Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance
Abstract
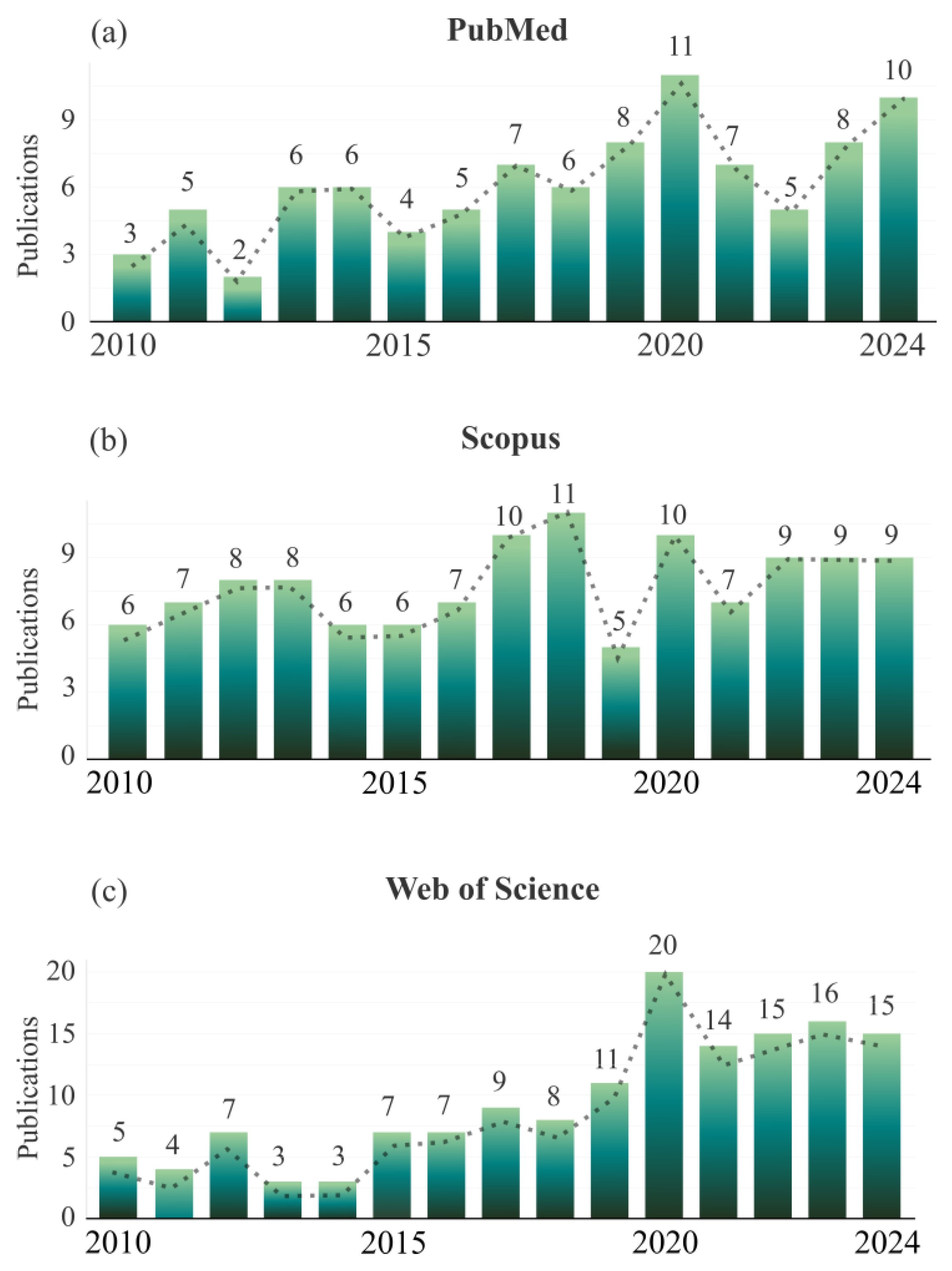
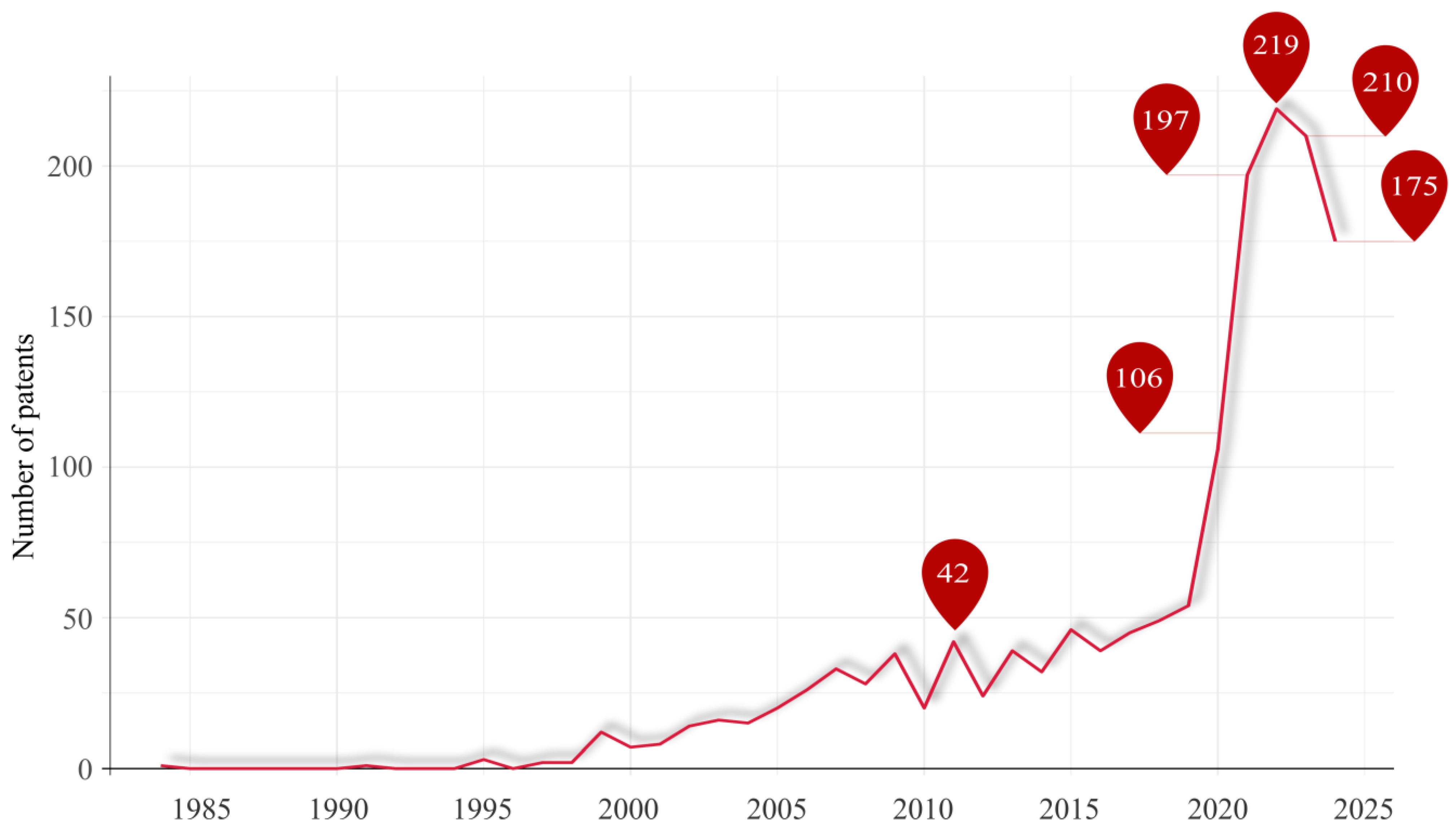
1. Introduction
2. Factors Influencing Bioaerosols’ Composition
3. Spatial Distribution
4. The One Health Approach
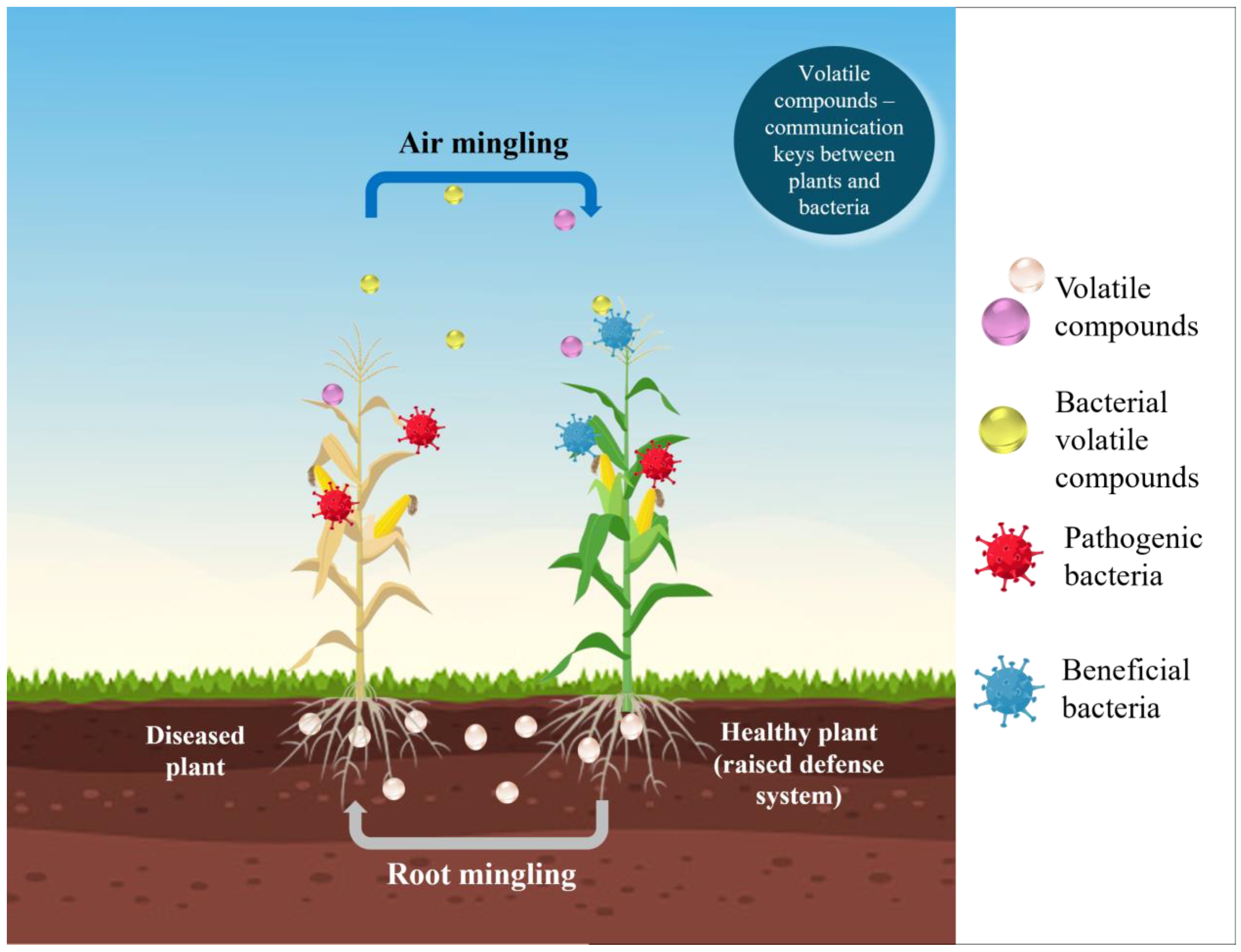
5. Behavioral and Communication Pathways
5.1. Bacterial Volatile Compounds (BVCs)
5.2. Volatile Organic Compounds (VOCs)
6. Practical Importance of Bioaerosols in Agriculture
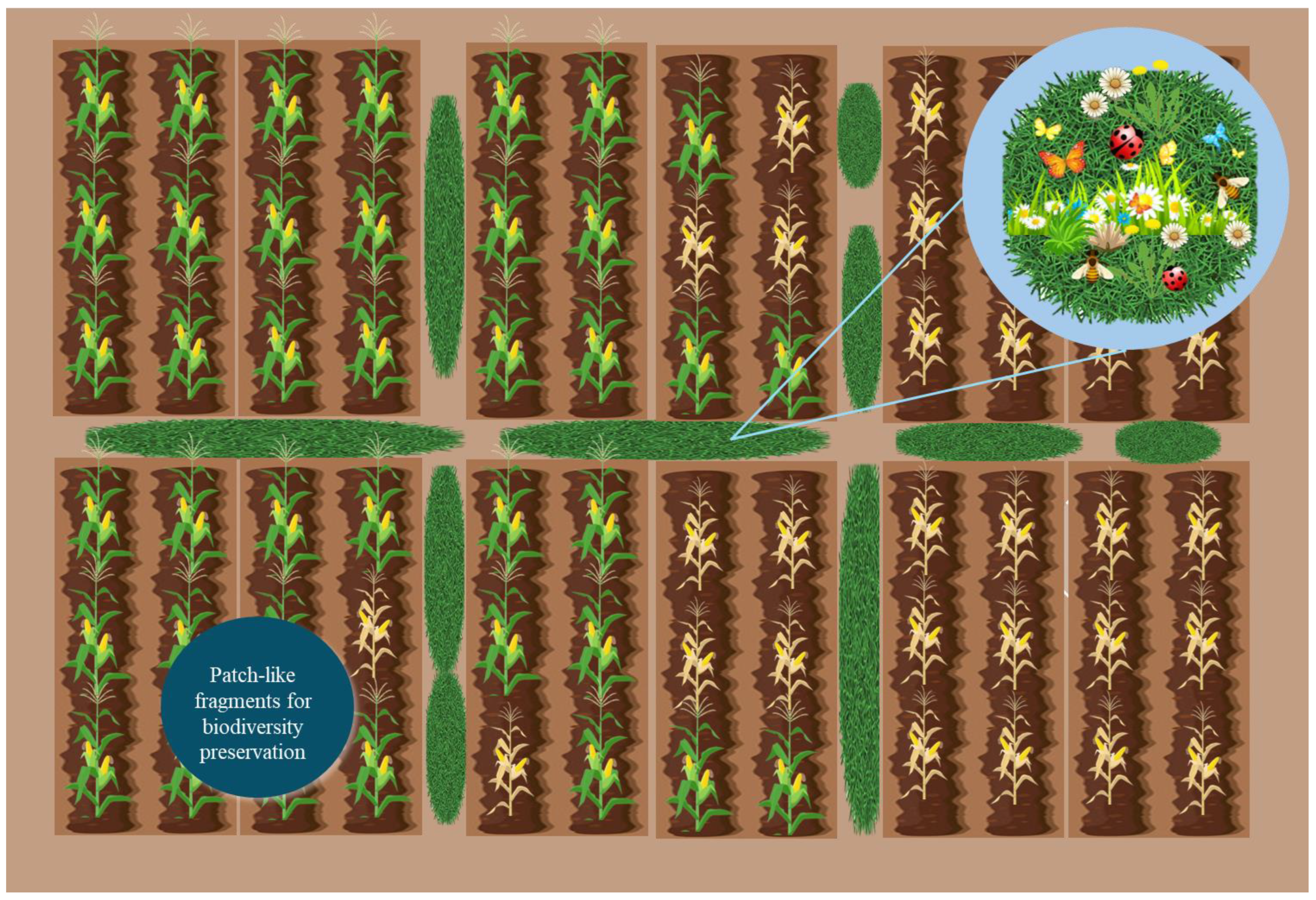
6.1. Combating Biodiversity Loss
6.2. Direct Role of Bioaerosols in Crops’ Health
| Crop | Disease | Symptoms | Responsible microorganism | References |
|---|---|---|---|---|
| Cucumber, pumpkin, zucchini, and watermelon | Powdery Mildew of Cucurbits (Podosphaera xanthii) | Brittle yellow leaves, malformed fruit (not directly attacked), and white colonies on the surface of leaves and stems | Powdery Mildew of Cucurbits (Podosphaera xanthii) | [171] |
| Tomatoe | Bacterial speck of tomatoe | Leaves with brown–black spots, dark spots on fruit, low yield | Pseudomonas syringae | [172] |
| Potato | Alternaria disease of potato | Yield decrease, damage of all green parts of the plant | Alternaria solani | [173] |
| Wheat | Pyricularia disease | Partial or total loss of the heads, many infection cycles per season | Pyricularia grisea | [174] |
| Winter wheat | Eyespot | Loss in yield, weakened stems, elliptical lesions on stems | Oculimacula acuformis and Oculimacula yallundae | [174] |
| Corn | Tar Spot Complex (TSC) of maize | Dark, oval-to-round lesions on leaves (0.5–2.0 mm diameter), slightly raised black points randomly distributed on the leaf | Phyllachora maydis, Monographella maydis, and the hypeparasite Coniothyrium phyllachorae | [175,176] |
| Grape | Pierce’s disease | Leaf marginal necrosis, decline of plant vigor, wilting, and fruit drying | Xylella fastidiosa | [177,178,179] |
6.3. Bioaerosol-Based Disease Control
7. Bioaerosol Collection Principles and Analysis Methods
| Sampling Method | Filter Samplers | Liquid-Based Samplers (Impingers) | Impaction Samplers | Cyclones | Electrostatic Precipitators |
|---|---|---|---|---|---|
| Filtration Technique | Inertial forces, resistance forces, diffusion forces, and electrostatic attraction forces are responsible for collecting particles on a filter surface [194]. | Use inertia for separating air particles into a liquid collection medium [185]. | Inertial forces pull air through the perforated sampling head, and as air changes direction, microbes are collected on a Petri dish due to their inertia [207]. | Bioaerosol capturing into a liquid system using centrifugal force [196]. | Particles are collected through an electrostatic charge and deposited in a collection medium [185]. |
| Air Volume Processed * | From 1 to 50 L/min [194] | 12.5 L/min [208] | 20–80 L/min [209] | 100–1250 L/min [210] | 75 L/min [211] |
| Particle Size | From 0.01 to 10 mm [194] | 0.30–8 µm [212] | <2.5 µm [213] | From 1.7 to 9.8 µm [210] | From 30 nm to 10 mm [214] |
| Collection efficiency ** | >93% [215] | 80–90% [216] | <70% [217] | 50–90% [218] | >80% [211] |
| Availability | Europe and USA [219] | USA [220] | USA and Europe [221] | USA [222] | Asia [223] |
| Category | Method | Advantages | Disadvantages | References | |
|---|---|---|---|---|---|
| Conventional | Culture-Based Methods |
|
| [20,224] | |
| PCR * |
|
| [20,224] | ||
| Advanced | Fluorescence Detection (Epifluorescence microscopy and Flow cytometry) |
|
| [20,225] | |
| Mass Spectrometry (MALDI-TOF **) |
|
| [20,225] | ||
| ATP Bioluminescence |
|
| [20] | ||
| NGS *** (high-throughput sequencing) | rRNA sequencing |
|
| [20,224] | |
| Shotgun sequencing |
| ||||
8. Current Challenges in Bioaerosol Studies
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma Ghimire, P.; Tripathee, L.; Kang, S. Modification and Coupled Use of Technologies Are an Essential Envisioned Need for Bioaerosol Study—An Emerging Public Health Concern. Fundam. Res. 2022, 2, 218–221. [Google Scholar] [CrossRef]
- Kummer, V.; Thiel, W.R. Bioaerosols–Sources and Control Measures. Int. J. Hyg. Environ. Health 2008, 211, 299–307. [Google Scholar] [CrossRef]
- Clauß, M. Emission of Bioaerosols from Livestock Facilities—Methods and Results from Available Bioaerosol Investigations in and Around Agricultural Livestock Farming; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2020. [Google Scholar]
- Huffman, J.A.; Sinha, B.; Garland, R.M.; Snee-Pollmann, A.; Gunthe, S.S.; Artaxo, P.; Martin, S.T.; Andreae, M.O.; Pöschl, U. Size Distributions and Temporal Variations of Biological Aerosol Particles in the Amazon Rainforest Characterized by Microscopy and Real-Time UV-APS Fluorescence Techniques during AMAZE-08. Atmos. Chem. Phys. 2012, 12, 11997–12019. [Google Scholar] [CrossRef]
- Dommergue, A.; Amato, P.; Tignat-Perrier, R.; Magand, O.; Thollot, A.; Joly, M.; Bouvier, L.; Sellegri, K.; Vogel, T.; Sonke, J.E.; et al. Methods to Investigate the Global Atmospheric Microbiome. Front. Microbiol. 2019, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The Source and Transport of Bioaerosols in the Air: A Review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef]
