Ultraviolet Radiation Effect on Seed Germination and Seedling Growth of Common Species from Northeastern Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. UV-B and UV-C Radiation Doses
2.2. Standard Germination Tests
2.3. Accelerated Aging Test and Vigor of Seedlings
2.4. Experimental Design and Statistical Data Analysis
3. Results
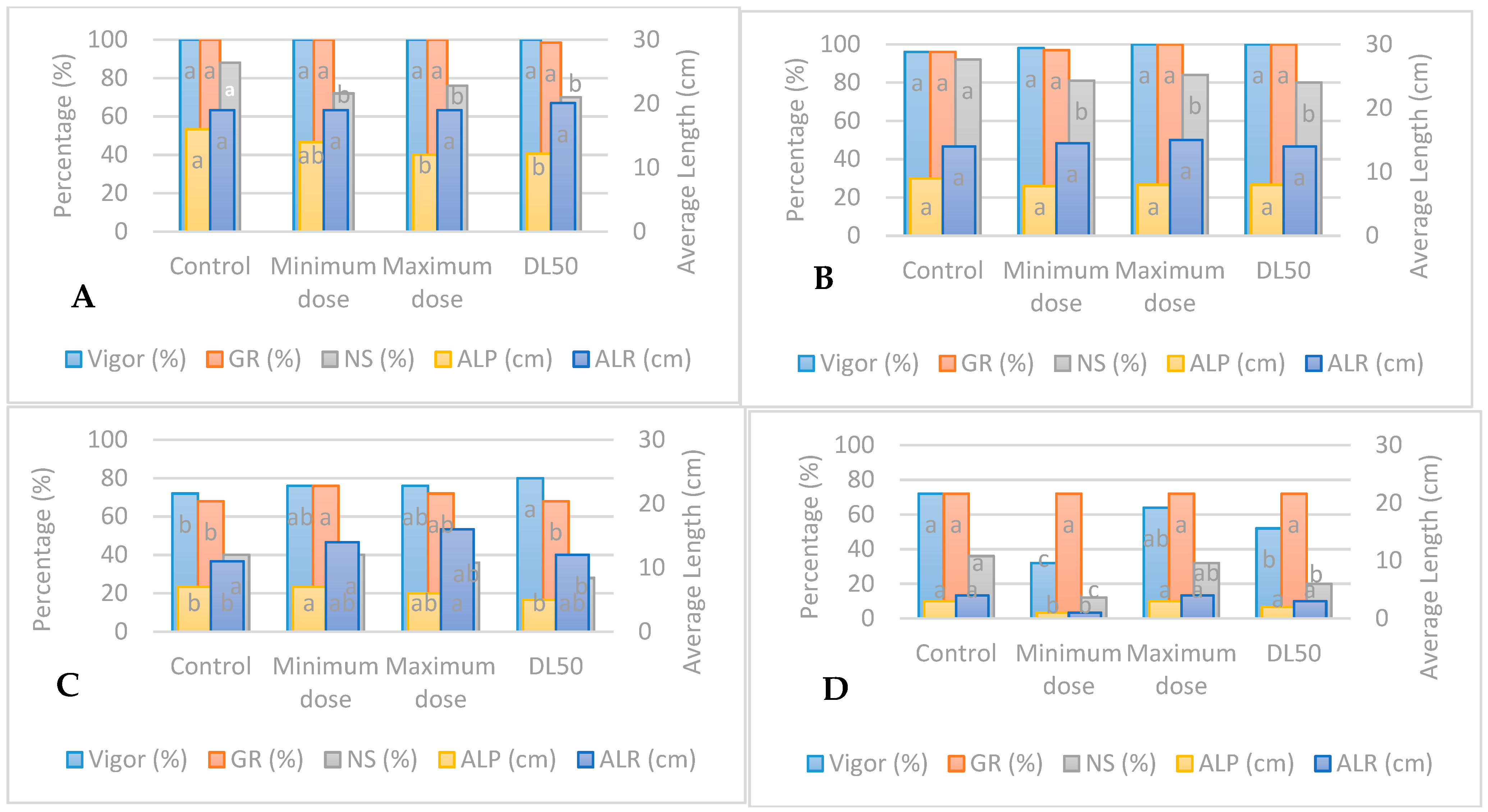
3.1. Standard Germination Test under UV-B Radiation
3.2. Accelerated Aging Test under UV-B Radiation
3.3. Standard Germination under UV-C
3.4. Accelerated Aging Test under UV-C Radiation
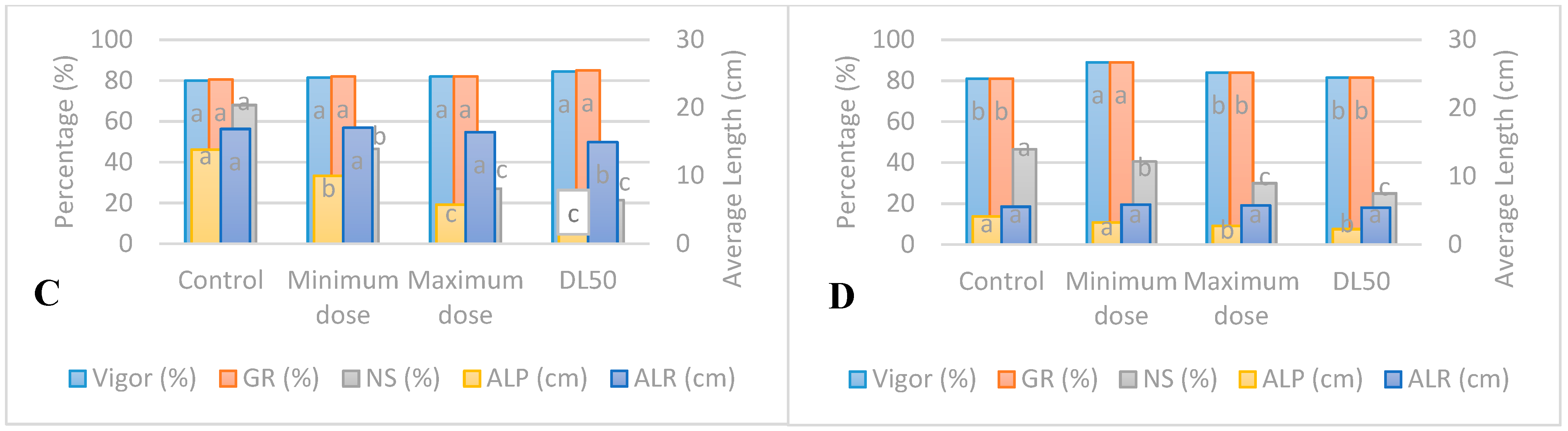
3.5. Responses of Seedlings to UV-B and UV-C Radiations
4. Discussion
4.1. Standard Germination of Seeds Irradiated with UV-B
4.2. Standard Germination of Seeds Irradiated with UV-C
4.3. Accelerated Aging of Seedlings from Seeds Irradiated with UV-B
4.4. Accelerated Aging of Seedlings from Seeds Irradiated with UV-C
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kerr, J.B.; Mcelroy, C.T. Evidence for large upward trends of Ultraviolet-B radiation linked to ozone depletion. Science 1993, 262, 1032–1034. [Google Scholar] [CrossRef] [PubMed]
- Winter, T.R.; Rostas, M. Ambient ultraviolet radiation induces protective responses in soybean but does not attenuate indirect defense. Environ. Pollut. 2008, 155, 290–297. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M. Irradiación de frutillas con UV-C: Efecto sobre la síntesis de proteínas, degradación de la pared celular y mecanismos de defensa. Doctoral Thesis, Universidad Nacional de San Martín (UNSAM), Laboratorio de Bioquímica y Fisiología de la Maduración y Senescencia (UB-4) IIB-INTECH (Chascomús), San Martín, Argentina, 2009. [Google Scholar]
