 3′-hydroxylase |
| CYP93C | Isoflavone synthase |
| DEG | Differentially expressed gene |
| Dxs | 1-Deoxy-D-Xylulose-5-Phosphate Pyruvate-Lyase |
| E | EliceVakcina |
| E20 | EliceVakcina treatment of dosage at 20 g ha−1 |
| E240 | EliceVakcina treatment of dosage at 240 g ha−1 |
| ERF5 | Ethylene-responsive transcription factor 5 |
| ET | Ethylene |
| F | Fitokondi treatment of dosage of 4 L ha−1 |
| Fop | Fusarium oxysporum f. sp. pisi |
| GA | Gibberellin |
| GL | Garlic-lipo |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| GST | Glutathione transferase |
| HPLC-DAD | High-performance liquid chromatography with diode-array detection |
| HSP | Heat shock protein |
| IAA | Indole-3-acetic acid |
| Inoc. | Inoculated |
| IscS | Cysteine Desulfurases |
| JA | Jasmonic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes database |
| NDVI | Normalized Difference Vegetation Index |
| NES | Normalized Enrichment Score |
| NGS | Next-generation sequencing |
| NIR | Near-infrared light |
| PB | Plant biostimulants |
| POD | Peroxidase |
| PR | Pathogenesis-related |
| RED | Red light |
| RIMPH Ltd. | Research Institute for Medicinal Plants and Herbs Ltd. |
| SA | Salicylic acid |
| SC-CO2 | Supercritical carbon dioxide extraction |
| SnRK | Sucrose non-fermenting1-related protein kinase |
| SPAD | Soil-Plant Analysis Development |
| SRA | Sequence Read Archive NCBI |
| TCA | Tricarboxylic acid cycle |
| TSA | Transcriptome Shotgun Assembly NCBI |
| ZEP | Zeaxanthin epoxidase |
References
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs) an Introduction. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; ElSayed, A.I.; Merwad, A.-R.M.; Rady, M.M. Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef]
- Ismaiel, S.A.R.; Khedr, F.G.; Metwally, A.G.; Soror, A.F.S. Effect of biostimulants on soil characteristics, plant growth and yield of Pea (Pisum sativum L.) under field conditions. Plant Sci. Today 2022, 9, 650–657. [Google Scholar] [CrossRef]
- Merwad, A.-R.M. Using Moringa oleifera extract as biostimulant enhancing the growth, yield and nutrients accumulation of pea plants. J. Plant Nutr. 2018, 41, 425–431. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Kliszcz, A.; Slizowska, A.; Kot, D. Application of biostimulants influences shoot and root characteristics of seedlings of winter pea (Pisum sativum L.). Acta Agrobot. 2019, 72, 1771. [Google Scholar] [CrossRef]
- Sulewska, H.; Niewiadomska, A.; Ratajczak, K.; Budka, A.; Panasiewicz, K.; Faligowska, A.; Wolna-Maruwka, A.; Dryjański, L. Changes in Pisum sativum L. plants and in soil as a result of application of selected foliar fertilizers and biostimulators. Agronomy 2020, 10, 1558. [Google Scholar] [CrossRef]
- Szpunar-Krok, E. Physiological Response of Pea (Pisum sativum L.) Plants to Foliar Application of Biostimulants. Agronomy 2022, 12, 3189. [Google Scholar] [CrossRef]
- Manchikanti, P. Bioavailability and environmental safety of nanobiopesticides. In Nano-Biopesticides Today and Future Perspectives; Academic Press: Cambridge, MA, USA, 2019; pp. 207–222. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Sayyad, G.S.; Abdelaziz, A.M.; Gaber, S.E.; Khalil, A.M.A.; Saleh, A.M.; Al zoubi, O.M.; Hashem, A.H. Protective role of Mycosynthesized Bimetallic ZnO-CuO nanoparticles as therapeutic nutrients to enhance the resistance of Vicia faba against Fusarium Wilt Disease. Agronomy 2023, 13, 2725. [Google Scholar] [CrossRef]