- Womack, A.M.; Bohannan, B.J.M.; Green, J.L. Biodiversity and Biogeography of the Atmosphere. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3645–3653. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Amato, P.; Casamayor, E.O.; Lee, P.K.H.; Pointing, S.B. Microbial Ecology of the Atmosphere. FEMS Microbiol. Rev. 2022, 46, fuac009. [Google Scholar] [CrossRef]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol Generation by Raindrops on Soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef]
- Kumari, K.; Yadav, S. Unveiling the Role of Bioaerosols in Climate Processes: A Mini Review. Int. J. Environ. Res. 2024, 18, 81. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Zhang, J.; Zhu, B.; Xiang, D.; Zhao, X.; Liu, X. Bioaerosol Seasonal Variation and Contribution to Airborne Particulate Matter in Huangshi City of Central China. Atmosphere 2022, 13, 909. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Marr, L.C.; Randall, K.; Ewing, E.T.; Tufekci, Z.; Greenhalgh, T.; Tellier, R.; Tang, J.W.; Li, Y.; Morawska, L.; et al. What Were the Historical Reasons for the Resistance to Recognizing Airborne Transmission during the COVID-19 Pandemic? Indoor Air 2022, 32, e13070. [Google Scholar] [CrossRef]
- Osbiston, K.; Oxbrough, A.; Fernández-Martínez, L.T. Antibiotic Resistance Levels in Soils from Urban and Rural Land Uses in Great Britain. Access Microbiol. 2020, 3, acmi000181. [Google Scholar] [CrossRef]
- Abu-Ashour, J.; Joy, D.M.; Lee, H.; Whiteley, H.R.; Zelin, S. Transport of Microorganisms through Soil. Water Air Soil. Pollut. 1994, 75, 141–158. [Google Scholar] [CrossRef]
- Kakde, U. Fungal bioaerosols: Global diversity, distribution and its impact on human beings and agricultural crops. Bionano Front. 2012, 5, 323–329. [Google Scholar]
- Afzal, A.; Syed, S.; Ahmad, R.; Zeeshan, M.; Nabi, G.; Afzal, A.; Syed, S.; Ahmad, R.; Zeeshan, M.; Nabi, G. The Menace of Aflatoxin: Understanding the Effects of Contamination by Aspergillus Species on Crops and Human Health and Advancements in Managing These Toxic Metabolites. In Aspergillus and Aspergillosis—Advances in Genomics, Drug Development, Diagnosis and Treatment; IntechOpen: London, UK, 2023. [Google Scholar]
- Qu, Z.; Ren, X.; Du, Z.; Hou, J.; Li, Y.; Yao, Y.; An, Y. Fusarium Mycotoxins: The Major Food Contaminants. mLife 2024, 3, 176–206. [Google Scholar] [CrossRef]
- Sajjad, B.; Hussain, S.; Rasool, K.; Hassan, M.; Almomani, F. Comprehensive Insights into Advances in Ambient Bioaerosols Sampling, Analysis and Factors Influencing Bioaerosols Composition. Environ. Pollut. 2023, 336, 122473. [Google Scholar] [CrossRef]
- Heikkinen, M.S.A.; Hjelmroos-Koski, M.K.; Häggblom, M.M.; Macher, J.M. Bioaerosols. In Aerosols Handbook; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Buiarelli, F.; Sonego, E.; Uccelletti, D.; Bruni, E.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Perrino, C.; Marcovecchio, F.; Simonetti, G. Determination of the Main Bioaerosol Components Using Chemical Markers by Liquid Chromatography–Tandem Mass Spectrometry. Microchem. J. 2019, 149, 103974. [Google Scholar] [CrossRef]
- Löndahl, J. Physical and Biological Properties of Bioaerosols. In Bioaerosol Detection Technologies; Jonsson, P., Olofsson, G., Tjärnhage, T., Eds.; Springer: New York, NY, USA, 2014; pp. 33–48. [Google Scholar]
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Singh, R.L., Ed.; Springer: Singapore, 2017; pp. 43–84. [Google Scholar]
- Nair, A.T. Bioaerosols in the Landfill Environment: An Overview of Microbial Diversity and Potential Health Hazards. Aerobiologia 2021, 37, 185–203. [Google Scholar] [CrossRef]
- Zhong, X.; Qi, J.; Li, H.; Dong, L.; Gao, D. Seasonal Distribution of Microbial Activity in Bioaerosols in the Outdoor Environment of the Qingdao Coastal Region. Atmos. Environ. 2016, 140, 506–513. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Pastuszka, J.S. Influence of Meteorological Factors on the Level and Characteristics of Culturable Bacteria in the Air in Gliwice, Upper Silesia (Poland). Aerobiologia 2018, 34, 241–255. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.B.; Singh, R. Seasonal Variation and Size Distribution in the Airborne Indoor Microbial Concentration of Residential Houses in Delhi and Its Impact on Health. Aerobiologia 2021, 37, 719–732. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, C.; Lu, R.; Liu, P.; Wang, B.; Du, S.; Jin, C.; Deng, S.; Li, Y. Characteristics of Ambient Bioaerosols during Haze Episodes in China: A Review. Environ. Pollut. 2018, 243, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, X.; Liu, Q.; Maki, T.; Alam, K.; Wang, Y.; Xue, F.; Tang, S.; Du, P.; Dong, Q.; et al. Bioaerosols in the Atmosphere: A Comprehensive Review on Detection Methods, Concentration and Influencing Factors. Sci. Total Environ. 2024, 912, 168818. [Google Scholar] [CrossRef]
