Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Biostimulant and Auxin Treatments and Propagation Conditions
2.3. Plant Growth Measurements
2.4. Measurements of Root Morphological Traits
2.5. Primary Metabolite Extraction and Qualitative Analysis from Biostimulant
2.6. Quantification of BRs in Plant-Derived Biostimulant and Plant Samples
2.7. Amino Acid Quantification of Vegetal-Biostimulant
2.8. Total Phenolic Content and Antioxidant Capacity
2.9. Experimental Design and Statistical Analysis
3. Results
3.1. The Effects of Biostimulant on Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum
3.2. The Effects of Biostimulant on Root Diameter Class Distribution
3.3. The Effects of Biostimulant on Shoot Growth
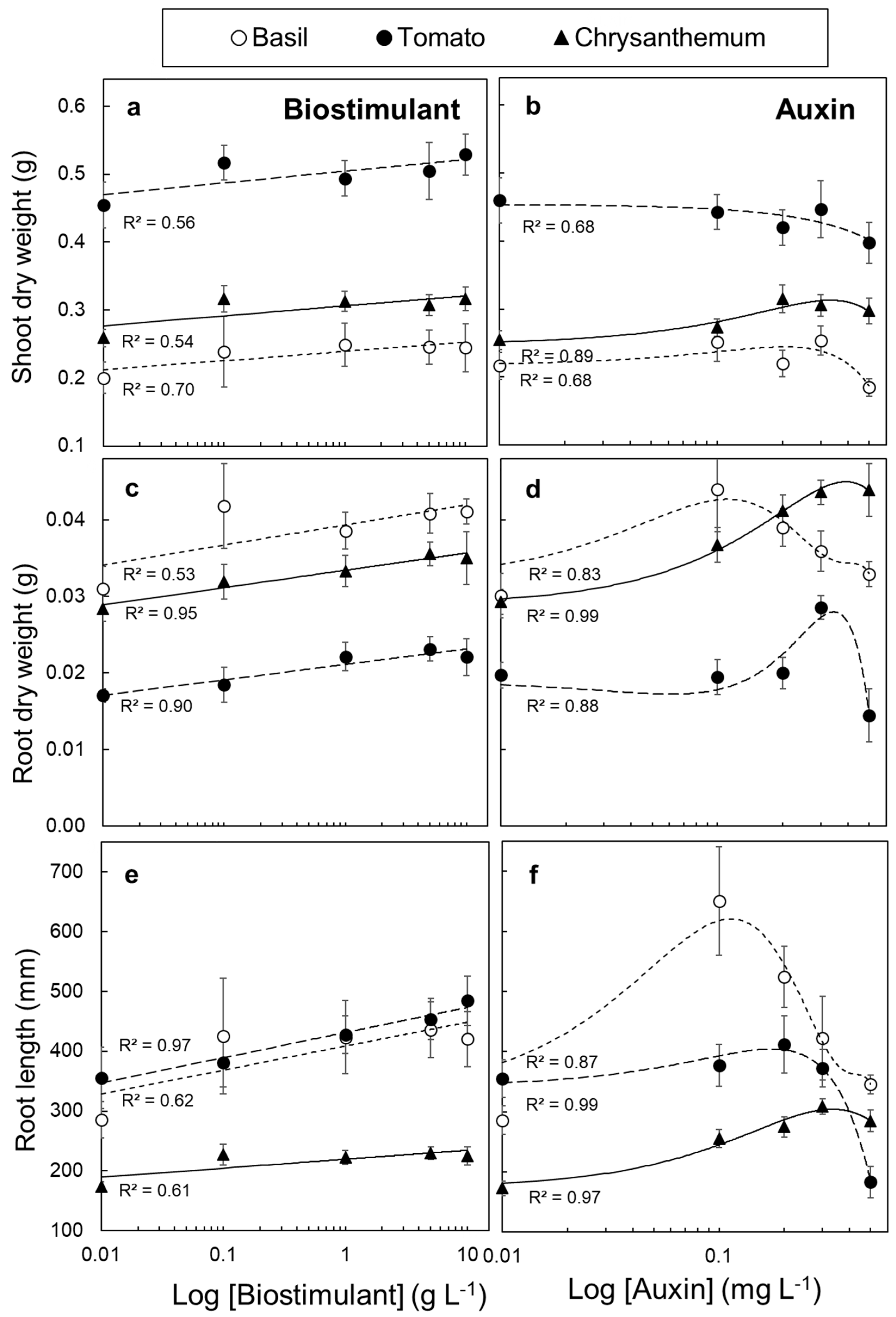
3.4. Tissue Responsiveness to Biostimulant and Auxin
3.5. BRs in Roots and Shoots of Cuttings
3.6. Antioxidant Capacities and Total Phenolic Content of Cuttings
4. Discussion
4.1. Biostimulant Promotes Adventitious Rooting Responses of Stem Cuttings Similar to Auxin, but to a Lesser Extent
4.2. Biostimulant Induces Adventitious Rooting of Stem Cuttings Primarily via BR-Mediated Processes
4.3. Biostimulant-Induced BRs and Auxin Have Overlapping Functions in Adventitious Root Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
References
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean; InTech Europe: Rijeka, Croatia, 2011; pp. 215–230. [Google Scholar]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop. Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. Hortscience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nalchel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nakanomyo, I.; Motose, H.; Iwamoto, K.; Sawa, S.; Dohmae, N.; Fukuda, H. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 2006, 313, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawa, S.; Kinoshita, A.; Mizuno, S.; Kakimoto, T.; Fukuda, H.; Sakagami, Y. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 2006, 313, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Pearce, G. Polypeptide hormones. Plant Physiol. 2001, 125, 65–68. [Google Scholar] [CrossRef]
- Ryan, C.A.; Pearce, G.; Scheer, J.; Moura, D.S. Polypeptide hormones. Plant Cell 2002, 14, S251–S264. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Monterumici, C.M.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Paradikovic, N.; Vinkovic, T.; Vrcek, I.V.; Zuntar, I.; Bojic, M.; Medic-Saric, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; Garcia-Martinez, A.; Gonzalez-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic vegetable extract with bio-active components: Influence of fertiliser on the colour and anthocyanins of red grapes. J. Sci. Food Agric. 