- Attia, M.S.; Abdelaziz, A.M.; Hassanin, M.M.; Al-Askar, A.A.; Marey, S.A.; Abdelgawad, H.; Hashem, A.H. Eco-friendly preparation of thyme essential oil nano emulsion: Characterization, antifungal activity and resistance of Fusarium wilt disease of Foeniculum vulgare. Not. Bot. Horti Agrobot. 2023, 51, 13312. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Elshaer, M.A.; Abd-Elraheem, M.A.; Ali, O.M.O.M.; Haggag, M.I.; El-Sayyad, G.S.; Attia, M.S. Ziziphus spina-christi extract-stabilized novel silver nanoparticle synthesis for combating Fusarium oxysporum-causing pepper wilt disease: In vitro and in vivo studies. Arch. Microbiol. 2023, 205, 69. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; El-Sayyad, G.S.; Al-Shammari, B.M.; Abdelaziz, A.M.; Nofel, M.M.; Gobara, M.; Elkhatib, W.F.; Eid, N.A.; Salem, M.S.; Attia, M.S. Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microb. Pathog. 2023, 180, 106131. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal ABA in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Wu, H.-C.; Jinn, T.-L. Coordination of ABA and chaperone signaling in plant stress responses. Trends Plant Sci. 2019, 24, 636–651. [Google Scholar] [CrossRef]
- Kumar, M.; Kesawat, M.S.; Ali, A.; Lee, S.-C.; Gill, S.S.; Kim, H.U. Integration of abscisic acid signaling with other signaling pathways in plant stress responses and development. Plants 2019, 8, 592. [Google Scholar] [CrossRef]
- Humplík, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- van Staden, J.; Bornman, C.H. Inhibition and promotion by abscisic acid of growth in Spirodela. Planta 1969, 85, 157–159. [Google Scholar] [CrossRef]
- McWha, J.; Jackson, D. Some growth promotive effects of abscisic acid. J. Exp. Bot. 1976, 27, 1004–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W. Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants? J. Exp. Bot. 1990, 41, 1125–1132. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Yamburenko, M.V.; Zubo, Y.O.; Börner, T. Abscisic acid affects transcription of chloroplast genes via protein phosphatase 2C-dependent activation of nuclear genes: Repression by guanosine-3′-5′-bisdiphosphate and activation by sigma factor 5. Plant J. 2015, 82, 1030–1041. [Google Scholar] [CrossRef]
- Atif, M.J.; Amin, B.; Ghani, M.I.; Ali, M.; Liu, X.; Zhang, Y.; Cheng, Z. Allium sativum L. (Garlic) bulb enlargement as influenced by differential combinations of photoperiod and temperature. Food Chem. 2021, 338, 127991. [Google Scholar] [CrossRef]
- Latif, H.H. Physiological responses of Pisum sativum plant to exogenous ABA application under drought conditions. Pak. J. Bot. 2014, 46, 973–982. [Google Scholar]
- Abdelaziz, A.M.; Elrefaey, A.A.; Sharaf, M.H.; Al-Qthanin, R.N.; Attia, M.S. Enhancing tomato plant immune responses to Fusarium wilt disease by red seaweed Jania sp. Sci. Rep. 2024, 14, 18052. [Google Scholar] [CrossRef]
- Attia, M.S.; Salem, M.S.; Abdelaziz, A.M. Endophytic fungi Aspergillus spp. reduce fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Convers. Biorefinery 2024, 14, 16603–16613. [Google Scholar] [CrossRef]
- Kraft, J. Fusarium wilt of peas (a review). Agronomie 1994, 14, 561–567. [Google Scholar] [CrossRef]
- Bishop, C.; Cooper, R.M. An ultrastructural study of vascular colonization in three vascular wilt diseases I. Colonization of susceptible cultivars. Physiol. Plant Pathol. 1983, 23, 323–343. [Google Scholar] [CrossRef]