- Beeby, M.; Gumbart, J.C.; Roux, B.; Jensen, G.J. Architecture and Assembly of the Gram-Positive Cell Wall. Mol. Microbiol. 2013, 88, 664–672. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and Size Distribution of Culturable Bacteria in Ambient Air during Spring and Winter in Gliwice: A Typical Urban Area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-L.; Kalume, A.; Wang, C.; Santarpia, J. Atmospheric Aging Processes of Bioaerosols under Laboratory-Controlled Conditions: A Review. J. Aerosol Sci. 2021, 155, 105767. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet Radiation From a Plant Perspective: The Plant-Microorganism Context. Front. Plant Sci. 2020, 11, 597642. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Tseng, C.-H.; Wang, H.-C.; Dai, C.-F.; Shih, Y.-H. The Study of an Ultraviolet Radiation Technique for Removal of the Indoor Air Volatile Organic Compounds and Bioaerosol. Int. J. Environ. Res. Public. Health 2019, 16, 2557. [Google Scholar] [CrossRef]
- Wan, A.; Chen, X.; Yuen, J. Races of Puccinia Striiformis f. Sp. Tritici in the United States in 2011 and 2012 and Comparison with Races in 2010. Plant Dis. 2016, 100, 966–975. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Liu, D.; Zhang, T.; Zhang, Z.; Zhao, J.; Zhang, B.; Cao, S.; Xu, X.; Yao, Q.; et al. Migration of Wheat Stripe Rust from the Primary Oversummering Region to Neighboring Regions in China. Commun. Biol. 2025, 8, 350. [Google Scholar] [CrossRef]
- Islam, M.T.; Gupta, D.R.; Hossain, A.; Roy, K.K.; He, X.; Kabir, M.R.; Singh, P.K.; Khan, M.A.R.; Rahman, M.; Wang, G.-L. Wheat Blast: A New Threat to Food Security. Phytopathol. Res. 2020, 2, 28. [Google Scholar] [CrossRef]
- Xiong, Y.; McCarthy, C.; Humpal, J.; Percy, C. A Review on Common Root Rot of Wheat and Barley in Australia. Plant Pathol. 2023, 72, 1347–1364. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Islam, S.S.; Adhikary, S.; Sannal, A.; Al Emran Hosen, M.; et al. Plant Disease Dynamics in a Changing Climate: Impacts, Molecular Mechanisms, and Climate-Informed Strategies for Sustainable Management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Ruiz-Gil, T.; Acuña, J.J.; Fujiyoshi, S.; Tanaka, D.; Noda, J.; Maruyama, F.; Jorquera, M.A. Airborne Bacterial Communities of Outdoor Environments and Their Associated Influencing Factors. Environ. Int. 2020, 145, 106156. [Google Scholar] [CrossRef]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.R.; Junier, P. A Combination of Extreme Environmental Conditions Favor the Prevalence of Endospore-Forming Firmicutes. Front. Microbiol. 2016, 7, 1707. [Google Scholar] [CrossRef]
- Vaz, C.M.P.; de Freitas Iossi, M.; de Mendonça Naime, J.; Macedo, Á.; Reichert, J.M.; Reinert, D.J.; Cooper, M. Validation of the Arya and Paris Water Retention Model for Brazilian Soils. Soil. Sci. Soc. Am. J. 2005, 69, 577–583. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Schillinger, W.F.; Bary, A.I.; Sharratt, B.; Paulitz, T.C. Dust-Associated Microbiomes from Dryland Wheat Fields Differ with Tillage Practice and Biosolids Application. Atmos. Environ. 2018, 185, 29–40. [Google Scholar] [CrossRef]
- Pi, H.; Sharratt, B.; Schillinger, W.F.; Bary, A.; Cogger, C. Chemical Composition of Windblown Dust Emitted from Agricultural Soils Amended with Biosolids. Aeolian Res. 2018, 32, 102–115. [Google Scholar] [CrossRef]
- Dombrowski, N.; Schlaeppi, K.; Agler, M.T.; Hacquard, S.; Kemen, E.; Garrido-Oter, R.; Wunder, J.; Coupland, G.; Schulze-Lefert, P. Root Microbiota Dynamics of Perennial Arabis Alpina Are Dependent on Soil Residence Time but Independent of Flowering Time. ISME J. 2017, 11, 43–55. [Google Scholar] [CrossRef]
- Ginnan, N.A.; De Anda, N.I.; Campos Freitas Vieira, F.; Rolshausen, P.E.; Roper, M.C. Microbial Turnover and Dispersal Events Occur in Synchrony with Plant Phenology in the Perennial Evergreen Tree Crop Citrus Sinensis. mBio 2022, 13, e0034322. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The Plant Microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Jabeen, R.; Ahmed, M.E.; Hamouda, M.A.; Aly Hassan, A. Bioaerosols in Wastewater Treatment Plants: Trends, Recent Advances, and the Influence of SARS-CoV-2 Outbreak. Water 2023, 15, 4208. [Google Scholar] [CrossRef]
- Pepper, I.L.; Gerba, C.P. Aeromicrobiology. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; p. 89. [Google Scholar]
- Chiang, C.-F.; Yang, H.-H.; Chi, T.-W. Monitoring of Bioaerosol Emission from a Sludge Composting Facility. Int. J. Appl. Sci. Eng. 2003, 1, 148–159. [Google Scholar]
- Hameed, A.A.A.; Khodr, M.I. Suspended Particulates and Bioaerosols Emittedfrom an Agricultural Non-Point Source. J. Environ. Monit. 2001, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the Global Transport of Desert Dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric Transport of Viable Fungi and Bacteria from Africa to the Caribbean with Soil Dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef]
- Rodó, X.; Pozdniakova, S.; Borràs, S.; Matsuki, A.; Tanimoto, H.; Armengol, M.-P.; Pey, I.; Vila, J.; Muñoz, L.; Santamaria, S.; et al. Microbial Richness and Air Chemistry in Aerosols above the PBL Confirm 2,000-Km Long-Distance Transport of Potential Human Pathogens. Proc. Natl. Acad. Sci. USA 2024, 121, e2404191121. [Google Scholar] [CrossRef]
- Abrego, N.; Norros, V.; Halme, P.; Somervuo, P.; Ali-Kovero, H.; Ovaskainen, O. Give Me a Sample of Air and I Will Tell Which Species Are Found from Your Region: Molecular Identification of Fungi from Airborne Spore Samples. Mol. Ecol. Resour. 2018, 18, 511–524. [Google Scholar] [CrossRef]