2007, 87, 2310–2318. [Google Scholar] [CrossRef]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- de Vasconcelos, A.C.F.; Zhang, X.Z.; Ervin, E.H.; Kiehl, J.D. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agric. 2009, 66, 395–402. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. In I World Congress on the Use of Biostimulants in Agriculture; Silva, S.S., Brown, P., Ponchet, M., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2013; Volume 1009, pp. 29–35. [Google Scholar]
- Colla, G.; Svecova, E.; Cardarelli, M.; Rouphael, Y.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a plant-derived protein hydrolysate to improve crop performances under different growing conditions. In I World Congress on the Use of Biostimulants in Agriculture; Silva, S.S., Brown, P., Ponchet, M., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2013; Volume 1009, pp. 175–179. [Google Scholar]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Ludwigmuller, J. Indole-3-butyric acid in plants—Occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Ludwig-Muller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Nordstrom, A.C.; Jacobs, F.A.; Eliasson, L. Effect of exogenous indole-3-acetic-acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic-acid during adventitious root-formation in pea cuttings. Plant Physiol. 1991, 96, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Pal, M. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New Forests. 2007, 33, 309–323. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Van der Krieken, W.; De Jong, J.C. Review—The formation of adventitious roots: New concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant. 1999, 35, 189–199. [Google Scholar] [CrossRef]
- da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Fabijan, D.; Taylor, J.S.; Reid, D.M. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings.2. Action of gibberellins, cytokinins, auxins and ethylene. Physiol. Plant. 1981, 53, 589–597. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F., Jr.; Geneve, R.L. Plant Propagation: Principles and Practices, 8th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Liu, J.H.; Reid, D.M. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings.4. The role of changes in endogenous free and conjugated indole-3-acetic-acid. Physiol. Plant. 1992, 86, 285–292. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Manoli, A.; Ravazzolo, L.; Franceschi, C.; Quaggiotti, S. mRNA-sequencing analysis reveals transcriptional changes in root of maize seedlings treated with two increasing concentrations of a new biostimulant. J. Agric. Food Chem. 2017, 65, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Falasca, G.; Zaghi, D.; Possenti, M.; Altamura, M.M. Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep. 2004, 23, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G.; McKhann, H.; Garcion, C.; Vaucheret, H.; Sandberg, G.; Bellini, C. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005, 17, 1343–1359. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inze, D.; Sandberg, G.; Casero, P.J.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001, 13, 843–852. [Google Scholar] [CrossRef]
- Malamy, J.E.; Benfey, P.N. Down and out in Arabidopsis: The formation of lateral roots. Trends Plant Sci. 1997, 2, 390–396. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Gu, D.X.; Zhen, F.X.; Hannaway, D.B.; Zhu, Y.; Liu, L.L.; Cao, W.X.; Tang, L. Quantitative classification of rice (Oryza sativa L.) root length and diameter using image analysis. PloS ONE 2017, 12, e0169968. [Google Scholar] [CrossRef] [PubMed]
- Zobel, R.W.; Waisel, Y. A plant root system architectural taxonomy: A framework for root nomenclature. Plant Biosyst. 2010, 144, 507–512. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.E. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 1–16. [Google Scholar] [CrossRef]
- El-Bassiony, A.M.; Ghoname, A.A.A.; El-Awadi, M.E.; Fawzy, Z.F.; Gruda, N. Ameliorative effects of brassinosteroids on growth and productivity of snap beans grown under high temperature. Gesunde Pflanz. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma. 2018, 255, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Clouse, S.D. Brassinosteroids. The arabidopsis book 2011, 9, e0151. [Google Scholar] [CrossRef]
- Men, S.; Boutte, Y.; Ikeda, Y.; Li, X.; Palme, K.; Stierhof, Y.-D.; Hartmann, M.-A.; Moritz, T.; Grebe, M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell. Biol. 2008, 10, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D.; Zurek, D.M.; McMorris, T.C.; Baker, M.E. Effect of brassinolide on gene-expression in elongating soybean epicotyls. Plant Physiol. 1992, 100, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Nemhauser, J.L.; Mockler, T.C.; Chory, J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004, 2, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Shen, J.J.; Brady, S.R.; Muday, G.K.; Asami, T.; Yang, Z.B. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 2004, 134, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Y.; Lv, B.S.; Ding, T.T.; Bai, M.Y.; Ding, Z.J. Auxin-BR interaction regulates plant growth and development. Front. Plant Sci. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2009, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Guan, H.Y.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Mussig, C.; Shin, G.H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef]
