The Influence of Management on the Content of Macro- and Microelements in Plant Shoots of a Meadow Sward of an Arrhenatheretalia Plant Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filed Study
2.2. Laboratory Study
2.3. Statistical Analysis
3. Results
3.1. The Relationship Between Soil Macro- and Micro-Nutrient Concentrations, Topographic Factors and Physical Properties of Soil
3.2. Concentrations of Macro- and Micro-Nutrient in Above-Ground Plant Shoots and Meadow Sward
3.3. The Influence of Different Management Methods on the Concentration of Macro- and Micro-Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pullin, A.S.; Báldi, A.; Emre Can, O.; Dieterich, M.; Kati, V.; Livoreil, B.; Lövei, G.; Mihók, B.; Nevin, O.; Selva, N.; et al. Conservation focus on Europe: Major conservation policy issues that need to be informed by conservation science. Conserv. Biol. 2009, 23, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Feurdean, A.; Ruprecht, E.; Molnár, Z.; Hutchinson, S.M.; Hickler, T. Biodiversity-rich European grasslands: Ancient, forgotten ecosystems. Biol. Conserv. 2018, 228, 224–232. [Google Scholar] [CrossRef]
- Fumy, F.; Schwarz, C.; Fartmann, T. Intensity of grassland management and landscape heterogeneity determine species richness of insects in fragmented hay meadows. Glob. Ecol. Conserv. 2023, 47, e02672. [Google Scholar] [CrossRef]
- Török, P.; Janišová, M.; Kuzemko, A.; Rūsiņa, S.; Dajić Stevanović, Z. Grasslands, Their Threats and Management in Eastern Europe. In Grasslands of the World: Diversity, Management and Conservation; Squires, V.R., Dengler, J., Hua, L., Feng, H., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 64–88. [Google Scholar]
- Marini, L.; Scotton, M.; Klimek, S.; Isselstein, J.; Pecile, A. Effects of local factors on plant species richness and composition of Alpine meadows. Agric. Ecosyst. Environ. 2007, 119, 281–288. [Google Scholar] [CrossRef]
- Merunková, K.; Chytrý, M. Environmental control of species richness and composition in upland grasslands of the southern Czech Republic. Plant Ecol. 2012, 213, 591–602. [Google Scholar] [CrossRef]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Klaus, V.H.; Hölzel, N.; Fischer, M. Direct and productivity-mediated indirect effects of fertilization, mowing and grazing on grassland species richness. J. Ecol. 2012, 100, 1391–1399. [Google Scholar] [CrossRef]
