Stability of Intracellular Protein Concentration under Extreme Osmotic Challenge
Abstract
:1. Introduction
2. Reagents and Methods
2.1. Cell Culture
2.2. Cell Volume and Protein Concentration Measurements
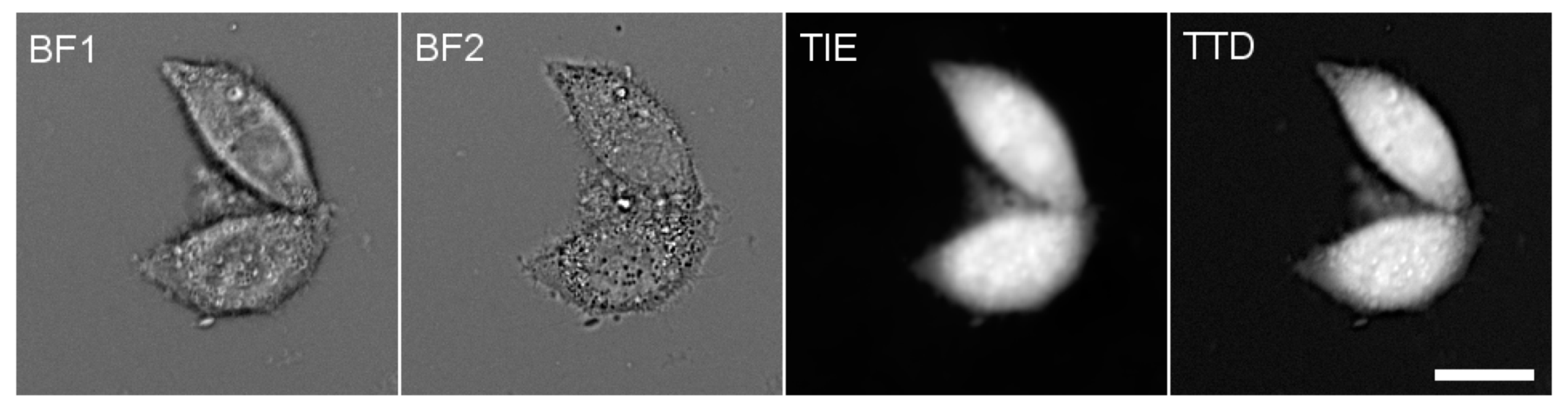
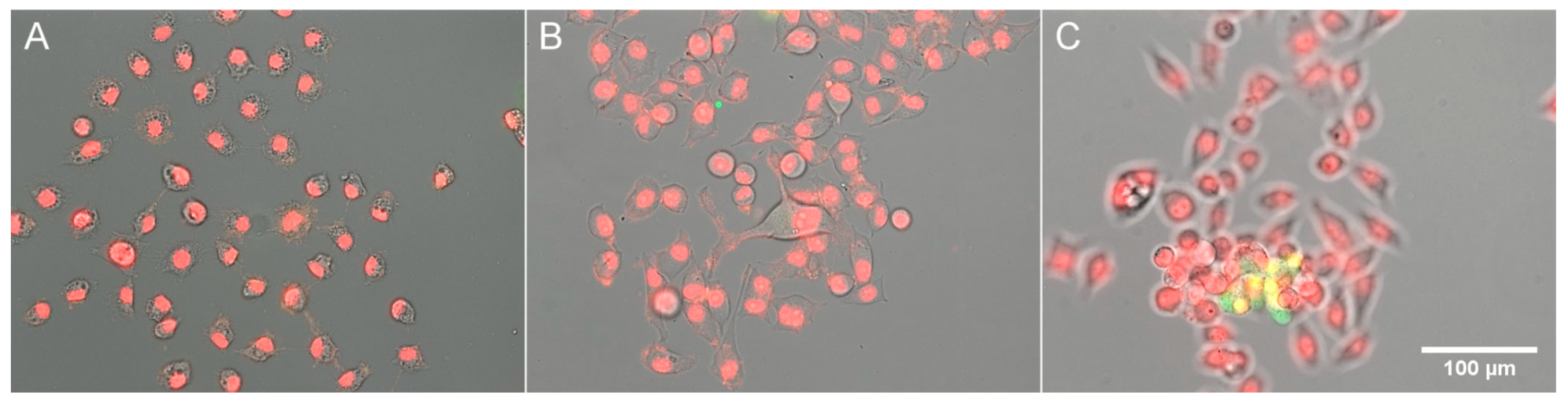
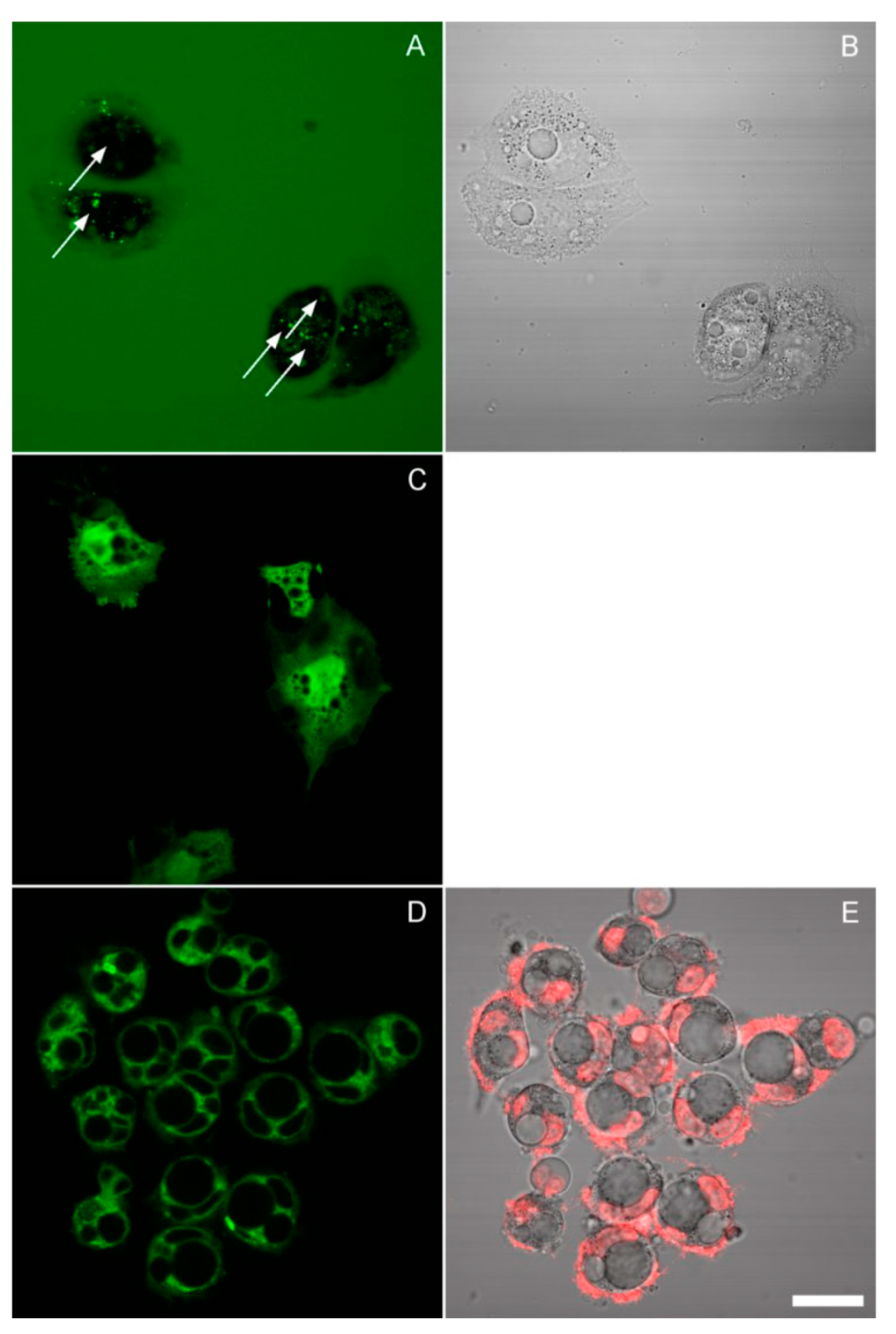
2.3. Fluorescence Microscopy
2.4. Estimation of Cell Survival
3. Results
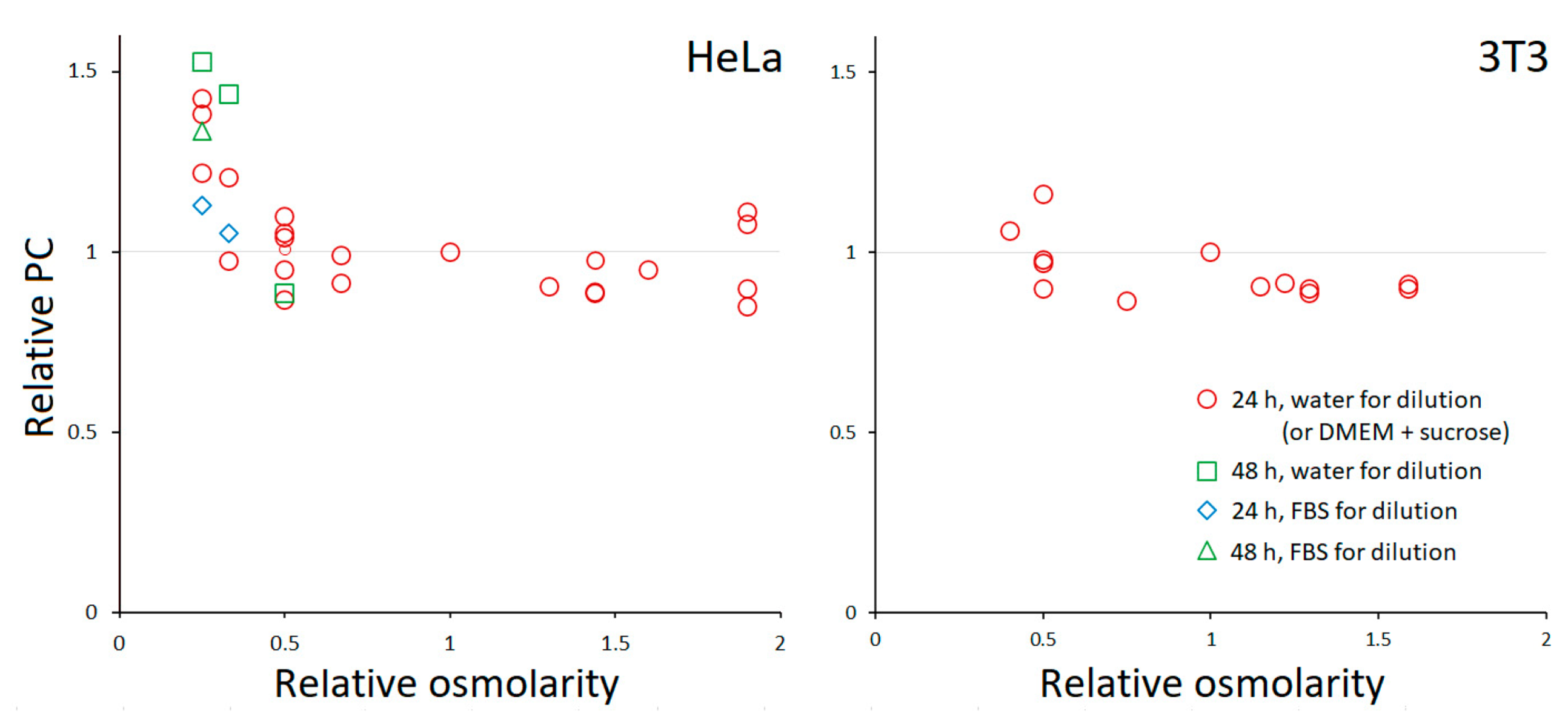
3.1. PC Homeostasis
3.2. Response of HeLa to Extreme Osmolarities
3.3. The Effect of Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 2001, 532, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Model, M.A.; Petruccelli, J.C. Intracellular macromolecules in cell volume control and methods of their quantification. Curr. Top. Membr. 2018, 81, 237–289. [Google Scholar]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Habori, M. Macromolecular crowding and its role as intracellular signalling of cell volume regulation. Int. J. Biochem. Cell Biol. 2001, 33, 844–864. [Google Scholar] [CrossRef]
- Zhou, H.X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef] [Green Version]
- Model, M.A.; Hollembeak, J.E.; Kurokawa, M. Macromolecular crowding: A hidden link between cell volume and everything else. Cell Physiol Biochem. 2021, 55 (Suppl. S1), 25–40. [Google Scholar]
- Liu, B.; Hasrat, Z.; Poolman, B.; Boersma, A.J. Decreased effective macromolecular crowding in Escherichia coli adapted to hyperosmotic stress. J. Bacteriol. 2019, 201, e00708-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, P.S.; Kurokawa, M.; Model, M.A. Evidence for macromolecular crowding as a direct apoptotic stimulus. J. Cell Sci. 2020, 133, jcs243931. [Google Scholar] [CrossRef]