- Grünwald, N.; Coffman, V.; Kraft, J. Sources of partial resistance to Fusarium root rot in the Pisum core collection. Plant Dis. 2003, 87, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.D.; Kraft, J.M.; Grünwald, N.J. Release of Pea Germplasm with Fusarium Resistance Combined with Desirable Yield and Anti-Lodging Traits. J. Plant Regist. 2014, 8, 191–194. [Google Scholar] [CrossRef]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Trusov, Y.; Sewelam, N.; Rookes, J.E.; Kunkel, M.; Nowak, E.; Schenk, P.M.; Botella, J.R. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene-and abscisic acid-mediated defense signaling. Plant J. 2009, 58, 69–81. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kozak, B.; Wojtasik, W.; Preisner, M.; Prescha, A.; Gola, E.M.; Lysh, D.; Dudek, B.; Szopa, J. Fusarium oxysporum infection activates the plastidial branch of the terpenoid biosynthesis pathway in flax, leading to increased ABA synthesis. Planta 2020, 251, 50. [Google Scholar] [CrossRef]
- Khedr, M.A.; Massarotti, A.; Mohamed, M.E. Rational discovery of (+)(S) abscisic acid as a potential antifungal agent: A repurposing approach. Sci. Rep. 2018, 8, 8565. [Google Scholar] [CrossRef]
- Virág, E.; Kiniczky, M.; Kutasy, B.; Nagy, Á.; Pallos, J.P.; Laczkó, L.; Freytag, C.; Hegedűs, G. Supplementation of the Plant Conditioner ELICE Vakcina® Product with β-Aminobutyric Acid and Salicylic Acid May Lead to Trans-Priming Signaling in Barley (Hordeum vulgare). Plants 2023, 12, 2308. [Google Scholar] [CrossRef]
- Hegedűs, G.; Kutasy, B.; Kiniczky, M.; Decsi, K.; Juhász, Á.; Nagy, Á.; Pallos, J.P.; Virág, E. Liposomal formulation of botanical extracts may enhance yield triggering PR genes and phenylpropanoid pathway in Barley (Hordeum vulgare). Plants 2022, 11, 2969. [Google Scholar] [CrossRef]
- Decsi, K.; Kutasy, B.; Hegedűs, G.; Alföldi, Z.P.; Kálmán, N.; Nagy, Á.; Virág, E. Natural immunity stimulation using ELICE16INDURES® plant conditioner in field culture of soybean. Heliyon 2023, 9, e12907. [Google Scholar] [CrossRef]
- Decsi, K.; Kutasy, B.; Kiniczky, M.; Hegedűs, G.; Virág, E. RNA-seq datasets of field soybean cultures conditioned by Elice16Indures® biostimulator. Data Brief. 2022, 42, 108182. [Google Scholar] [CrossRef]
- Kutasy, B.; Decsi, K.; Hegedűs, G.; Virág, E. Dataset of conditioning effect of herbal extract-based plant biostimulants in pea (Pisum sativum). Data Brief 2023, 46, 108800. [Google Scholar] [CrossRef] [PubMed]
- Kutasy, B.; Decsi, K.; Kiniczky, M.; Hegedűs, G.; Virág, E. Time-course gene expression profiling data of Triticum aestivum treated by supercritical CO2 garlic extract encapsulated in nanoscale liposomes. Data Brief 2022, 42, 108287. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, J.; Mena, C.; Budinich, M. Extraction of garlic with supercritical CO2 and conventional organic solvents. Braz. J. Chem. Eng. 2008, 25, 532–542. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Perkhofer, S.; Howard, S.J.; Garcia-Effron, G.; Vishukumar, A.; Perlin, D.; Lass-Flörl, C. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: The challenge of azoles versus echinocandins. Antimicrob. Agents Chemother. 2008, 52, 3504–3511. [Google Scholar] [CrossRef]
- Pfaller, M.; Messer, S.; Mills, K.; Bolmstrom, A. In vitro susceptibility testing of filamentous fungi: Comparison of Etest and reference microdilution methods for determining itraconazole MICs. J. Clin. Microbiol. 2000, 38, 3359–3361. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.; Deering, D. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NTRS—NASA Technical Reports Server: Washington, DC, USA, 1973. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef]