- Pruchniewicz, D. Abandonment of traditionally managed mesic mountain meadows affects plant species composition and diversity. Basic Appl. Ecol. 2017, 20, 10–18. [Google Scholar] [CrossRef]
- Steffens, M.; Köelbl, A.; Totsche, K.U.; Köegel-Knabner, I. Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (PR China). Geoderma 2008, 143, 63–72. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Song, X.; Chang, Q.; Frank, D.A.; Wang, D.; Li, J.; Lin, H.; Du, F. Towards a mechanistic understanding of the effect that different species of large grazers have on grassland soil N availability. J. Ecol. 2018, 106, 357–366. [Google Scholar] [CrossRef]
- Randall, K.; Brennan, F.; Clipson, N.; Creamer, R.; Griffiths, B.; Storey, S.; Doyle, E. Soil bacterial community structure and functional responses across a long-term mineral phosphorus (Pi) fertilisation gradient differ in grazed and cut grasslands. Appl. Soil Ecol. 2019, 138, 134–143. [Google Scholar] [CrossRef]
- Hou, D.; Guo, K.; Liu, C. Asymmetric effects of grazing intensity on macroelements and microelements in grassland soil and plants in Inner Mongolia Grazing alters nutrient dynamics of grasslands. Ecol. Evol. 2020, 10, 8916–8926. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, Y.; Wang, L.; Zhang, X.; Wang, J.; Wu, H.; Yan, Z.; Zhang, K.; Kang, X. Grazing significantly increases root shoot ratio but decreases soil organic carbon in Qinghai-Tibetan Plateau grasslands: A hierarchical meta-analysis. Land Degrad. Dev. 2020, 31, 2369–2378. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zu, J.; Wei, W.; Xue, P.; Yan, H. Response of soil nutrients and stoichiometry to grazing management in alpine grassland on the Qinghai-Tibet Plateau. Soil Tillage Res. 2021, 206, 104822. [Google Scholar] [CrossRef]
- Köhler, B.; Ryser, P.; Güsewell, S.; Gigon, A. Nutrient availability and limitation in traditionally mown and in abandoned limestone grasslands: A bioassay experiment. Plant Soil 2001, 230, 323–332. [Google Scholar] [CrossRef]
- Whitehead, D.C. Nutrient Elements in Grassland: Soil-Plant-Animal Relationships; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Zistl-Schlingmann, M.; Kwatcho Kengdo, S.; Kiese, R.; Dannenmann, M. Management intensity controls nitrogen-use-efficiency and flows in grasslands-a 15N tracing experiment. Agronomy 2020, 10, 606. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Żołnierz, L. The influence of environmental factors and management methods on the vegetation of mesic grasslands in a central European mountain range. Flora 2014, 209, 687–692. [Google Scholar] [CrossRef]