- Nawkar, G.M.; Maibam, P.; Parque, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-Induced Cell Death in Plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef] [Green Version]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A.K. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef]
- Sarghein, S.H.; Carapetian, J.; Khara, J. The Effects of UV Radiation on Some Structural and Ultrastructural Parameters in Pepper (Capsicum longum A.DC.). Turk. J. Biol. 2011, 35, 69–77. [Google Scholar]
- Yagura, T.; Makita, K.; Yamamoto, H.; Menck, C.F.; Schuch, A.P. Biological Sensors for Solar Ultraviolet Radiation. Sensors 2011, 11, 4277–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, L.G.A.; Qüesta, A.G.; Rodríguez, S.C. Efecto de luz UV-C sobre las propiedades antioxidantes y calidad sensorial de repollo mínimamente procesado. Rev. Iber. Tecnología Postcosecha 2010, 11, 101–108. [Google Scholar]
- Sgroppo, S.C.; Sosa, C.A. Zapallo anco (Cucurbita moschata, D.) fresco cortado tratado con luz UV-C. Facena 2009, 25, 7–19. [Google Scholar]
- Fonseca, J. Efecto de la luz UV-C en la calidad de hortalizas. Revista Productores de Hortalizas 2009, 2009. [Google Scholar]
- Rastogi, R.P.; Kumar, A.R.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 32. [Google Scholar] [CrossRef] [PubMed]
- Luckey, T.D. Hormesis with Ionizing Radiation; CRC press: Boca Rato, FL, USA, 1980; p. 222. [Google Scholar]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 32. [Google Scholar] [CrossRef]
- Rivera, P.D.M.; Gardea, B.M.; Martínez, T.A.; Rivera, D.M.; González, A. Efectos Bioquímicos postcosecha de la irradiación UV-C en frutas y hortalizas. Rev. Fitotec. Méx. 2007, 30, 361–372. [Google Scholar]