- Mudrak, N.J.; Rana, P.S.; Model, M.A. Calibrated brightfield-based imaging for measuring intracellular protein concentration. Cytom. Part A 2018, 93, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Model, M.A.; Mudrak, N.J.; Rana, P.S.; Clements, R.J. Staurosporine-induced apoptotic water loss is cell-and attachment-specific. Apoptosis 2018, 23, 449–455. [Google Scholar] [CrossRef]
- Barty, A.; Nugent, K.A.; Paganin, D.; Roberts, A. Quantitative optical phase microscopy. Opt. Lett. 1998, 23, 817–819. [Google Scholar] [CrossRef]
- Zuo, C.; Li, J.; Sun, J.; Fan, Y.; Zhang, J.; Lu, L.; Zhang, R.; Wang, B.; Huang, L.; Chen, Q. Transport of intensity equation: A tutorial. Opt. Lasers Eng. 2020, 135, 106187. [Google Scholar] [CrossRef]
- Model, M.A.; Khitrin, A.K.; Blank, J.L. Measurement of the absorption of concentrated dyes and their use for quantitative imaging of surface topography. J. Microsc. 2008, 231, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Khitrin, A.K.; Petruccelli, J.C.; Model, M.A. Bright-field microscopy of transparent objects: A ray tracing approach. Microsc. Microanal. 2017, 23, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Gorthi, S.S.; Schonbrun, E. Phase imaging flow cytometry using a focus-stack collecting microscope. Opt. Lett. 2012, 37, 707–709. [Google Scholar] [CrossRef]
- Stutzin, A.; Torres, R.; Oporto, M.; Pacheco, P.; Eguiguren, A.L.; Cid, L.P.; Sepúlveda, F.V. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am. J. Physiol. Cell Physiol. 1999, 277, C392–C402. [Google Scholar] [CrossRef]
- Hermoso, M.; Olivero, P.; Torres, R.; Riveros, A.; Quest, A.F.; Stutzin, A. Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase C α mutant lacking kinase activity. J. Biol. Chem. 2004, 279, 17681–17689. [Google Scholar] [CrossRef] [Green Version]