- Al-Shahrour, F.; Díaz-Uriarte, R.; Dopazo, J. FatiGO: A web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics 2004, 20, 578–580. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Lei, W.; Huang, S.; Tang, S.; Shui, X.; Chen, C. Determination of abscisic acid and its relationship to drought stress based on cowpea varieties with different capability of drought resistance. Am. J. Biochem. Biotechnol. 2016, 12, 79–85. [Google Scholar] [CrossRef]
- Ulusoy, S.; Akçay, M. Simultaneous determination of vitamins B1 and B2 in food samples by modified cloud point extraction method and HPLC-DAD. Food Anal. Methods 2018, 11, 260–269. [Google Scholar] [CrossRef]
- Abdelwahab, N.S.; Farid, N.F. Validated HPLC-DAD method for stability study of sulbutiamine HCl. RSC Adv. 2014, 4, 30523–30529. [Google Scholar] [CrossRef]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Hosseini, S.; Elfstrand, M.; Heyman, F.; Funck Jensen, D.; Karlsson, M. Deciphering common and specific transcriptional immune responses in pea towards the oomycete pathogens Aphanomyces euteiches and Phytophthora pisi. BMC Genom. 2015, 16, 627. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Myung, K.; Wang, N.; Johnson, A. Spray retention of crop protection agrochemicals on the plant surface. In Retention, Uptake, and Translocation of Agrochemicals in Plants; Myung, K., Satchivi, N.M., Kingston, C.K., Eds.; ACS Publications, American Chemical Society: Washington, DC, USA, 2014; pp. 1–22. [Google Scholar]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef]
- Talaat, I.; El-Metwaly, I.; Dawood, M.; El-Awadi, M.; El-Rokiek, K. Response of pea plants (Pisum sativum) to nano-salicylic acid and herbicides. J. Mater. Environ. Sci. 2022, 13, 1218–1226. [Google Scholar]
- Rahman, M.S.; Chakraborty, A.; Kibria, A.; Hossain, M.J. Effects of silver nanoparticles on seed germination and growth performance of pea (Pisum sativum). Plant Nano Biol. 2023, 5, 100042. [Google Scholar] [CrossRef]
- Kiani, B.H.; Arshad, I.; Nazir, S.; Saleh, I.A.; Kiani, S.H.; Zomot, N.; Al-Qahtani, W.H.; Alfuraydi, A.A.; Abdel-Maksoud, M.A. Innovative approaches for sustainable ZINC Nutrition and crop Yield enhancement in pea plants using zinc oxide nanoparticles. J. Soil Sci. Plant Nutr. 2024, 24, 5829–5840. [Google Scholar] [CrossRef]
- Sahani, R.; Saxena, A. Fungitoxic Properties of Medicinal and Aromatic Plants against Fusarium oxysporum f. sp. pisi. Ann. Plant Prot. Sci. 2009, 17, 146–148. [Google Scholar]
- Minz, S.; Samuel, C.; Tripathi, S. The effect of plant extracts on the growth of wilt causing fungi Fusarium oxysporum. J. Pharm. Biol. Sci. 2012, 4, 13–16. [Google Scholar] [CrossRef]
- Sagar, M.A.; Prasad, R.; Kumar, S.; Singh, M. Antifungal activity of some botanicals extracts against Fusarium oxysporum f. sp. pisi a causal agent of wilt of pea (Pisum sativum L.). J. Med. Plants 2021, 9, 38–41. [Google Scholar]
- Verma, S.; Dohroo, N.P. Evaluation of botanicals in vitro against Fusarium oxysporum f. sp. pisi causing wilt of pea. Plant Dis. Res. 2003, 18, 131–134. [Google Scholar]