- Häder, D.P.; Koumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285. [Google Scholar] [CrossRef]
- Castronuovo, D.; Sofo, A.; Lovelli, S.; Candido, V.; Scopa, A. Effects of UV-C radiation on common dandelion and purple coneflower: First results. Int. J. Plant Biol. 2017, 8, 7255. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Solar UV-B and UVA/B exclusion effects on intraspecific variations in crop growth and yield of wheat varieties. Field Crops Res. 2012, 125, 8–13. [Google Scholar] [CrossRef]
- Brown, J.E.; Lu, T.Y.; Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Collins, D.J.; Wilson, M.A.; Igwegbe, E.C.K.; Chalutz, E.; et al. The effect of low dose ultraviolet light-C seed treatment on induced resistance in cabbage to black rot (Xanthomonas campestris pv. campestris). Crop Prot. 2001, 20, 873–883. [Google Scholar] [CrossRef]
- Moreno, E.M. Análisis Físico y Fisiológico de Semillas Agrícolas, 3rd ed.; UNAM, Ciudad Universitaria: México, D.F., Mexico, 1996; p. 393. [Google Scholar]
- International Seed Testing Association (ISTA). International Rules for Seed Testing 1999, 2nd ed.; Seed Science and Technology 27, Supplement of vigor test methods, ISTA: Zurich, Switzerland, 1999; p. 117. [Google Scholar]
- López, R.V.; Artés, H.F.D.A.; Artés, C.F. Evaluación de la calidad de granadas tratadas con UV-C y almacenadas en atmósfera controlada. V Congreso iberoamericano de tecnología postcosecha y agroexportaciones. 2007, pp. 137–145. Available online: https://www.researchgate.net/publication/36720755_Evaluacion_de_la_calidad_de_granadas_tratadas_con_UV-C_y_almacenadas_en_atmosfera_controlada (accessed on 15 November 2018).