- Allen, S.E. (Ed.) Chemical Analysis of Ecological Materials; Second Edition Completely Revised; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Radojević, M.; Bashkin, V. Plant analysis. In Practical Environmental Analysis; Radojevic, M., Bashkin, V., Eds.; Royal Society of Chemistry Publishing: Cambridge, UK, 2006; p. 457. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. Available online: https://www.statistica.com/en/software/tibco-data-science-/-tibco-statistica (accessed on 2 October 2024).
- Grzegorczyk, S.; Olszewska, M.; Alberski, J. Accumulation of copper, zinc, manganese and iron by selected species of grassland legumes and herbs. J. Elem. 2014, 19, 109–118. [Google Scholar] [CrossRef]
- He, N.P.; Zhang, Y.H.; Yu, Q.; Chen, Q.S.; Pan, Q.M.; Zhang, G.M.; Han, X.G. Grazing intensity impacts soil carbon and nitrogen storage of continental steppe. Ecosphere 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Oliveira Filho, J.S.; Vieira, J.N.; Ribeiro da Silva, E.M.; Beserra de Oliveira, J.G.; Pereira, M.G.; Brasileiro, F.G. Assessing the effects of 17 years of grazing exclusion in degraded semi-arid soils: Evaluation of soil fertility, nutrients pools and stoichiometry. J. Arid Environ. 2019, 166, 1–10. [Google Scholar] [CrossRef]
- Reeder, J.D.; Schuman, G.E.; Morgan, J.A.; Lecain, D.R. Response of organic and inorganic carbon and nitrogen to long-term grazing of the shortgrass steppe. Environ. Manag. 2004, 33, 485–495. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Niu, H.; Wu, J.; Wan, S.; Schnug, E.; Rogasik, J.; Fleckenstein, J.; Tang, Y. Effect of long-term grazing on soil organic carbon content in semiarid steppes in Inner Mongolia. Ecol. Res. 2005, 20, 519–527. [Google Scholar] [CrossRef]
- Klaus, V.H.; Kleinebecker, T.; Hölzel, N.; Blüthgen, N.; Boch, S.; Müller, J.; Socher, S.A.; Prati, D.; Fischer, M. Nutrient concentrations and fibre contents of plant community biomass reflect species richness patterns along a broad range of land-useintensities among agricultural grasslands. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 287–295. [Google Scholar] [CrossRef]
- Du, C.; Gao, Y. Grazing exclusion alters ecological stoichiometry of plant and soil in degraded alpine grassland. Agric. Ecosyst. Environ. 2021, 308, 107256. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Davies, R.O.; Jones, D.I.H.; Milton, W.E.J. Factors affecting the composition and nutritive value of herbage from fescue and Molinia areas. J. Agric. Sci. 1959, 53, 268–285. [Google Scholar] [CrossRef]