- Guan, M.; Roddick, J.G. Epibrassinolide-inhibition of development of excised, adventitious and intact roots of tomato (Lycopersicon esculentum)-comparison with the effects of steroidal estrogens. Physiol Plant. 1988, 74, 720–726. [Google Scholar] [CrossRef]
- Guan, M.; Roddick, J.G. Comparison of the effects of epibrassinolide and steroidal estrogens on adventitious root-growth and early shoot development in mung bean cuttings. Physiol. Plant 1988, 73, 426–431. [Google Scholar] [CrossRef]
- Ren, C.M.; Han, C.Y.; Peng, W.; Huang, Y.; Peng, Z.H.; Xiong, X.Y.; Zhu, Q.; Gao, B.D.; Xie, D.X. A leaky mutation in DWARF4 reveals an antagonistic role of brassinosteroid in the inhibition of root growth by jasmonate in Arabidopsis. Plant Physiol. 2009, 151, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.N.; Seeta Ram Rao, S. Influence of brassinosteroids on rooting and growth of geranium (Pelargonium sp.) stem cuttings. Asian J. Plant Sci. 2006, 5, 619–622. [Google Scholar]
- Swamy, K.N.; Seeta Ram Rao, S. Effect of brassinosteroids on rooting and early vegetative growth of Coleus [Plectranthus forskohlii (Wild.) Briq.] stem cuttings. Indian J. Plant Sci. 2010, 1, 68–73. [Google Scholar]

| Treatment | Root Number | Root Dry Mass (g plant−1) | Total Root Length (mm) | Root Surface Area (mm2) | Root Volume (mm3) | Root Diameter (mm) |
|---|---|---|---|---|---|---|
| Basil | ||||||
| Control | 39.4 | 0.033 a | 286 b | 48.7 b | 0.67 b | 0.55 |
| B5 | 52.0 | 0.041 a | 436 a | 69.0 a | 0.88 a | 0.51 |
| B10 | 51.9 | 0.041 a | 420 ab | 66.4 a | 0.84 a | 0.52 |
| IBA+NAA0.2 | 42.4 | 0.034 a | 322 ab | 60.1 ab | 0.89 a | 0.60 |
| IBA+NAA0.5 | 49.8 | 0.033 a | 346 ab | 60.9 ab | 0.87 a | 0.57 |
| Significance | ns | ns | * | * | * | ns |
| Tomato | ||||||
| Control | 22.2 c | 0.017 ab | 355 b | 41.8 a | 0.39 | 0.38 b |
| B5 | 36.9 a | 0.023 a | 454 ab | 50.2 a | 0.44 | 0.35 bc |
| B10 | 36.2 a | 0.022 a | 484 a | 50.7 a | 0.42 | 0.33 c |
| IBA+NAA0.2 | 28.3 b | 0.021 ab | 362 ab | 42.4 a | 0.40 | 0.38 b |
| IBA+NAA0.5 | 16.2 c | 0.014 b | 183 c | 28.0 b | 0.34 | 0.48 a |
| Significance | *** | * | *** | *** | ns | *** |
| Chrysanthemum | ||||||
| Control | 16.4 c | 0.029 d | 173 c | 35.0 c | 0.57 b | 0.63 |
| B5 | 20.1 bc | 0.033 cd | 223 b | 45.7 b | 0.75 a | 0.65 |
| B10 | 21.1 bc | 0.036 bc | 230 b | 47.0 b | 0.77 a | 0.66 |
| IBA+NAA0.2 | 39.8 a | 0.041 ab | 275 ab | 53.4 ab | 0.83 a | 0.63 |
| IBA+NAA0.5 | 25.1 b | 0.046 a | 285 a | 57.1 a | 0.92 a | 0.64 |
| Significance | *** | *** | *** | *** | *** | ns |
| Treatment | Root diameter class (mm) | ||||