- Huron. Foro del Parque Natural de las Sierras de Cazorla, Segura y las Villas. Reproducción de semillas de las principales especies de pinos del parque. February 2009. Available online: www.cazorlaturismo.com (accessed on 15 November 2018).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall, Inc.: Saddle River, NJ, USA, 2010; p. 947. [Google Scholar]
- Ma, M.; Wang, P.; Yang, R.; Gu, Z. Effects of UV-B radiation on the isoflavone accumulation and physiological-biochemical changes of soybean during germination: Physiological-biochemical change of germinated soybean induced by UV-B. Food Chem. 2018, 250, 259–267. [Google Scholar] [CrossRef]
- Shinkle, J.R.; Atkins, A.K.; Humphrey, E.E.; Rodgers, C.W.; Wheeler, S.L.; Barnes, P.W. Growth and morphological responses to different UV wavebands in cucumber (Cucumis sativum) and other dicotyledonous seedlings. Physiol. Plant. 2004, 120, 240–248. [Google Scholar] [CrossRef]
- Lizana, X.C.; Hess, S.; Calderini, D.F. Crop phenology modifies wheat responses to increased UV-B radiation. Agric. For. Meteorol. 2009, 149, 1964–1974. [Google Scholar] [CrossRef]
- Mazza, C.A.; Boccalandro, H.E.; Giordano, C.V.; Ballaré, C.L. Significación funcional y de inducción de la radiación solar ultravioleta de protectores solares que absorben en los cultivos de soja cultivadas en el campo. Plant Physiol. 2000, 122, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Selezneva, E.M.; Goncharova, L.I.; Tikhonov, V.N. The effects of UV-irradiation of barley plants on the morphophysiological parameters and productivity of the offsprings. Radiats Biol. Radioecol. 2008, 48, 487–492. [Google Scholar] [PubMed]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Luo, M.; Gu, C.B.; Fu, Y.J.; Ma, W. Ultraviolet radiation-elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in Astragalus membranaceus hairy root cultures. J. Agric. Food Chem. 2015, 63, 8216–8224. [Google Scholar] [CrossRef]
- Jansen, M.A.K. Ultraviolet-B radiation effects on plants: Induction of morphogenic responses. Physiol. Plant. A. 2002, 116, 423–429. [Google Scholar] [CrossRef]
- Turtola, S.; Sallas, L.; Holopainen, J.K.; Julkunen-Tiitto, R.; Kainulainen, P. La exposición prolongada a la radiación UV-B mejorada no tiene efectos significativos sobre el crecimiento o compuestos secundarios de pino Silvestre y exterior crecido y Noruega plántulas de piceas. Environ. Contam. 2006, 144, 166–171. [Google Scholar]
- Kanash, E.V.; Savin, V.N. The sensitivity of agricultural plants to short-term UV-stress. Kosm. Biol. Aviakosm. Med. 1991, 25, 18–20. [Google Scholar] [PubMed]
- Thomas, T.T.D.; Puthur, J.T. UV radiation priming: A means of amplifying the inherent potential for abiotic stress tolerance in crop plants. Environ. Exp. Bot. 2017, 138, 57–66. [Google Scholar] [CrossRef]
- Aguirrezabal, L.A.N.; Orioli, G.A.; Hernandez, L.; Pereyra, V.R.; Mirave, J.P. Girasol: Aspectos Fisiológicos que Determinan el Rendimiento; Unidad Integrada Balcarce (ISBN N°950-9853-71-2). Offset Vega: Buenos Aires, Argentina, 1996; p. 127. [Google Scholar]
- Neelamegam, R.; Sutha, T. UV-C irradiation effect on seed germination, seedling growth and productivity of groundnut (Arachis hypogaea L.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 430–443. [Google Scholar]
- Reglinski, T.; Taylor, J.T.; Chee, A.A.; Northcott, G.; Spiers, M. Biochemical responses to Ultraviolet-C radiation and methyl jasmonate in Pinus radiata seedlings that accompany induced resistance to Diplodia pinea. Plant Pathol. 2012, 62, 851–858. [Google Scholar] [CrossRef]
- Torres, M.; Frutos, G.; Duran, J.M. Sunflower seed deterioration from exposure to U.V.-C radiation. Environ. Exp. Bot. 1991, 31, 201–207. [Google Scholar] [CrossRef]
- Cerero, H.N. Girasol, situación actual, mundial y nacional. CONASIPRO. Available online: http://www.oleaginosas.org/art_237.shtml (accessed on 7 January 2019).