- Hou, S.L.; Yi, J.X.; Sistla, S.; Yang, J.J.; Sun, Y.; Li, Y.Y.; Lü, X.T.; Han, X.G. Long-term mowing did not alter the impacts of nitrogen deposition on litter quality in a temperate steppe. Ecol. Eng. 2017, 102, 404–410. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Lomba, Ȃ.; Żołnierz, L.; Dradrach, A.; Honrado, J.P. The impact of environmental factors and management on the fitness of Carlina acaulis subsp. caulescens (Lam.) Schübl. et G. Martens in mountain mesic meadows. Turk. J. Bot. 2023, 47, 586–594. [Google Scholar]
- Stockdale, C.R. Effects of season and time since defoliation on the nutritive characteristics of three irrigated perennial pasture species in northern Victoria. 2. Macro-minerals. Aust. J. Exp. Agric. 1999, 39, 567–577. [Google Scholar] [CrossRef]
- Wheeler, D.M. Investigation into the mechanisms causing lime responses in a grass/clover pasture on a clay loam soil. N. Z. J. Agric. Res. 1998, 41, 497–515. [Google Scholar] [CrossRef]
- Scott, D. Sustainability of New Zealand high-country pastures under contrasting development inputs. 1. Site and shoot nutrients. N. Z. J. Agric. Res. 1999, 42, 365–383. [Google Scholar] [CrossRef]
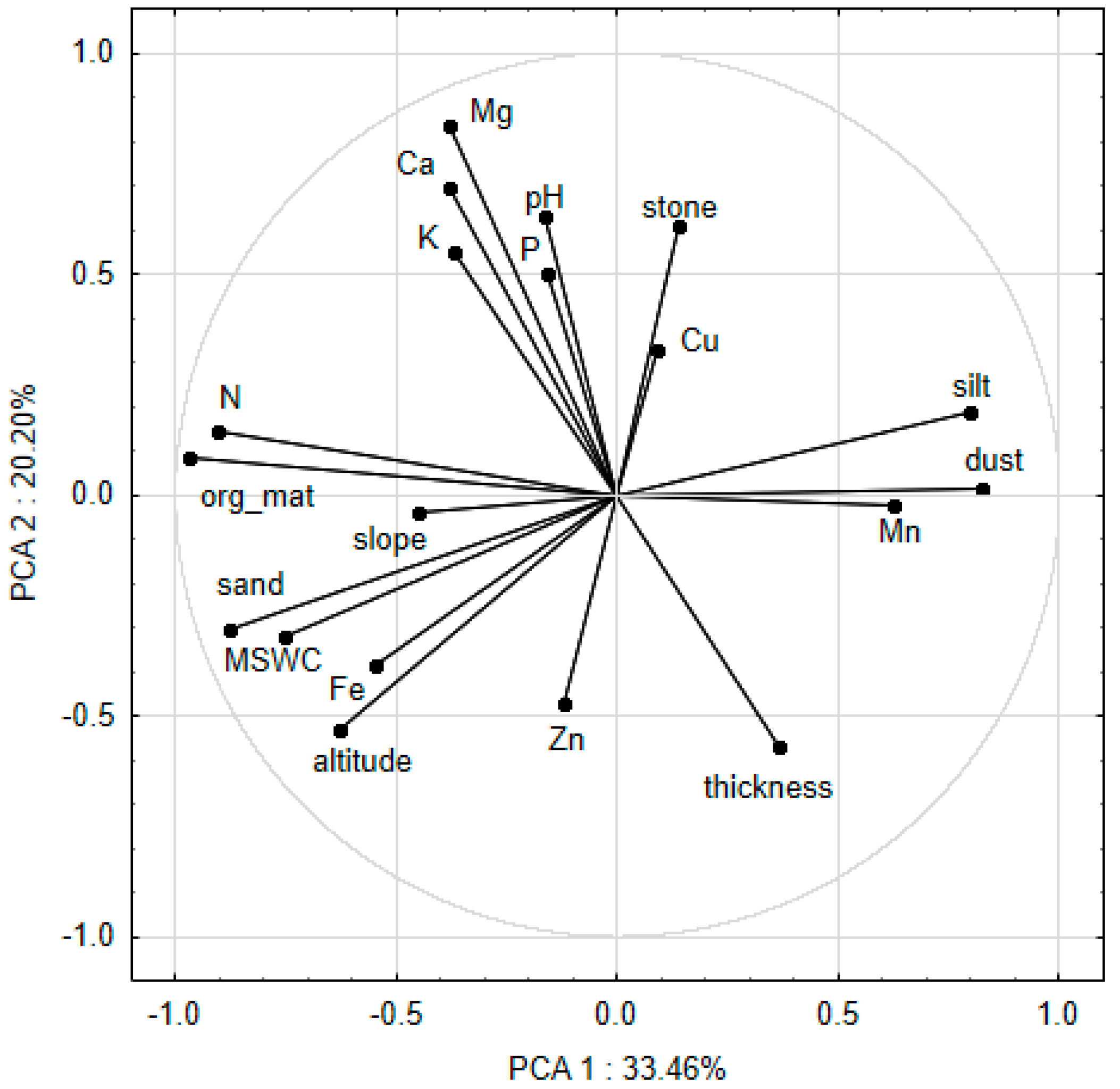

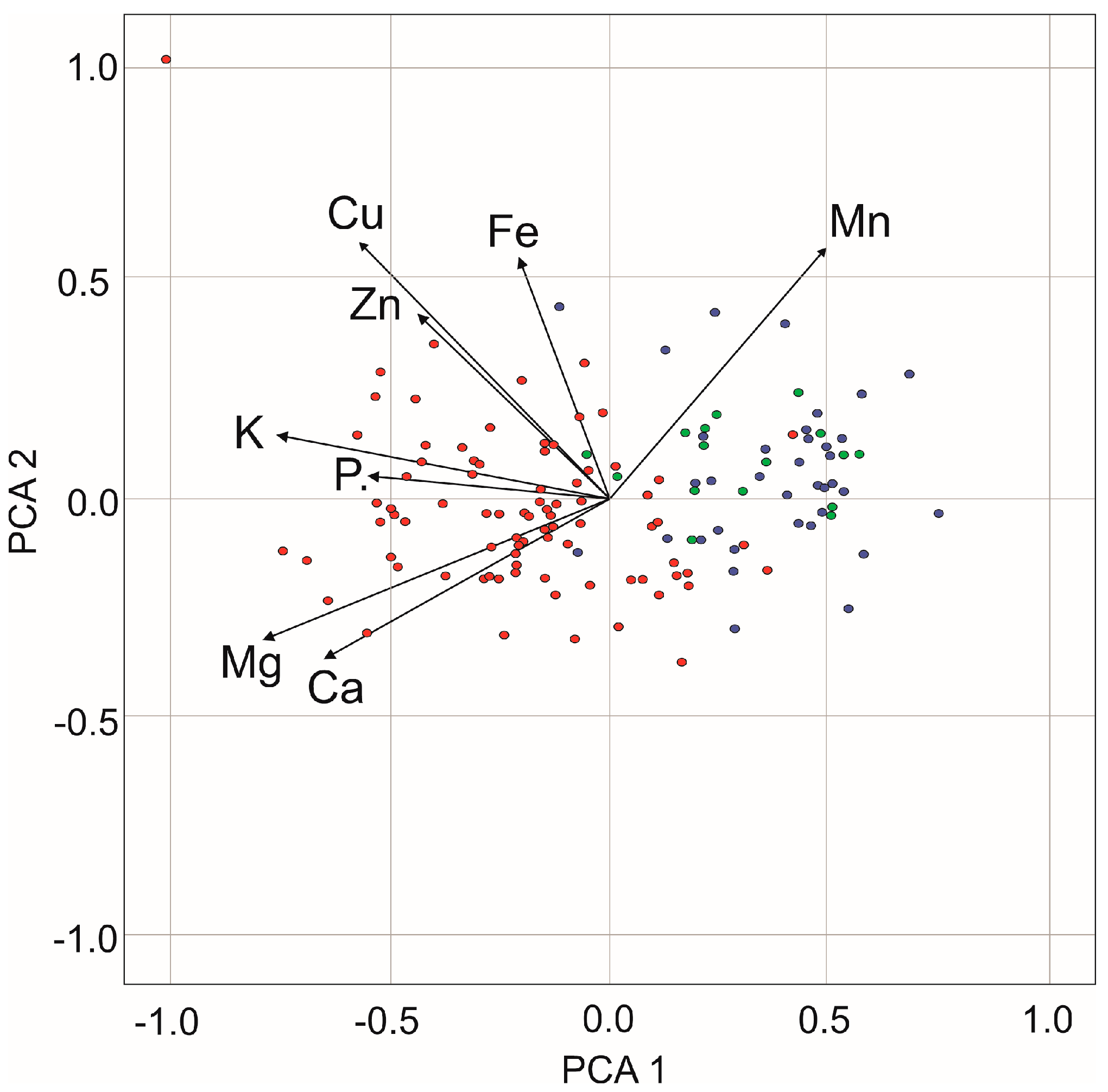
| Altitude | Slope | Thickness | pH | MSWC | Org_Mat | Stone | Sand | Dust | Silt | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | |||||||||||
| K | −0.461 * | ||||||||||
| Ca | 0.480 * | 0.788 *** | 0.505 * | ||||||||
| Mg | −0.685 *** | 0.496 * | 0.532 * | ||||||||
| Cu | |||||||||||
| Fe | 0.448 * | 0.726 *** | 0.810 *** | 0.741 *** | −0.614 ** | −0.631 ** | 0.671 *** | ||||
| Mn | −0.798 *** | −0.551 * | 0.509 * | −0.587 ** | |||||||
| Zn | −0.673 *** |
| Mowing | Mowing-Pasture | Fallowing | ||||
|---|---|---|---|---|---|---|
| x | SE | x | SE | x | SE | |
| Phosphorus [mg·kg−1] | ||||||
| Achillea millefolium | 8.02 | 1.09 | 7.07 | 0.89 | 8.67 | 1.65 |
| Lotus corniculatus | 4.82 | 0.57 | 5.42 | 0.77 | 3.94 | 0.71 |
| Plantago lanceolata | 6.16 | 0.79 | 7.69 | 0.93 | 5.76 | 1.19 |
| Rumex acetosa | 8.29 | 1.21 | 8.64 | 2.00 | 7.00 | 0.74 |
| Veronica chamaedrys | 6.07 | 0.71 | 5.87 | 0.75 | 6.29 | 0.92 |
| Agrostis capillaris | 3.63 | 0.32 | 4.50 | 0.98 | 3.91 | 0.63 |