- Li, W.-R.; Shi, Q.-S.; Liang, Q.; Huang, X.-M.; Chen, Y.-B. Antifungal effect and mechanism of garlic oil on Penicillium funiculosum. Appl. Microbiol. Biotechnol. 2014, 98, 8337–8346. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Shi, Q.-S.; Dai, H.-Q.; Liang, Q.; Xie, X.-B.; Huang, X.-M.; Zhao, G.-Z.; Zhang, L.-X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W.; Yoon, J.H.; Kim, S.H.; Hong, S.B.; Cheon, Y.; Ko, S.J. Detection of Extracellular Enzyme Activities in Various Fusarium spp. Mycobiology 2007, 35, 162–165. [Google Scholar] [CrossRef]
- Turco, E.; Broggio, M.; Ragazzi, A. Enzymes produced by Fusarium oxysporum f. sp. vasinfectum compared on a saline and a non-saline substrate. Z. Für Pflanzenkrankh. Und Pflanzenschutz/J. Plant Dis. Prot. 1999, 106, 333–341. [Google Scholar]
- Arney, S.; Mitchell, D. The effect of abscisic acid on stem elongation and correlative inhibition. New Phytol. 1969, 68, 1001–1015. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Liu, L.; Zhao, L.; Shen, X.; Rao, G.; Li, T. Effects of exogenous salicylic acid and abscisic acid on growth, photosynthesis and antioxidant system of rice. Chil. J. Agric. Res. 2022, 82, 21–32. [Google Scholar] [CrossRef]
- Travaglia, C.; Reinoso, H.; Cohen, A.; Luna, C.; Tommasino, E.; Castillo, C.; Bottini, R. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul. 2010, 29, 366–374. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Hayat, K.; Khan, M.A.; Cheng, Z. Aqueous garlic extract as a plant biostimulant enhances physiology, improves crop quality and metabolite abundance, and primes the defense responses of receiver plants. Appl. Sci. 2018, 8, 1505. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Ali, M.; Ren, K.; Cheng, Z. Aqueous garlic extract stimulates growth and antioxidant enzymes activity of tomato (Solanum lycopersicum). Sci. Hortic. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Al-Araby, A.A.; Xuan, T.D. Growth traits, physiological parameters and hormonal status of snap bean (Phaseolus vulgaris L.) sprayed with garlic cloves extract. Arch. Agron. Soil. Sci. 2018, 64, 1068–1082. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Badr, E.A.; Sadak, M.S.; Khedr, H.H. Effect of garlic extract, ascorbic acid and nicotinamide on growth, some biochemical aspects, yield and its components of three faba bean (Vicia faba L.) cultivars under sandy soil conditions. Bull. Natl. Res. Cent. 2020, 44, 100. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant–pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Virág, E.; Nagy, Á.; Tóth, B.B.; Kutasy, B.; Pallos, J.P.; Szigeti, Z.M.; Máthé, C.; Kardos, G.; Hegedűs, G. Master Regulatory Transcription Factors in β-Aminobutyric Acid-Induced Resistance (BABA-IR): A Perspective on Phytohormone Biosynthesis and Signaling in Arabidopsis thaliana and Hordeum vulgare. Int. J. Mol. Sci. 2024, 25, 9179. [Google Scholar] [CrossRef]
- Kumar, S.; Röder, M.S.; Singh, R.P.; Kumar, S.; Chand, R.; Joshi, A.K.; Kumar, U. Mapping of spot blotch disease resistance using NDVI as a substitute to visual observation in wheat (Triticum aestivum L.). Mol. Breed. 2016, 36, 95. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2018; pp. 265–281. [Google Scholar]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1, 3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef]
- Caporale, C.; Di Berardino, I.; Leonardi, L.; Bertini, L.; Cascone, A.; Buonocore, V.; Caruso, C. Wheat pathogenesis-related proteins of class 4 have ribonuclease activity. FEBS Lett. 2004, 575, 71–76. [Google Scholar] [CrossRef]