- Tripathi, R.; Rai, K.; Singh, S.; Agrawal, M.; Agrawal, S.B. Role of supplemental UV-B in changing the level of ozone toxicity in two cultivars of sunflower: Growth, seed yield and oil quality. Ecotoxicology 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cechin, I.; De Fátima, F.T.; Ligia Dokkedal, A. Crecimiento y respuestas fisiológicas de las plantas de girasol expuestos a la radiación ultravioleta-B. Universidad Federal de Santa Maria. Centro de Ciencias Rurais. Cienc. Rural 2007, 37. [Google Scholar]
- Boccalandro, H.E.; Mazza, C.A.; Ballaré, C.L. La radiación ultravioleta B mejora la respuesta photomorphogenic fitocromo-B mediada en Arabidopsis. Plant Physiol. 2001, 126, 780–788. [Google Scholar] [CrossRef]
- Beena, A.K.; Jayaram, K.M. Electrophoretic variations in seed protein profile of green pea (Pisum Sativum L.) and soybean [Glycine max (L.) MERR.] seedlings during early stages of germination under heat-stress. Legume Res. 2010, 33, 171–177. [Google Scholar]
- Minuzzi, A.; Mora, F.; Sedrez Rangel, M.A.; Scapim, C.A. Características Fisiológicas, Contenido de Aceite y Proteína en Genotipos de Soya, Evaluadas en Diferentes Sitios y Épocas de Cosecha, Brasil. Agric. Téc. 2007, 67, 353–361. [Google Scholar] [CrossRef]
- Rai, R.; Meena, R.P.; Smita, S.S.; Shukla, A.; Rai, S.K.; Pandey-Rai, S. UV-B and UV-C pre-treatments induce physiological changes and artemisinin biosynthesis in Artemisia annua L. an antimalarial plant. J. Photochem Photobiol B. 2011, 105, 216–225. [Google Scholar] [CrossRef]
- Erdoğdu, S.B.; Ekiz, H.I. Effect of Ultraviolet and Far Infrared Radiation on Microbial Decontamination and Quality of Cumin Seeds. J. Food Sci. 2011, 76, M284–M292. [Google Scholar] [CrossRef]
- Mpoloka, S.W. Effects of prolonged UV-B exposure in plants. Afr. J. Biotechnol. 2008, 7, 4874–4883. [Google Scholar] [CrossRef]
- Deng, N.; Liu, C.; Chang, E.; Ji, J.; Yao, X.; Yue, J.; Bartish, I.V.; Chen, L.; Jiang, Z.; Shi, S. High temperature and UV-C treatments affect stilbenoid accumulation and related gene expression levels in Gnetum parvifolium. Electron. J. Biotechnol. 2017, 25, 43–49. [Google Scholar] [CrossRef]
- Rocha, A.B.; Honório, S.L.; Messias, C.L.; Otón, M.; Gómez, P.A. Effect of UV-C radiation and fluorescent light to control postharvest soft rot in potato seed tubers. Sci. Hortic. 2015, 181, 174–181. [Google Scholar] [CrossRef]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants, growth, antioxidant activity and phenolic compounds. Plant Phys. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef]






| Species | UV Radiation Type | Treatments | ||||||
|---|---|---|---|---|---|---|---|---|
| Control (T1) | Minimum Dose (T2) | Maximum Dose (T3) | Lethal Dose (T4) | |||||
| kJ/m2 | Time (s) | kJ/m2 | Time (s) | kJ/m2 | Time (s) | |||
| Glycine max | UV-B | Without UV radiation | 5.4 | 3600 | 43.2 | 28800 | 56.7 | 37800 |
| UV-C | Without UV radiation | 1.44 | 900 | 43.2 | 27000 | 57.60 | 36000 | |
| Triticum aestivum | UV-B | Without UV radiation | 1.35 | 900 | 8.1 | 5400 | 37.8 | 25200 |
| UV-C | Without UV radiation | 2.88 | 1800 | 17.28 | 10800 | 43.2 | 27000 | |
| Helianthus annuus | UV-B | Without UV radiation | 1.35 | 900 | 0.27 | 180 | 8.1 | 5400 |
| UV-C | Without UV radiation | 2.88 | 1800 | 8.64 | 5400 | 43.2 | 27000 | |