- Rieux, A.; Soubeyrand, S.; Bonnot, F.; Klein, E.K.; Ngando, J.E.; Mehl, A.; Ravigne, V.; Carlier, J.; de Lapeyre de Bellaire, L. Long-Distance Wind-Dispersal of Spores in a Fungal Plant Pathogen: Estimation of Anisotropic Dispersal Kernels from an Extensive Field Experiment. PLoS ONE 2014, 9, e103225. [Google Scholar] [CrossRef]
- Bernal, R.F.; Berger, R.D. The Spread of Epiphytic Populations of Xanthomonas Campestris Pv. Vesicatoria on Pepper in the Field. J. Phytopathol. 1996, 144, 479–484. [Google Scholar] [CrossRef]
- Tang, D.; Wei, T.; Yuan, J.; Xia, H.; Dou, X. Observation of Bioaerosol Transport Using Wideband Integrated Bioaerosol Sensor and Coherent Doppler Lidar. Atmos. Meas. Tech. 2022, 15, 2819–2838. [Google Scholar] [CrossRef]
- Métris, K.L.; Métris, J. Aircraft Surveys for Air eDNA: Probing Biodiversity in the Sky. PeerJ 2023, 11, e15171. [Google Scholar] [CrossRef]
- Basapathi Raghavendra, J.; Mathanlal, T.; Zorzano, M.-P.; Martin-Torres, J. An Optimized Active Sampling Procedure for Aerobiological DNA Studies. Sensors 2023, 23, 2836. [Google Scholar] [CrossRef]
- Van Leuken, J.P.G.; Swart, A.N.; Havelaar, A.H.; Van Pul, A.; Van der Hoek, W.; Heederik, D. Atmospheric Dispersion Modelling of Bioaerosols That Are Pathogenic to Humans and Livestock—A Review to Inform Risk Assessment Studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Mbareche, H.; Morawska, L.; Duchaine, C. On the Interpretation of Bioaerosol Exposure Measurements and Impacts on Health. J. Air Waste Manag. Assoc. 2019, 69, 789–804. [Google Scholar] [CrossRef]
- Odeyemi, D.A.; Alao, J.O.; Kayode, T.A.; Durugbo, E.U. Assessment of Bioaerosol Composition and Public Health Implications in High-Traffic Urban Areas of Southwest, Nigeria. Environ. Res. Commun. 2024, 6, 121008. [Google Scholar] [CrossRef]
- Cabo Verde, S.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological Assessment of Indoor Air Quality at Different Hospital Sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Ereth, M.H.; Fine, J.; Stamatatos, F.; Mathew, B.; Hess, D.; Simpser, E. Healthcare-Associated Infection Impact with Bioaerosol Treatment and COVID-19 Mitigation Measures. J. Hosp. Infect. 2021, 116, 69–77. [Google Scholar] [CrossRef]
- Grzyb, J.; Lenart-Boroń, A. Bacterial Bioaerosol Concentration and Size Distribution in the Selected Animal Premises in a Zoological Garden. Aerobiologia 2019, 35, 253–268. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Hao, P.; Ji, S.; Gao, Y. Characteristics and Health Impacts of Bioaerosols in Animal Barns: A Comprehensive Study. Ecotoxicol. Environ. Saf. 2024, 278, 116381. [Google Scholar] [CrossRef]
- Grewling, Ł.; Magyar, D.; Chłopek, K.; Grinn-Gofroń, A.; Gwiazdowska, J.; Siddiquee, A.; Ianovici, N.; Kasprzyk, I.; Wójcik, M.; Lafférsová, J.; et al. Bioaerosols on the Atmospheric Super Highway: An Example of Long Distance Transport of Alternaria Spores from the Pannonian Plain to Poland. Sci. Total Environ. 2022, 819, 153148. [Google Scholar] [CrossRef]
- Kobuszewska, A.; Wysok, B. Pathogenic Bacteria in Free-Living Birds, and Its Public Health Significance. Animals 2024, 14, 968. [Google Scholar] [CrossRef]
- Al-Shaarani, A.A.Q.A.; Pecoraro, L. A Review of Pathogenic Airborne Fungi and Bacteria: Unveiling Occurrence, Sources, and Profound Human Health Implication. Front. Microbiol. 2024, 15, 1428415. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Lindemann, J.; Upper, C.D. Aerial Dispersal of Epiphytic Bacteria over Bean Plants. Appl. Environ. Microbiol. 1985, 50, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Yondon, M.; Bailey, E.S.; Duman, E.K.; Simmons, R.A.; Greer, A.G.; Gray, G.C. Environmental Bioaerosol Surveillance as an Early Warning System for Pathogen Detection in North Carolina Swine Farms: A Pilot Study. Transbound. Emerg. Dis. 2021, 68, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Melogno, L.; Ginn, O.; Bailey, E.S.; Soria, F.; Andrade, M.; Bergin, M.H.; Brown, J.; Gray, G.C.; Deshusses, M.A. Bioaerosol Sampling Optimization for Community Exposure Assessment in Cities with Poor Sanitation: A One Health Cross-Sectional Study. Sci. Total Environ. 2020, 738, 139495. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, C.; Jiang, G.; Ma, J.; Li, Y.; Chen, H.; Guo, J. Bioaerosol Is an Important Transmission Route of Antibiotic Resistance Genes in Pig Farms. Environ. Int. 2021, 154, 106559. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance and Primary Health Care; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- IACG. No Time to Wait: Securing the Future from Drug Resistant Infections; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- O’neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Open J. Epidemiol. 2016, 9, 84. [Google Scholar]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in Understanding Antimicrobial Resistance across Domesticated Animal, Human, and Environmental Systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the Impact of Manure on the Environmental Antibiotic Residues and Resistance Pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef]