- Vigers, A.J.; Wiedemann, S.; Roberts, W.K.; Legrand, M.; Selitrennikoff, C.P.; Fritig, B. Thaumatin-like pathogenesis-related proteins are antifungal. Plant Sci. 1992, 83, 155–161. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, Z.; Chen, E.; Sun, G.; He, S.; Butt, H.I.; Zhang, C.; Zhang, X.; Yang, Z.; Du, X. A phi-class glutathione S-transferase gene for Verticillium wilt resistance in Gossypium arboreum identified in a genome-wide association study. Plant Cell Physiol. 2018, 59, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sukhova, E.; Sherstneva, O.; Grinberg, M.; Ladeynova, M.; Vodeneev, V.; Sukhov, V. Exogenous abscisic acid can influence photosynthetic processes in peas through a decrease in activity of H+-ATP-ase in the plasma membrane. Biology 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Goyer, A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Miller, G.; Song, L.; Kim, J.; Sodek, A.; Koussevitzky, S.; Misra, A.N.; Mittler, R.; Shintani, D. Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 2009, 151, 421–432. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Wolak, N.; Kujda, M.; Banas, A.K. The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Dakora, F.D.; Matiru, V.N.; Kanu, A.S. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants. Front. Plant Sci. 2015, 6, 700. [Google Scholar] [CrossRef]
- Ahn, I.-P.; Kim, S.; Lee, Y.-H.; Suh, S.-C. Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol. 2007, 143, 838–848. [Google Scholar] [CrossRef]
- Khozaei, M.; Fisk, S.; Lawson, T.; Gibon, Y.; Sulpice, R.; Stitt, M.; Lefebvre, S.C.; Raines, C.A. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 2015, 27, 432–447. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Chapman, L.M. The importance of thiamine (vitamin B1) in plant health: From crop yield to biofortification. J. Biol. Chem. 2020, 295, 12002–12013. [Google Scholar] [CrossRef]


| Product Name | Abbr. | Manufacturers | Formulation | Garlic Content (%) | ABA Content (µg g−1) | References |
|---|---|---|---|---|---|---|
| * EliceVakcina | E | RIMPH | nanoliposome, SC-CO2 extract | ~65 | 6.3 ± 1.2 | [39,40,41,42] |
| Fitokondi | F | RIMPH | aqueous extract of herbs | ~1 | <LOD | [43] |
| * Garlic-lipo | GL | RIMPH | nanoliposome, SC-CO2 extract | ~90 | 80.4 ± 2.2 | [44] |
| Garlic-oil | G | Flavex | SC-CO2 extract | 100 | 81.0 ± 2.4 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutasy, B.; Hegedűs, G.; Kiniczky, M.; Pallos, J.P.; Nagy, Á.; Pócsi, I.; Pákozdi, K.; Kállai, M.; Weingart, C.; Andor, K.; et al. Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum. Agronomy 2025, 15, 991. https://doi.org/10.3390/agronomy15040991
Kutasy B, Hegedűs G, Kiniczky M, Pallos JP, Nagy Á, Pócsi I, Pákozdi K, Kállai M, Weingart C, Andor K, et al. Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum. Agronomy. 2025; 15(4):991. https://doi.org/10.3390/agronomy15040991
Chicago/Turabian StyleKutasy, Barbara, Géza Hegedűs, Márta Kiniczky, József Péter Pallos, Ágnes Nagy, István Pócsi, Klaudia Pákozdi, Máté Kállai, Csaba Weingart, Katalin Andor, and et al. 2025. "Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum" Agronomy 15, no. 4: 991. https://doi.org/10.3390/agronomy15040991
APA StyleKutasy, B., Hegedűs, G., Kiniczky, M., Pallos, J. P., Nagy, Á., Pócsi, I., Pákozdi, K., Kállai, M., Weingart, C., Andor, K., Kovács, B., & Virág, E. (2025). Garlic Extracts Nanoliposome as an Enhancer of Bioavailability of ABA and Thiamine Content and as an Antifungal Agent Against Fusarium oxysporum f. sp. pisi Infecting Pisum sativum. Agronomy, 15(4), 991. https://doi.org/10.3390/agronomy15040991