| 0–0.25 | 0.25–0.50 | 0.50–0.75 | 0.75–1.00 | >1.00 | |
| Relative root diameter class length (%) | |||||
| Basil | |||||
| Control | 15.7 b | 27.0 | 41.0 | 13.8 | 2.5 |
| B5 | 22.7 a | 28.5 | 40.0 | 11.7 | 3.8 |
| B10 | 21.6 a | 30.3 | 38.2 | 11.4 | 4.5 |
| IBA+NAA0.2 | 20.6 ab | 26.8 | 33.4 | 11.3 | 3.1 |
| IBA+NAA0.5 | 15.5 b | 25.3 | 32.2 | 15.5 | 3.6 |
| Significance | ** | ns | ns | ns | ns |
| Tomato | |||||
| Control | 35.2 b | 40.1 | 22.4 b | 1.2 b | 0.3 b |
| B5 | 39.2 ab | 42.3 | 17.3 bc | 1.0 b | 0.2 b |
| B10 | 43.4 a | 41.6 | 13.8 c | 1.0 b | 0.2 b |
| IBA+NAA0.2 | 33.1 b | 45.7 | 18.6 bc | 1.9 b | 0.7 b |
| IBA+NAA0.5 | 21.6 c | 38.1 | 34.3 a | 4.0 a | 2.1 a |
| Significance | *** | ns | *** | *** | *** |
| Chrysanthemum | |||||
| Control | 10.4 b | 30.1 a | 29.9 b | 19.1 a | 10.4 ab |
| B5 | 11.5 ab | 29.8 a | 27.2 b | 20.1 a | 11.4 a |
| B10 | 11.9 ab | 26.9 ab | 30.4 b | 19.3 a | 11.5 a |
| IBA+NAA0.2 | 14.0 a | 27.2 ab | 32.0 b | 17.7 ab | 8.9 b |
| IBA+NAA0.5 | 12.8 ab | 24.8 b | 38.7 a | 15.0 b | 8.6 b |
| Significance | * | ** | *** | ** | ** |
| Treatment | Dry Mass (g plant−1) | Stem Length (cm) | SPAD Index | Total N (%) | Root-to-Shoot Ratio | |||
|---|---|---|---|---|---|---|---|---|
| Total | Shoots | Leaves | Stems | |||||
| Basil | ||||||||
| Control | 0.228 ab | 0.200 ab | 0.145 | 0.055 | 6.0 | 35.1 | 1.42 | 0.144 |
| B5 | 0.287 a | 0.246 a | 0.185 | 0.061 | 6.3 | 35.1 | 1.39 | 0.170 |
| B10 | 0.285 a | 0.244 a | 0.177 | 0.059 | 6.6 | 31.7 | 1.56 | 0.177 |
| IBA+NAA0.2 | 0.234 ab | 0.200 ab | 0.115 | 0.050 | 5.8 | 35.5 | 1.44 | 0.175 |
| IBA+NAA0.5 | 0.218 b | 0.185 b | 0.137 | 0.049 | 5.9 | 32.5 | 1.45 | 0.183 |
| Significance | ** | * | ns | ns | ns | ns | ns | ns |
| Tomato | ||||||||
| Control | 0.473 bc | 0.457 bc | 0.323 ab | 0.133 b | 7.2 ab | 41.5 | 2.53 | 0.038 b |
| B5 | 0.526 ab | 0.505 ab | 0.360 a | 0.146 ab | 7.5 ab | 41.4 | 2.54 | 0.046 a |
| B10 | 0.551 a | 0.530 a | 0.358 a | 0.173 a | 7.8 a | 40.8 | 2.47 | 0.042 a |
| IBA+NAA0.2 | 0.414 c | 0.399 c | 0.283 bc | 0.111 b | 7.0 b | 39.5 | 2.22 | 0.046 a |
| IBA+NAA0.5 | 0.412 c | 0.398 c | 0.262 c | 0.134 b | 6.8 b | 39.2 | 2.38 | 0.036 b |
| Significance | ** | ** | ** | * | * | ns | ns | ns |
| Chrysanthemum | ||||||||
| Control | 0.287 b | 0.259 b | 0.182 | 0.077 b | 6.9 b | 34.1 c | 3.34 | 0.104 c |
| B5 | 0.346 a | 0.313 a | 0.209 | 0.099 a | 7.6 ab | 35.4 bc | 3.00 | 0.109 c |
| B10 | 0.343 ab | 0.307 ab | 0.206 | 0.101 a | 8.3 a | 34.6 bc | 3.02 | 0.118 bc |
| IBA+NAA0.2 | 0.356 a | 0.315 a | 0.212 | 0.103 a | 6.9 b | 37.6 a | 2.93 | 0.136 b |
| IBA+NAA0.5 | 0.343 ab | 0.297 ab | 0.210 | 0.087 ab | 7.3 ab | 36.4 ab | 2.97 | 0.159 a |
| Significance | * | * | ns | *** | *** | *** | ns | *** |