| Pinus maximartinezii | UV-B | Without UV radiation | 0.63 | 420 | 0.99 | 660 | 1.17 | 780 |
| UV-C | Without UV radiation | 0.288 | 180 | 0.864 | 540 | 1.056 | 660 | |
| Source of variation | Df | Vigor (%) | Fcal | GR | Fcal | NS | Fcal | ALP | Fcal | ALR | Fcal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 3 | 3.80 | 2.01 | 3.35 | 1.58 | 272.30 | 52.61 ** | 67.30 | 25.93 ** | 8.69 | 3.62 ** |
| Species | 3 | 64.34 | 33.98 ** | 61.77 | 29.1 ** | 470.28 | 90.87 ** | 121.83 | 46.94 ** | 907.62 | 378.68 ** |
| T×S | 9 | 1.21 | 0.64 | 0.98 | 0.46 | 46.57 | 9.00 ** | 17.28 | 6.66 ** | 11.62 | 4.85 ** |
| S.E. | 1.89 | 2.12 | 5.18 | 2.60 | 2.40 | ||||||
| C.V.% | 5.84 | 6.19 | 15.51 | 21.36 | 10.73 |
| Source of Variation | Df | Vigor (%) | F | GR (%) | F | NS (%) | F | ALP (cm) | F | ALR (cm) | F |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 3 | 1.84 | 0.62 | 3.13 | 1.14 | 456.75 | 92.81 ** | 134.78 | 91.95 ** | 7.36 | 1.54 |
| Species | 3 | 106.80 | 35.89 ** | 120.97 | 44.05 ** | 890.11 | 180.8 ** | 161.49 | 110.17 ** | 784.31 | 164.23 ** |
| T×S | 9 | 3.07 | 1.03 | 2.64 | 0.96 | 76.00 | 15.44 ** | 39.66 | 27.06 ** | 6.46 | 1.35 |
| S.E. | 2.97 | 2.74 | 4.92 | 1.46 | 4.77 | ||||||
| C.V.% | 7.74 | 7.38 | 18.43 | 20.64 | 16.56 |
| Source of Variation | Df | Vigor (%) | Fcal | GR (%) | Fcal | NS | Fcal | ALP | Fcal | ALR | Fcal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 3 | 27.65 | 6.36 ** | 25.84 | 5.47 ** | 75.55 | 13.55 ** | 22.71 | 9.77 ** | 14.53 | 2.78 ** |
| Species | 3 | 913.47 | 210.02 ** | 940.64 | 199.2 ** | 1698.63 | 304.7 ** | 729.99 | 313.9 ** | 1473.10 | 2818 ** |
| T×S | 9 | 37.65 | 8.66 ** | 39.11 | 8.29 ** | 15.40 | 2.76 ** | 7.86 | 3.38 ** | 14.02 | 2.68 ** |
| S.E. | 4.35 | 4.72 | 5.57 | 2.33 | 5.23 | ||||||
| C.V.% | 10.14 | 10.77 | 17.16 | 20.22 | 18.27 |
| Source of variation | Df | Vigor % | Fcal | GR % | Fcal | NS | Fcal | ALP | Fcal | ALR | Fcal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 3 | 30.20 | 3.47 ** | 26.09 | 3.32 * | 127.65 | 2.87 * | 278609 | 4.95 ** | 10.76 | 0.30 |
| Species | 3 | 3907.88 | 448.52 ** | 3831.69 | 488.05 ** | 0 | 0 | 0.37 | 0.07 | 1080.58 | 30.62 ** |
| T×S | 9 | 121.25 | 13.92 ** | 127.24 | 16.21 ** | 54.51 | 1.22 | 14.17 | 2.52 | 2.05 | 0.06 |
| S.E. | 8.7 | 7.85 | 44.55 | 5.63 | 35.29 | ||||||
| C.V.% | 15.84 | 15.11 | 73.90 | 59.00 | 61.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foroughbakhch Pournavab, R.; Bacópulos Mejía, E.; Benavides Mendoza, A.; Salas Cruz, L.R.; Ngangyo Heya, M. Ultraviolet Radiation Effect on Seed Germination and Seedling Growth of Common Species from Northeastern Mexico. Agronomy 2019, 9, 269. https://doi.org/10.3390/agronomy9060269
Foroughbakhch Pournavab R, Bacópulos Mejía E, Benavides Mendoza A, Salas Cruz LR, Ngangyo Heya M. Ultraviolet Radiation Effect on Seed Germination and Seedling Growth of Common Species from Northeastern Mexico. Agronomy. 2019; 9(6):269. https://doi.org/10.3390/agronomy9060269
Chicago/Turabian StyleForoughbakhch Pournavab, Rahim, Elly Bacópulos Mejía, Adalberto Benavides Mendoza, Lidia Rosaura Salas Cruz, and Maginot Ngangyo Heya. 2019. "Ultraviolet Radiation Effect on Seed Germination and Seedling Growth of Common Species from Northeastern Mexico" Agronomy 9, no. 6: 269. https://doi.org/10.3390/agronomy9060269