- Storteboom, H.; Arabi, M.; Davis, J.G.; Crimi, B.; Pruden, A. Tracking Antibiotic Resistance Genes in the South Platte River Basin Using Molecular Signatures of Urban, Agricultural, And Pristine Sources. Environ. Sci. Technol. 2010, 44, 7397–7404. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Solehati, Q.; Zimmerling, U.; Kleineidam, K.; Schloter, M.; Müller, T.; Focks, A.; Thiele-Bruhn, S.; Smalla, K. Accumulation of Sulfonamide Resistance Genes in Arable Soils Due to Repeated Application of Manure Containing Sulfadiazine. Appl. Environ. Microbiol. 2011, 77, 2527–2530. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef]
- Ullah, H.; Hassan, S.H.A.; Yang, Q.; Salama, E.-S.; Liu, P.; Li, X. Dynamic Interaction of Antibiotic Resistance between Plant Microbiome and Organic Fertilizers: Sources, Dissemination, and Health Risks. World J. Microbiol. Biotechnol. 2024, 41, 4. [Google Scholar] [CrossRef]
- Cellini, A.; Spinelli, F.; Donati, I.; Ryu, C.-M.; Kloepper, J.W. Bacterial Volatile Compound-Based Tools for Crop Management and Quality. Trends Plant Sci. 2021, 26, 968–983. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.-M.; Ghigo, J.-M. Role of Bacterial Volatile Compounds in Bacterial Biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef]
- Audrain, B.; Létoffé, S.; Ghigo, J.-M. Airborne Bacterial Interactions: Functions Out of Thin Air? Front. Microbiol. 2015, 6, 1476. [Google Scholar] [CrossRef]
- Rani, A.; Rana, A.; Dhaka, R.K.; Singh, A.P.; Chahar, M.; Singh, S.; Nain, L.; Singh, K.P.; Minz, D. Bacterial Volatile Organic Compounds as Biopesticides, Growth Promoters and Plant-Defense Elicitors: Current Understanding and Future Scope. Biotechnol. Adv. 2023, 63, 108078. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Ryu, C.-M. Interspecific Bacterial Sensing through Airborne Signals Modulates Locomotion and Drug Resistance. Nat. Commun. 2013, 4, 1809. [Google Scholar] [CrossRef] [PubMed]
- Létoffé, S.; Wu, Y.; Darch, S.E.; Beloin, C.; Whiteley, M.; Touqui, L.; Ghigo, J.-M. Pseudomonas Aeruginosa Production of Hydrogen Cyanide Leads to Airborne Control of Staphylococcus Aureus Growth in Biofilm and In Vivo Lung Environments. mBio 2022, 13, e02154-22. [Google Scholar] [CrossRef]
- Tao, J.; Yu, S.; Jin, J.; Lu, P.; Yang, Z.; Xu, Y.; Chen, Q.; Li, Z.; Cao, P. The Wilt Pathogen Induces Different Variations of Root-Associated Microbiomes of Plant. Front. Plant Sci. 2022, 13, 1023837. [Google Scholar] [CrossRef] [PubMed]
- Enagbonma, B.J.; Fadiji, A.E.; Ayangbenro, A.S.; Babalola, O.O. Communication between Plants and Rhizosphere Microbiome: Exploring the Root Microbiome for Sustainable Agriculture. Microorganisms 2023, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Zhang, H.; Ryu, C.-M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The Emerging Molecular Analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Shulaev, V.; Silverman, P.; Raskin, I. Airborne Signalling by Methyl Salicylate in Plant Pathogen Resistance. Nature 1997, 385, 718–721. [Google Scholar] [CrossRef]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus Subtilis Impact on Plant Growth, Soil Health and Environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef]
- Cordovez, V.; Schop, S.; Hordijk, K.; Dupré de Boulois, H.; Coppens, F.; Hanssen, I.; Raaijmakers, J.M.; Carrión, V.J. Priming of Plant Growth Promotion by Volatiles of Root-Associated Microbacterium Spp. Appl. Environ. Microbiol. 2018, 84, e01865-18. [Google Scholar] [CrossRef]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A.; Tortella, G.; Diez, M.C.; Rubilar, O. Current Advances in Plant-Microbe Communication via Volatile Organic Compounds as an Innovative Strategy to Improve Plant Growth. Microbiol. Res. 2021, 247, 126726. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Revisiting Bacterial Volatile-Mediated Plant Growth Promotion: Lessons from the Past and Objectives for the Future. Ann. Bot. 2018, 122, 349–358. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Are Bacterial Volatile Compounds Poisonous Odors to a Fungal Pathogen Botrytis Cinerea, Alarm Signals to Arabidopsis Seedlings for Eliciting Induced Resistance, or Both? Front. Microbiol. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Kikuta, Y.; Matsuda, K. Plant Communication: Mediated by Individual or Blended VOCs? Plant Signal Behav. 2012, 7, 222–226. [Google Scholar] [CrossRef]
- Villamar-Torres, R.; Mehdi, S.; Liuba-Delfini, G.; García, L.; Viot, C.-R. Volatile organic compounds: Plant natural defense mechanisms against herbivorous arthropods and an opportunity for plant breeding of cotton. Sci. Agropecu. 2018, 9, 287–297. [Google Scholar] [CrossRef]
- Hedsén, M. Plant-Plant Communication—Possible Mechanisms and Benefits. 2019. Available online: https://stud.epsilon.slu.se/15017/1/hedsen_m_190909.pdf (accessed on 25 February 2025).
- Šimpraga, M.; Takabayashi, J.; Holopainen, J.K. Language of Plants: Where Is the Word? J. Integr. Plant Biol. 2016, 58, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile Terpenes—Mediators of Plant-to-Plant Communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef]
- Ramovs, V. Mechanisms Behind Interplant Communication-‘How Do Plants Talk?’. 2013. Available online: https://studenttheses.uu.nl/bitstream/handle/20.500.12932/14429/Mechanisms%20behind%20interplant%20communication_FINAL.pdf?sequence=1 (accessed on 25 February 2025).