- Tivey, D.R.; Simmons, N.L.; Aiton, J.F. Role of passive potassium fluxes in cell volume regulation in cultured HeLa cells. J. Membr. Biol. 1985, 87, 93–105. [Google Scholar] [CrossRef]
- Andersen, O.S. Gramicidin channels. Annu. Rev. Physiol. 1984, 46, 531–548. [Google Scholar] [CrossRef]
- Maltese, W.A.; Overmeyer, J.E.H. Methuosis: Nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 2014, 184, 1630–1642. [Google Scholar] [CrossRef] [Green Version]
- Ritter, M.; Bresgen, N.; Kerschbaum, H.H. From Pinocytosis to Methuosis—Fluid Consumption as a Risk Factor for Cell Death. Front. Cell Dev. Biol. 2021, 9, 651982. [Google Scholar] [CrossRef]
- Rana, P.S.; Mudrak, N.J.; Lopez, R.; Lynn, M.; Kershner, L.; Model, M.A. Phase separation in necrotic cells. Biochem. Biophys. Res. Commun. 2017, 492, 300–303. [Google Scholar] [CrossRef]
- Ozturk, S.S.; Palsson, B.Ø. Effect of medium osmolarity on hybridoma growth, metabolism, and antibody production. Biotechnol. Bioeng. 1991, 37, 989–993. [Google Scholar] [CrossRef] [Green Version]
- Takagi, M.; Hayashi, H.; Yoshida, T. The effect of osmolarity on metabolism and morphology in adhesion and suspension chinese hamster ovary cells producing tissue plasminogen activator. Cytotechnology 2000, 32, 171–179. [Google Scholar] [CrossRef]
- Boersma, A.J.; Zuhorn, I.S.; Poolman, B. A sensor for quantification of macromolecular crowding in living cells. Nat. Methods 2015, 12, 227–229. [Google Scholar] [CrossRef]
- Guigas, G.; Kalla, C.; Weiss, M. Probing the nanoscale viscoelasticity of intracellular fluids in living cells. Biophys. J. 2007, 93, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, J.; Boersma, A.J.; Poolman, B. Microorganisms maintain crowding homeostasis. Nat. Rev. Microbiol. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am. J. Physiol. Cell Physiol. 1996, 271, C950–C961. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Amon, A. Relevance and regulation of cell density. Trends Cell Biol. 2020, 30, 213–225. [Google Scholar] [CrossRef]