| Plant Tissue | Treatment | Stigmasterol | ß-Sitosterol | Campesterol | Total |
|---|---|---|---|---|---|
| (μg g−1 DW) | |||||
| Basil | |||||
| Roots | Control | 440 ± 10 | 454 ± 19 | 232 ± 10 | 1126 ± 36 |
| B5 | 474 ± 27 | 430 ± 17 | 231 ± 90 | 1136 ± 51 | |
| B10 | 410 ± 9 | 389 ± 32 | 214 ± 18 | 1013 ± 52 | |
| IBA+NAA0.2 | 420 ± 13 | 402 ± 15 | 217 ± 10 | 1039 ± 31 | |
| Significance | nsa | ns | ns | ns | |
| Shoots | Control | 59 ± 40 | 204 ± 13 | 64 ± 60 | 327 ± 23 |
| B5 | 59 ± 60 | 158 ± 14 | 55 ± 6 | 272 ± 25 | |
| B10 | 47 ± 20 | 164 ± 40 | 53 ± 2 | 264 ± 5 | |
| IBA+NAA0.2 | 47 ± 3 | 166 ± 16 | 53 ± 4 | 261 ± 22 | |
| Significance | ns | ns | ns | ns | |
| Tomato | |||||
| Roots | Control | 270 ± 16 a | 97 ± 12 | 30 ± 3 a | 397 ± 28 a |
| B5 | 197 ± 17 b | 79 ± 9 | 22 ± 3 ab | 298 ± 27 b | |
| B10 | 182 ± 5 b | 69 ± 6 | 20 ± 1 ab | 270 ± 90b | |
| IBA+NAA0.2 | 155 ± 17 b | 71 ± 6 | 17 ± 2 b | 243 ± 20 b | |
| Significance | *** | ns | * | ** | |
| Shoots | Control | 55 ± 8 | 17 ± 3 b | 3.0 ± 0.6 | 75 ± 12 |
| B5 | 54 ± 40 | 22 ± 2 ab | 5.7 ± 1.8 | 81 ± 6 | |
| B10 | 62 ± 90 | 31 ± 5 a | 4.0 ± 1.9 | 97 ± 15 | |
| IBA+NAA0.2 | 48 ± 50 | 32 ± 2 a | 3.1 ± 1.9 | 82 ± 7 | |
| Significance | ns | ** | ns | ns | |
| Chrysanthemum | |||||
| Roots | Control | 83 ± 7 | 111 ± 9 | 19.5 ± 2.3 | 213 ± 18 |
| B5 | 92 ± 5 | 115 ± 6 | 20.9 ± 1.5 | 228 ± 10 | |
| B10 | 104 ± 6 | 111 ± 5 | 20.7 ± 0.8 | 235 ± 7 | |
| IBA+NAA0.2 | 103 ± 6 | 114 ± 8 | 21.0 ± 1.9 | 238 ± 15 | |
| Significance | nsb | ns | ns | ns | |
| Shoots | Control | 89 ± 5 | 65 ± 5 b | 1.3 ± 0.5 b | 155 ± 10 |
| B5 | 95 ± 2 | 71 ± 2 ab | 1.8 ± 0.2 b | 168 ± 40 | |
| B10 | 99 ± 4 | 86 ± 4 a | 2.0 ± 0.4 b | 169 ± 7 | |
| IBA+NAA0.2 | 104 ± 7 | 78 ± 4 ab | 6.5 ± 1.6 a | 188 ± 13 | |
| Significance | nsb | * | ** | ns | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Ku, K.-M.; Choi, S.; Cardarelli, M. Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes. Agronomy 2019, 9, 74. https://doi.org/10.3390/agronomy9020074
Kim H-J, Ku K-M, Choi S, Cardarelli M. Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes. Agronomy. 2019; 9(2):74. https://doi.org/10.3390/agronomy9020074
Chicago/Turabian StyleKim, Hye-Ji, Kang-Mo Ku, Seunghyun Choi, and Mariateresa Cardarelli. 2019. "Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes" Agronomy 9, no. 2: 74. https://doi.org/10.3390/agronomy9020074
APA StyleKim, H.-J., Ku, K.-M., Choi, S., & Cardarelli, M. (2019). Vegetal-Derived Biostimulant Enhances Adventitious Rooting in Cuttings of Basil, Tomato, and Chrysanthemum via Brassinosteroid-Mediated Processes. Agronomy, 9(2), 74. https://doi.org/10.3390/agronomy9020074