- Meents, A.K.; Mithöfer, A. Plant–Plant Communication: Is There a Role for Volatile Damage-Associated Molecular Patterns? Front. Plant Sci. 2020, 11, 583275. [Google Scholar] [CrossRef]
- Erb, M. Volatiles as Inducers and Suppressors of Plant Defense and Immunity-Origins, Specificity, Perception and Signaling. Curr. Opin. Plant Biol. 2018, 44, 117–121. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-Mediated Plant–Plant Interactions: Volatile Organic Compounds as Modulators of Receiver Plant Defence, Growth, and Reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Heil, M. Plant Communication. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wang, L.; Wu, J. The Essential Role of Jasmonic Acid in Plant-Herbivore Interactions—Using the Wild Tobacco Nicotiana Attenuata as a Model. J. Genet. Genom. 2013, 40, 597–606. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M.; Posthumus, M.A. Induction of Indirect Defence Aganist Spider-Mites in Uninfested Lima Bean Leaves. Phytochemistry 1991, 30, 1459–1462. [Google Scholar] [CrossRef]
- He, J.; Halitschke, R.; Schuman, M.C.; Baldwin, I.T. Light Dominates the Diurnal Emissions of Herbivore-Induced Volatiles in Wild Tobacco. BMC Plant Biol. 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole Is an Essential Herbivore-Induced Volatile Priming Signal in Maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Ruther, J.; Kleier, S. Plant-Plant Signaling: Ethylene Synergizes Volatile Emission in Zea Mays Induced by Exposure to (Z)-3-Hexen-1-Ol. J. Chem. Ecol. 2005, 31, 2217–2222. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Hagiwara, T.; Ishihara, M.I.; Takabayashi, J.; Hiura, T.; Shiojiri, K. Effective Distance of Volatile Cues for Plant-Plant Communication in Beech. Ecol. Evol. 2021, 11, 12445–12452. [Google Scholar] [CrossRef]
- Mullu, D. A Review on the Effect of Habitat Fragmentation on Ecosystem. J. Nat. Sci. Res. 2016, 6, 1–15. [Google Scholar]
- Decocq, G.; Andrieu, E.; Brunet, J.; Chabrerie, O.; De Frenne, P.; De Smedt, P.; Deconchat, M.; Diekmann, M.; Ehrmann, S.; Giffard, B.; et al. Ecosystem Services from Small Forest Patches in Agricultural Landscapes. Curr. For. Rep. 2016, 2, 30–44. [Google Scholar] [CrossRef]
- Leimu, R.; Vergeer, P.; Angeloni, F.; Ouborg, N.J. Habitat Fragmentation, Climate Change, and Inbreeding in Plants. Ann. N. Y Acad. Sci. 2010, 1195, 84–98. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Bianchelli, S.; Candela, M.; Dell’Anno, A.; Gambi, C.; Rastelli, E.; Varrella, S.; Danovaro, R. Microbiome-Assisted Restoration of Degraded Marine Habitats: A New Nature-Based Solution? Front. Mar. Sci. 2023, 10, 1227560. [Google Scholar] [CrossRef]
- Warren, S.D.; St. Clair, L.L.; Leavitt, S.D. Aerobiology and Passive Restoration of Biological Soil Crusts. Aerobiologia 2019, 35, 45–56. [Google Scholar] [CrossRef]
- Lindenmayer, D. Small Patches Make Critical Contributions to Biodiversity Conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Eycott, A.; Watts, K. Biodiversity in Fragmented Landscapes. 2011. Available online: https://cdn.forestresearch.gov.uk/2011/11/fcrn010.pdf (accessed on 25 February 2025).
- Takkis, K.; Kull, T.; Hallikma, T.; Jaksi, P.; Kaljund, K.; Kauer, K.; Kull, T.; Kurina, O.; Külvik, M.; Lanno, K.; et al. Drivers of Species Richness and Community Integrity of Small Forest Patches in an Agricultural Landscape. J. Veg. Sci. 2018, 29, 978–988. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global Synthesis of Conservation Studies Reveals the Importance of Small Habitat Patches for Biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Tiang, D.C.F.; Morris, A.; Bell, M.; Gibbins, C.N.; Azhar, B.; Lechner, A.M. Ecological Connectivity in Fragmented Agricultural Landscapes and the Importance of Scattered Trees and Small Patches. Ecol. Process. 2021, 10, 20. [Google Scholar] [CrossRef]
- Duff, H.; Debinski, D.; Maxwell, B.D. Landscape Context Affects Patch Habitat Contributions to Biodiversity in Agroecosystems. Ecosphere 2024, 15, e4879. [Google Scholar] [CrossRef]
- Benton, T.G.; Bieg, C.; Harwatt, H.; Pudasaini, R.; Wellesley, L. Food System Impacts on Biodiversity Loss: Three Levers for Food System Transformation in Support of Nature. 2021. Available online: https://www.ciwf.com/media/7443948/food-system-impacts-on-biodiversity-loss-feb-2021.pdf (accessed on 25 February 2025).
- Omae, N.; Tsuda, K. Plant-Microbiota Interactions in Abiotic Stress Environments. Mol. Plant Microbe Interact. 2022, 35, 511–526. [Google Scholar] [CrossRef]
- Weber, R.W. Meteorologic Variables in Aerobiology. Immunol. Allergy Clin. N. Am. 2003, 23, 411–422. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V.S. Interference of Climate Change on Plant-Microbe Interaction: Present and Future Prospects. Front. Agron. 2022, 3, 153–179. [Google Scholar] [CrossRef]
- Tastassa, A.C.; Sharaby, Y.; Lang-Yona, N. Aeromicrobiology: A Global Review of the Cycling and Relationships of Bioaerosols with the Atmosphere. Sci. Total Environ. 2024, 912, 168478. [Google Scholar] [CrossRef]
- van Leuken, J.P.G.; Swart, A.N.; Droogers, P.; van Pul, A.; Heederik, D.; Havelaar, A.H. Climate Change Effects on Airborne Pathogenic Bioaerosol Concentrations: A Scenario Analysis. Aerobiologia 2016, 32, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Beggs, P.J.; Clot, B.; Sofiev, M.; Johnston, F.H. Climate Change, Airborne Allergens, and Three Translational Mitigation Approaches. EBioMedicine 2023, 93, 104478. [Google Scholar] [CrossRef] [PubMed]
- Rajput, P.; Chauhan, A.S.; Gupta, T. Bioaerosols Over the Indo-Gangetic Plain: Influence of Biomass Burning Emission and Ambient Meteorology. In Environmental Contaminants. Energy, Environment, and Sustainability; Gupta, T., Agarwal, A., Agarwal, R., Labhsetwar, N., Eds.; Springer: Singapore, 2017; pp. 93–121. [Google Scholar]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar]
- Frączek, K.; Ropek, D. Municipal Waste Dumps as the Microbiological Threat to the Natural Environment. Ecol. Chem. Eng. S 2011, 18, 93–110. [Google Scholar]
- Chattopadhyay, P.; Banerjee, G.; Mukherjee, S. Recent Trends of Modern Bacterial Insecticides for Pest Control Practice in Integrated Crop Management System. 3 Biotech 2017, 7, 60. [Google Scholar] [CrossRef]
- de Maagd, R.A. Bacillus Thuringiensis-Based Products for Insect Pest Control. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 185–192. [Google Scholar]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus Thuringiensis: A Story of a Successful Bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Kumar, P.; Kamle, M.; Borah, R.; Mahato, D.K.; Sharma, B. Bacillus Thuringiensis as Microbial Biopesticide: Uses and Application for Sustainable Agriculture. Egypt. J. Biol. Pest. Control 2021, 31, 95. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Lorenzo-Santiago, M.A.; Gumiere, T.; Aid, L.; Rodriguez-Campos, J.; Contreras-Ramos, S.M. Glyphosate Biodegradation by Airborne Plant Growth-Promoting Bacteria: Influence on Soil Microbiome Dynamics. Agriculture 2025, 15, 362. [Google Scholar] [CrossRef]
- Lymperopoulou, D.S.; Adams, R.I.; Lindow, S.E. Contribution of Vegetation to the Microbial Composition of Nearby Outdoor Air. Appl. Environ. Microbiol. 2016, 82, 3822–3833. [Google Scholar] [CrossRef]
- Li, B.-B.; Roley, S.S.; Duncan, D.S.; Guo, J.; Quensen, J.F.; Yu, H.-Q.; Tiedje, J.M. Long-Term Excess Nitrogen Fertilizer Increases Sensitivity of Soil Microbial Community to Seasonal Change Revealed by Ecological Network and Metagenome Analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Greenwald, R.; Bergin, M.H.; Xu, J.; Cohan, D.; Hoogenboom, G.; Chameides, W.L. The Influence of Aerosols on Crop Production: A Study Using the CERES Crop Model. Agric. Syst. 2006, 89, 390–413. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Ghosh, P.; Kumar, A. Endophytic and Epiphytic Modes of Microbial Interactions and Benefits. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 1: Fundamental Mechanisms, Methods and Functions; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 227–253. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cavagnaro, T.R.; Watts-Williams, S.J. The Effects of Soil Phosphorus and Zinc Availability on Plant Responses to Mycorrhizal Fungi: A Physiological and Molecular Assessment. Sci. Rep. 2019, 9, 14880. [Google Scholar] [CrossRef] [PubMed]
- Rajapitamahuni, S.; Kang, B.R.; Lee, T.K. Exploring the Roles of Arbuscular Mycorrhizal Fungi in Plant–Iron Homeostasis. Agriculture 2023, 13, 1918. [Google Scholar] [CrossRef]
- Hanson, M.C.; Petch, G.M.; Ottosen, T.-B.; Skjøth, C.A. Climate Change Impact on Fungi in the Atmospheric Microbiome. Sci. Total Environ. 2022, 830, 154491. [Google Scholar] [CrossRef] [PubMed]
- Arnault, G.; Mony, C.; Vandenkoornhuyse, P. Plant Microbiota Dysbiosis and the Anna Karenina Principle. Trends Plant Sci. 2023, 28, 18–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhang, Z.; Liu, Y.; Wei, Y. Tomato Endophytic Bacteria Composition and Mechanism of Suppressiveness of Wilt Disease (Fusarium Oxysporum). Front. Microbiol. 2021, 12, 731764. [Google Scholar] [CrossRef]
- Fry, W. Phytophthora Infestans: The Plant (and R Gene) Destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Leonard, K.J.; Szabo, L.J. Stem Rust of Small Grains and Grasses Caused by Puccinia Graminis. Mol. Plant Pathol. 2005, 6, 99–111. [Google Scholar] [CrossRef]
- Djurle, A.; Young, B.; Berlin, A.; Vågsholm, I.; Blomström, A.-L.; Nygren, J.; Kvarnheden, A. Addressing Biohazards to Food Security in Primary Production. Food Sec. 2022, 14, 1475–1497. [Google Scholar] [CrossRef]
- Marcelloni, A.M.; Pigini, D.; Chiominto, A.; Gioffrè, A.; Paba, E. Exposure to Airborne Mycotoxins: The Riskiest Working Environments and Tasks. Ann. Work. Expo. Health 2024, 68, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.A.; Ford, R. Biosensor Technologies for Early Detection and Quantification of Plant Pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul Rahman, N.S.N. Plant–Microbe Interaction: Aboveground to Belowground, from the Good to the Bad. Int. J. Mol. Sci. 2021, 22, 10388. [Google Scholar] [CrossRef]
- Li, Y. Powdery Mildrew of Cucurbitus. 2012. Available online: https://portal.ct.gov/-/media/CAES/DOCUMENTS/Publications/Fact_Sheets/Plant_Pathology_and_Ecology/POWDERYMILDEWOFCUCURBITS103112pdf.pdf (accessed on 25 February 2025).