- Minton, A.P. Water loss in aging erythrocytes provides a clue to a general mechanism of cellular senescence. Biophys. J. 2020, 119, 2039–2044. [Google Scholar] [CrossRef]
- Fraser, J.A.; Huang, C.L.H. Quantitative techniques for steady-state calculation and dynamic integrated modelling of membrane potential and intracellular ion concentrations. Prog. Biophys. Mol. Biol. 2007, 94, 336–372. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflügers Arch. 2004, 448, 313–318. [Google Scholar] [CrossRef]
- Model, M.A. Possible causes of apoptotic volume decrease: An attempt at quantitative review. Am. J. Physiol. Cell Physiol. 2014, 306, C417–C424. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.P. Regulation and critical role of potassium homeostasis in apoptosis. Prog. Neurobiol. 2003, 70, 363–386. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. Role of ion transport in control of apoptotic cell death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar]
- Kunzelmann, K. Ion channels in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2387–2403. [Google Scholar] [CrossRef]
- Rana, P.S.; Model, M.A. A reverse-osmosis model of apoptotic shrinkage. Front. Cell Dev. Biol. 2020, 8, 588721. [Google Scholar] [CrossRef]
- Nunez, R.; Sancho-Martinez, S.M.; Novoa, J.M.L.; Lopez-Hernandez, F.J. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 2010, 17, 1665–1671. [Google Scholar] [CrossRef]
- Yurinskaya, V.E.; Moshkov, A.V.; Wibberley, A.V.; Lang, F.; Model, M.A.; Vereninov, A.A. Dual response of human leukemia U937 cells to hypertonic shrinkage: Initial regulatory volume increase (RVI) and delayed apoptotic volume decrease (AVD). Cell. Physiol. Biochem. 2012, 30, 964–973. [Google Scholar] [CrossRef]