| Festuca rubra | 3.76 | 0.63 | 3.20 | 0.27 | 2.95 | 0.33 |
| Meadow sward | 4.26 | 0.40 | 3.99 | 0.32 | 4.48 | 1.09 |
| Necromass | 3.96 | 0.40 | 3.06 | 0.54 | 3.75 | 0.23 |
| Exchangeable forms | 14.23 | 1.40 | 14.53 | 1.08 | 27.99 | 9.04 |
| Potassium [mg·kg−1] | ||||||
| Achillea millefolium | 35,774.24 | 1730.84 | 39,484.14 | 4954.71 | 31,123.15 | 2521.99 |
| Lotus corniculatus | 19,310.43 | 2054.75 | 17,468.04 | 2316.25 | 24,015.90 | 5374.46 |
| Plantago lanceolata | 29,156.68 | 3050.82 | 28,940.65 | 1906.01 | 29,175.00 | 2318.02 |
| Rumex acetosa | 41,730.88 | 3658.04 | 39,464.25 | 3016.31 | 29,076.20 | 5534.57 |
| Veronica chamaedrys | 21,843.72 | 936.24 | 22,514.55 | 850.42 | 28,637.08 | 7405.31 |
| Agrostis capillaris | 11,616.06 | 1119.60 | 13,118.21 | 1433.42 | 14,800.90 | 1232.31 |
| Festuca rubra | 15,059.93 | 1976.29 | 16,752.67 | 2610.29 | 13,672.38 | 3561.87 |
| Meadow sward | 21,378.34 | 2561.05 | 18,631.33 | 2123.12 | 18,290.75 | 2861.62 |
| Necromass | 9935.78 | 1969.97 | 8488.17 | 1197.22 | 8898.00 | 647.14 |
| Total content | 10,928.27 | 430.62 | 10,180.60 | 1005.88 | 10,699.83 | 1704.58 |
| Exchangeable forms | 124.99 | 11.23 | 144.00 | 31.61 | 316.87 | 129.30 |
| Magnesium [mg·kg−1] | ||||||
| Achillea millefolium | 3415.97 | 305.78 | 3333.90 | 395.82 | 3406.22 | 265.74 |
| Lotus corniculatus | 3375.01 | 396.88 | 5085.53 | 611.81 | 3925.85 | 730.80 |
| Plantago lanceolata | 4498.00 | 641.62 | 3915.48 | 355.92 | 4491.92 | 879.44 |
| Rumex acetosa | 4445.72 | 577.98 | 5053.55 | 729.74 | 3116.77 | 599.85 |
| Veronica chamaedrys | 4152.34 | 363.30 | 4693.61 | 683.16 | 4793.10 | 325.54 |
| Agrostis capillaris | 1732.67 | 465.26 | 1772.78 | 464.61 | 3122.02 | 691.36 |
| Festuca rubra | 2243.16 | 397.69 | 2619.04 | 273.65 | 1970.97 | 422.58 |
| Meadow sward | 1996.22 | 189.43 | 1764.76 | 134.49 | 1947.93 | 257.06 |
| Necromass | 2918.24 | 678.84 | 2574.81 | 444.91 | 2493.58 | 260.95 |
| Total content | 4552.69 | 629.33 | 4105.13 | 784.38 | 4597.84 | 593.90 |
| Exchangeable forms | 141.82 | 21.93 | 107.01 | 19.80 | 171.29 | 61.33 |
| Calcium [mg·kg−1] | ||||||
| Achillea millefolium | 21,547.36 | 2705.41 | 14,682.14 | 1560.77 | 18,224.00 | 1836.71 |
| Lotus corniculatus | 17,858.93 | 3103.17 | 24,205.00 | 3936.78 | 14,357.00 | 2365.49 |
| Plantago lanceolata | 26,672.14 | 5420.83 | 22,020.50 | 1712.22 | 23,441.88 | 4692.74 |
| Rumex acetosa | 9595.00 | 786.79 | 7683.33 | 1468.74 | 9049.00 | 1412.97 |