- Preston, G.M. Pseudomonas Syringae Pv. Tomato: The Right Pathogen, of the Right Plant, at the Right Time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yuldashova, Z.Z.; Sodikov, B.S.; Khamiraev, U.K. Alternaria disease of potato and its control-review. EPRA Int. J. Res. Dev. (IJRD) 2023, 8, 168–172. [Google Scholar] [CrossRef]
- Serfling, A.; Kopahnke, D.; Habekuss, A.; Novakazi, F.; Ordon, F. Wheat Diseases: An Overview. In Achieving Sustainable Cultivation of Wheat; Burleigh Dodds Science Publishing: Cambridge, UK, 2016. [Google Scholar]
- Mottaleb, K.A.; Loladze, A.; Sonder, K.; Kruseman, G.; San Vicente Garcia, F.M. Threats of Tar Spot Complex Disease of Maize in the United States of America and Its Global Consequences. Mitig. Adapt. Strateg. Glob. Change 2019, 24, 281–300. [Google Scholar] [CrossRef]
- Mahuku, G.; Shrestha, R.; San Vicente, F.M. Tar Spot Complex of Maize: Facts and Actions. 2013. Available online: https://www.researchgate.net/publication/266732736_Tar_Spot_Complex_of_Maize_Facts_and_Actions (accessed on 25 February 2025).
- Hopkins, D.L.; Purcell, A.H. Xylella Fastidiosa: Cause of Pierce’s Disease of Grapevine and Other Emergent Diseases. Plant Dis. 2002, 86, 1056–1066. [Google Scholar] [CrossRef]
- Berisha, B.; Chen, Y.D.; Zhang, G.Y.; Xu, B.Y.; Chen, T.A. Isolation of Peirce’s Disease Bacteria from Grapevines in Europe. Eur. J. Plant Pathol. 1998, 104, 427–433. [Google Scholar] [CrossRef]
- Aguilar Alvarez, E.; Moreira, L.; Rivera, C. Confirmation of Xylella Fastidiosa Infecting Grapes Vitis Vinifera in Costa Rica. Trop. Plant Pathol. 2008, 33, 444–448. [Google Scholar]
- Correa-Garcia, S.; Constant, P.; Yergeau, E. The Forecasting Power of the Microbiome. Trends Microbiol. 2023, 31, 444–452. [Google Scholar] [CrossRef]
- Schrijver, R.; Poppe, K.; Daheim, C. Precision Agriculture and the Future of Farming in Europe. 2016. Available online: https://op.europa.eu/en/publication-detail/-/publication/40fe549e-cb49-11e7-a5d5-01aa75ed71a1/language-en (accessed on 25 February 2025).
- Maggio, E. Air Monitoring Systems and Methods. WO2022101510, 19 May 2022.
- Hoon, B.J.; Ho, H.J.; Rae, K.H.; Gwon, A.S. Apparatus for Capturing. Bioaerosols. Patent Number US11833527B2, 5 December 2023. [Google Scholar]
- Zhengyu, J.; Li, M. Bioaerosol Monitoring Equipment. CN114563382A, 31 May 2022.
- Mainelis, G. Bioaerosol Sampling: Classical Approaches, Advances, and Perspectives. Aerosol Sci. Technol. 2020, 54, 496–519. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.E.W.; Zur Nieden, A.; Jankofsky, M.; Stilianakis, N.I.; Boedeker, R.-H.; Eikmann, T.F. Effects of Bioaerosol Polluted Outdoor Air on Airways of Residents: A Cross Sectional Study. Occup. Environ. Med. 2003, 60, 336–342. [Google Scholar] [CrossRef]
- Sjoholm, P.; Ingham, D.B.; Lehtimaki, M.; Perttu-Roiha, L.; Goodfellow, H.; Torvela, H. Gas-Cleaning Technology. In Industrial Ventilation Design Guidebook; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1197–1316. [Google Scholar]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol Sampling: Sampling Mechanisms, Bioefficiency and Field Studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef]
- Manibusan, S.; Mainelis, G. Passive Bioaerosol Samplers: A Complementary Tool for Bioaerosol Research. A Review. J. Aerosol Sci. 2022, 163, 105992. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.J.; Gouin, T.; Wania, F. Comparison of Four Active and Passive Sampling Techniques for Pesticides in Air. Environ. Sci. Technol. 2010, 44, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Balyan, P.; Ghosh, C.; Das, S.; Banerjee, B.D. Comparison of Efficiency of Active and Passive Methods of Bioaerosols’ Estimation. In Indoor Environmental Quality; Sharma, A., Goyal, R., Mittal, R., Eds.; Springer: Singapore, 2020; pp. 85–94. [Google Scholar]
- Rastmanesh, A.; Boruah, J.S.; Lee, M.-S.; Park, S. On-Site Bioaerosol Sampling and Airborne Microorganism Detection Technologies. Biosensors 2024, 14, 122. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Green, B.J.; Blachere, F.M.; Martin, S.B.; Law, B.F.; Jensen, P.A.; Schafer, M.P. Sampling and Characterization of Bioaerosols, 5th ed.; National Institute for Occupational Safety and Health: Washington, DC, USA, 2017. [Google Scholar]
- Gilbert, Y.; Duchaine, C. Bioaerosols in Industrial Environments: A reviewThis Article Is One of a Selection of Papers Published in This Special Issue on Biological Air Treatment. Can. J. Civ. Eng. 2009, 36, 1873–1886. [Google Scholar] [CrossRef]
- Ghosh, B.; Lal, H.; Srivastava, A. Review of Bioaerosols in Indoor Environment with Special Reference to Sampling, Analysis and Control Mechanisms. Environ. Int. 2015, 85, 254–272. [Google Scholar] [CrossRef]
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for Bioaerosol Characterization: Limits and Perspectives for Human Health Risk Assessment in Organic Waste Treatment. Atmosphere 2020, 11, 452. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Sudharsanam, S.; Swaminathan, S.; Ramalingam, A.; Thangavel, G.; Annamalai, R.; Steinberg, R.; Balakrishnan, K.; Srikanth, P. Characterization of Indoor Bioaerosols from a Hospital Ward in a Tropical Setting. Afr. Health Sci. 2012, 12, 217–225. [Google Scholar] [CrossRef]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field Sampling of Indoor Bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Mescioglu, E.; Paytan, A.; Mitchell, B.W.; Griffin, D.W. Efficiency of Bioaerosol Samplers: A Comparison Study. Aerobiologia 2021, 37, 447–459. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, H.R.; Hwang, J. Continuous and Real-Time Bioaerosol Monitoring by Combined Aerosol-to-Hydrosol Sampling and ATP Bioluminescence Assay. Anal. Chim. Acta 2016, 941, 101–107. [Google Scholar] [CrossRef]
- Zucker, B.-A.; Draz, A.M.; Müller, W. Comparison of Filtration and Impingement for Sampling Airborne Endotoxin. J. Aerosol Sci. 2000, 31, 751–755. [Google Scholar] [CrossRef]
- Wang, Z.; Reponen, T.; Grinshpun, S.A.; Górny, R.L.; Willeke, K. Effect of Sampling Time and Air Humidity on the Bioefficiency of Filter Samplers for Bioaerosol Collection. J. Aerosol Sci. 2001, 32, 661–674. [Google Scholar] [CrossRef]
- Chen, P.; Du, Y.; Xu, Y.Z.; Liu, Z.; Yan, K. Review: Bioaerosol Collection. Int. J. Plasma Environ. Sci. Technol. 2017, 11, 52–55. [Google Scholar]
- Han, B.; Hudda, N.; Ning, Z.; Kim, Y.-J.; Sioutas, C. Efficient Collection of Atmospheric Aerosols with a Particle Concentrator—Electrostatic Precipitator Sampler. Aerosol Sci. Technol. 2009, 43, 757–766. [Google Scholar] [CrossRef]
- Bieber, P.; Seifried, T.M.; Burkart, J.; Gratzl, J.; Kasper-Giebl, A.; Schmale, D.G.; Grothe, H. A Drone-Based Bioaerosol Sampling System to Monitor Ice Nucleation Particles in the Lower Atmosphere. Remote Sens. 2020, 12, 552. [Google Scholar] [CrossRef]
- Environment Agency. Environmental Monitoring of Bioaerosols at Regulated Facilities; Environment Agency: London, UK, 2018. [Google Scholar]
- Kesavan, J.; Schepers, D.; McFarland, A.R. Sampling and Retention Efficiencies of Batch-Type Liquid-Based Bioaerosol Samplers. Aerosol Sci. Technol. 2010, 44, 817–829. [Google Scholar] [CrossRef]
- Rissler, J.; Asking, L.; Dreyer, J.K. A Methodology to Study Impactor Particle Reentrainment and a Proposed Stage Coating for the NGI. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.R.; Haglund, J.S.; King, M.D.; Hu, S.; Phull, M.S.; Moncla, B.W.; Seo, Y. Wetted Wall Cyclones for Bioaerosol Sampling. Aerosol Sci. Technol. 2010, 44, 241–252. [Google Scholar] [CrossRef]
- Pirhadi, M.; Mousavi, A.; Sioutas, C. Evaluation of a High Flow Rate Electrostatic Precipitator (ESP) as a Particulate Matter (PM) Collector for Toxicity Studies. Sci. Total Environ. 2020, 739, 140060. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, A.; Noakes, C.J. A Comparison of the Sampling Efficiency of Bioaerosol Samples and Particle Counters in Natural and Controlled Environments; White Rose Research Online, 2014. In Proceedings of the 13th International Conference on Indoor Air Quality and Climate, Hong Kong, China, 7–12 July 2014. [Google Scholar]
- SKC. PEM Monitor, 2.5 µm, 2 L/minPEM Monitor, 2.5 µm, 2 L/min. Available online: https://www.skcinc.com/products/pem-monitor-25-m-2-l-min (accessed on 25 February 2025).