- Maeno, E.; Ishizaki, Y.; Kanaseki, T.; Hazama, A.; Okada, Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9487–9492. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl. Acad. Sci. USA 2015, 112, 15790–15797. [Google Scholar] [CrossRef] [Green Version]
- Friard, J.; Tauc, M.; Cougnon, M.; Compan, V.; Duranton, C.; Rubera, I. Comparative effects of chloride channel inhibitors on LRRC8/VRAC-mediated chloride conductance. Front. Pharmacol. 2017, 8, 328. [Google Scholar] [CrossRef] [Green Version]


| Gramicidin Concentration (μM) | PC (Relative) | ||
|---|---|---|---|
| 25 min | 1 h | 24 h | |
| 0.1 | 0.93 | 0.945 | 1.085 ± 0.054 (3) |
| 0.5 | 0.87 | 0.99 | 1.121 ± 0.122 (3) |
| 2.5 | 0.71 | 0.60 | 1.234 ± 0.135 (3) |
| Inhibitor | PC (Relative) Mean ± SD (n) |
|---|---|
| torin 1 (20–100 nM) or rapamycin (100 nM) | 0.93 + 0.24 (6) |
| DCPIB (10 μM) or DIDS (0.2–1 mM) | 0.99 + 0.17 (5) |
| TEA (10 mM) | 0.95 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollembeak, J.E.; Model, M.A. Stability of Intracellular Protein Concentration under Extreme Osmotic Challenge. Cells 2021, 10, 3532. https://doi.org/10.3390/cells10123532
Hollembeak JE, Model MA. Stability of Intracellular Protein Concentration under Extreme Osmotic Challenge. Cells. 2021; 10(12):3532. https://doi.org/10.3390/cells10123532
Chicago/Turabian StyleHollembeak, Jordan E., and Michael A. Model. 2021. "Stability of Intracellular Protein Concentration under Extreme Osmotic Challenge" Cells 10, no. 12: 3532. https://doi.org/10.3390/cells10123532
APA StyleHollembeak, J. E., & Model, M. A. (2021). Stability of Intracellular Protein Concentration under Extreme Osmotic Challenge. Cells, 10(12), 3532. https://doi.org/10.3390/cells10123532