| Veronica chamaedrys | 17,721.25 | 2969.12 | 21,576.98 | 4195.19 | 14,822.50 | 4148.19 |
| Agrostis capillaris | 5338.89 | 1236.95 | 11,111.00 | 3052.31 | 14,238.50 | 3669.30 |
| Festuca rubra | 7772.86 | 1988.23 | 11,915.42 | 1619.51 | 5531.25 | 2765.82 |
| Meadow sward | 7474.06 | 1378.87 | 5545.83 | 743.51 | 5972.50 | 990.13 |
| Necromass | 13,382.50 | 2956.65 | 12,445.83 | 2789.52 | 10,877.50 | 3095.58 |
| Total content | 1191.60 | 168.17 | 1363.75 | 283.05 | 1063.33 | 152.72 |
| Exchangeable forms | 1606.93 | 246.77 | 1357.58 | 246.30 | 1061.20 | 208.75 |
| Manganese [mg·kg−1] | ||||||
| Achillea millefolium | 397.13 | 70.11 | 452.30 | 75.62 | 393.78 | 76.94 |
| Lotus corniculatus | 134.77 | 30.26 | 213.29 | 66.53 | 101.09 | 19.93 |
| Plantago lanceolata | 147.90 | 27.59 | 199.14 | 22.58 | 193.50 | 13.93 |
| Rumex acetosa | 324.83 | 84.85 | 262.35 | 63.22 | 310.65 | 88.85 |
| Veronica chamaedrys | 311.21 | 37.05 b | 157.00 | 15.81 a | 185.41 | 33.11 ab |
| Agrostis capillaris | 698.24 | 64.07 | 631.11 | 113.01 | 601.13 | 83.27 |
| Festuca rubra | 519.13 | 59.85 | 450.05 | 132.35 | 415.39 | 46.05 |
| Meadow sward | 502.80 | 66.52 | 472.79 | 112.48 | 457.33 | 84.10 |
| Necromass | 1091.72 | 153.33 b | 496.34 | 57.53 a | 865.75 | 196.34 ab |
| Total content | 1282.87 | 125.06 | 954.85 | 97 59 | 928.33 | 92.89 |
| Exchangeable forms | 95.21 | 8.39 | 77.93 | 19.11 | 92.23 | 10.94 |
| Copper [mg·kg−1] | ||||||
| Achillea millefolium | 16.24 | 1.64 | 21.53 | 3.98 | 14.07 | 2.07 |
| Lotus corniculatus | 9.64 | 0.67 | 9.85 | 1.13 | 9.19 | 0.81 |
| Plantago lanceolata | 12.54 | 0.56 | 14.96 | 1.49 | 15.63 | 3.16 |
| Rumex acetosa | 8.78 | 1.28 | 9.73 | 1.35 | 10.15 | 1.76 |
| Veronica chamaedrys | 11.83 | 0.91 | 12.13 | 1.63 | 12.47 | 1.80 |
| Agrostis capillaris | 11.78 | 2.01 b | 6.33 | 0.41 a | 7.60 | 0.94 ab |
| Festuca rubra | 7.64 | 0.67 | 8.30 | 1.26 | 7.30 | 1.59 |
| Meadow sward | 12.23 | 1.30 | 12.91 | 1.03 | 11.05 | 1.20 |
| Necromass | 11.42 | 1.79 | 9.93 | 0.79 | 11.50 | 1.99 |
| Total content | 27.51 | 4.58 | 25.08 | 3.13 | 21.00 | 2.80 |
| Exchangeable forms | 0.28 | 0.05 | 0.77 | 0.57 | 0.33 | 0.10 |
| Iron [mg·kg−1] | ||||||
| Achillea millefolium | 194.31 | 34.31 | 400.11 | 214.38 | 203.76 | 51.18 |
| Lotus corniculatus | 137.60 | 18.80 | 272.44 | 68.72 | 168.36 | 26.36 |
| Plantago lanceolata | 318.44 | 74.61 | 295.04 | 61.32 | 165.04 | 33.89 |
| Rumex acetosa | 371.88 | 150.80 | 360.31 | 113.33 | 198.30 | 36.34 |