- Xu, Y.; Zheng, C.; Liu, Z.; Yan, K. Electrostatic Precipitation of Airborne Bio-Aerosols. J. Electrost. 2013, 71, 204–207. [Google Scholar] [CrossRef]
- Burton, N.C.; Grinshpun, S.A.; Reponen, T. Physical Collection Efficiency of Filter Materials for Bacteria and Viruses. Ann. Occup. Hyg. 2007, 51, 143–151. [Google Scholar]
- Grinshpun, S.A.; Willeke, K.; Ulevicius, V.; Juozaitis, A.; Terzieva, S.; Donnelly, J.; Stelma, G.N.; Brenner, K.P. Effect of Impaction, Bounce and Reaerosolization on the Collection Efficiency of Impingers. Aerosol Sci. Technol. 1997, 26, 326–342. [Google Scholar] [CrossRef]
- Tsai, C.; Lin, T. Particle Collection Efficiency of Different Impactor Designs. Sep. Sci. Technol. 2000, 35, 2639–2650. [Google Scholar] [CrossRef]
- Sung, G.; Kim, H.-U.; Shin, D.; Shin, W.G.; Kim, T. High Efficiency Axial Wet Cyclone Air Sampler. Aerosol Air Qual. Res. 2018, 18, 2529–2537. [Google Scholar] [CrossRef]
- SKC. Bioaerosol Sampler Selection Guide. Available online: https://www.skcltd.com/knowledge-library/product-selection-guides/bioaerosol-sampler-selection-guide.html (accessed on 25 February 2025).
- SKC. BioSampler, 20 mL, 4 Pack. Available online: https://www.skcinc.com/products/biosampler-20-ml-4-pack (accessed on 25 February 2025).
- TCR Tecora. Andersen Cascade Impactor 6 Stage Viable. Available online: https://tcr-tecora.com/en/laboratory-instruments/andersen-cascade-impactor-6-stage-viable/ (accessed on 25 February 2025).
- TISCH. TE-BC251 NIOSH Bioaerosol Cyclone. Available online: https://tisch-env.com/product/te-bc251/ (accessed on 25 February 2025).
- KLEANLAND. Manufacturer of Commercial & Industrial Oil Smoke Filter. Available online: https://www.klean-esp.com/ (accessed on 25 February 2025).
- Yang, S.; Yin, Y.; Zhang, W.; Li, H.; Wang, X.; Chen, R. Advances in Understanding Bioaerosol Release Characteristics and Potential Hazards during Aerobic Composting. Sci. Total Environ. 2024, 926, 171796. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Gautam, S.; Santosh, M.; Sudan, H.A.; Gandhi, R.; Sam Jebadurai, V.; Shu, C.-M. Bioaerosols: Characterization, Pathways, Sampling Strategies, and Challenges to Geo-Environment and Health. Gondwana Res. 2021, 99, 178–203. [Google Scholar] [CrossRef]
- Wang, L.; Qi, W.; Liu, Y.; Essien, D.; Zhang, Q.; Lin, J. Recent Advances on Bioaerosol Collection and Detection in Microfluidic Chips. Anal. Chem. 2021, 93, 9013–9022. [Google Scholar] [CrossRef]
- Lee, B.G.; Jeong, K.H.; Kim, H.E.; Yeo, M.-K. Machine Learning Models for Predicting Indoor Airborne Fungal Concentrations in Public Facilities Utilizing Environmental Variables. Environ. Pollut. 2025, 368, 125684. [Google Scholar] [CrossRef]
- Tian, J.; Yan, C.; Nasir, Z.A.; Alcega, S.G.; Tyrrel, S.; Coulon, F. Real Time Detection and Characterisation of Bioaerosol Emissions from Wastewater Treatment Plants. Sci. Total Environ. 2020, 721, 137629. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef]
- Madhwal, S.; Prabhu, V.; Sundriyal, S.; Shridhar, V. Ambient Bioaerosol Distribution and Associated Health Risks at a High Traffic Density Junction at Dehradun City, India. Environ. Monit. Assess. 2020, 192, 196. [Google Scholar] [CrossRef] [PubMed]
- Miffre, A.; Cholleton, D.; Genoud, A.P.; Spanu, A.; Rairoux, P. Polarization Optics to Differentiate Among Bioaerosols for Lidar Applications. Photonics 2024, 11, 1067. [Google Scholar] [CrossRef]
- Billington, C.; Kingsbury, J.M.; Rivas, L. Metagenomics Approaches for Improving Food Safety: A Review. J. Food Prot. 2022, 85, 448–464. [Google Scholar] [CrossRef]
| Method | Sampling Instrument | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Passive | Agar settle plates |
|
| [189] |
| Petri dish |
|
| [30,191] | |
| Durham-type passive spore trap |
|
| [191,192] | |
| Filter |
|
| [193,194] | |
| Electrostatic precipitators |
|
| [185,192] | |
| Active | Impingers |
|
| [195] |
| Impactors |
|
| [196,197] | |
| Cyclones |
|
| [20,196] |
| Sampling Method | Filter Samplers | Impingers | Impaction Samplers | Cyclones | Electrostatic Precipitators | References |
|---|---|---|---|---|---|---|
| Multi-environmental | ✓ | ✓ | ✓ | ✓ | ✓ | [195,198] |
| Ease of use | ✓ | ✓ | ✓ | ✓ | ✘ | [192,195,199] |
| Cost effectiveness | ✓ | ✘ | ✓ | ✘ | ✓ | [192,200] |
| Maintenance | ✓ | ✘ | ✘ | ✘ | ✓ | [192,195] |
| Real-time monitoring | ✘ | ✓ | ✘ | ✘ | ✓ | [192,201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashi, N.; Szőke, Z.; Fauszt, P.; Dávid, P.; Mikolás, M.; Gál, F.; Stündl, L.; Remenyik, J.; Paholcsek, M. Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance. Agronomy 2025, 15, 1003. https://doi.org/10.3390/agronomy15051003
Gashi N, Szőke Z, Fauszt P, Dávid P, Mikolás M, Gál F, Stündl L, Remenyik J, Paholcsek M. Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance. Agronomy. 2025; 15(5):1003. https://doi.org/10.3390/agronomy15051003
Chicago/Turabian StyleGashi, Njomza, Zsombor Szőke, Péter Fauszt, Péter Dávid, Maja Mikolás, Ferenc Gál, László Stündl, Judit Remenyik, and Melinda Paholcsek. 2025. "Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance" Agronomy 15, no. 5: 1003. https://doi.org/10.3390/agronomy15051003
APA StyleGashi, N., Szőke, Z., Fauszt, P., Dávid, P., Mikolás, M., Gál, F., Stündl, L., Remenyik, J., & Paholcsek, M. (2025). Bioaerosols in Agriculture: A Comprehensive Approach for Sustainable Crop Health and Environmental Balance. Agronomy, 15(5), 1003. https://doi.org/10.3390/agronomy15051003