| Veronica chamaedrys | 206.04 | 33.49 | 140.94 | 16.30 | 243.80 | 45.89 |
| Agrostis capillaris | 463.26 | 180.22 | 137.13 | 28.32 | 155.29 | 30.93 |
| Festuca rubra | 253.04 | 64.57 | 281.50 | 54.12 | 175.47 | 54.03 |
| Meadow sward | 133.14 | 13.91 | 263.04 | 64.73 | 174.00 | 31.28 |
| Necromass | 579.23 | 170.62 | 409.26 | 77.47 | 963.04 | 432.24 |
| Total content | 27,629.33 | 1636.96 | 30,843.29 | 3129.27 | 29,291.00 | 3528.17 |
| Exchangeable forms | 4.04 | 3.50 | 20.35 | 12.77 | 0.73 | 0.19 |
| Zinc [mg·kg−1] | ||||||
| Achillea millefolium | 64.52 | 8.21 | 99.25 | 12.31 | 95.35 | 16.46 |
| Lotus corniculatus | 48.19 | 7.48 | 77.52 | 24.77 | 77.83 | 13.55 |
| Plantago lanceolata | 82.52 | 5.09 | 88.93 | 11.81 | 103.23 | 11.91 |
| Rumex acetosa | 53.70 | 2.16ab | 79.33 | 8.47b | 73.83 | 4.76b |
| Veronica chamaedrys | 84.29 | 7.79 | 92.39 | 10.03 | 98.03 | 19.57 |
| Agrostis capillaris | 74.77 | 4.69 | 76.12 | 8.55 | 69.22 | 9.08 |
| Festuca rubra | 54.91 | 6.69 | 67.11 | 9.37 | 42.10 | 7.99 |
| Meadow sward | 61.93 | 5.59 | 69.08 | 5.46 | 64.01 | 9.11 |
| Necromass | 81.49 | 5.00 | 87.55 | 7.01 | 90.49 | 22.06 |
| Total content | 87.54 | 5.34 | 107.46 | 8.02 | 106.14 | 12.53 |
| Exchangeable forms | 2.83 | 0.35 | 4.16 | 1.34 | 4.68 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruchniewicz, D.; Łobas, Z.; Dradrach, A.; Żołnierz, L. The Influence of Management on the Content of Macro- and Microelements in Plant Shoots of a Meadow Sward of an Arrhenatheretalia Plant Community. Agronomy 2025, 15, 1020. https://doi.org/10.3390/agronomy15051020
Pruchniewicz D, Łobas Z, Dradrach A, Żołnierz L. The Influence of Management on the Content of Macro- and Microelements in Plant Shoots of a Meadow Sward of an Arrhenatheretalia Plant Community. Agronomy. 2025; 15(5):1020. https://doi.org/10.3390/agronomy15051020
Chicago/Turabian StylePruchniewicz, Daniel, Zbigniew Łobas, Agnieszka Dradrach, and Ludwik Żołnierz. 2025. "The Influence of Management on the Content of Macro- and Microelements in Plant Shoots of a Meadow Sward of an Arrhenatheretalia Plant Community" Agronomy 15, no. 5: 1020. https://doi.org/10.3390/agronomy15051020
APA StylePruchniewicz, D., Łobas, Z., Dradrach, A., & Żołnierz, L. (2025). The Influence of Management on the Content of Macro- and Microelements in Plant Shoots of a Meadow Sward of an Arrhenatheretalia Plant Community. Agronomy, 15(5), 1020. https://doi.org/10.3390/agronomy15051020







