Molecular-Morphological Relationships of the Scaffold Protein FKBP51 and Inflammatory Processes in Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Samples
2.3. Immunohistochemistry
2.4. Microscopy
2.5. Antibodies
2.6. Secondary Antibodies
2.7. Image Analysis and Statistics
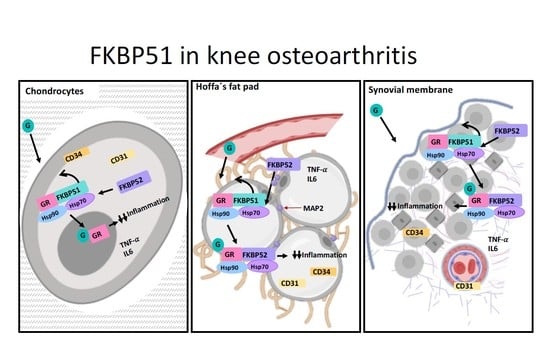
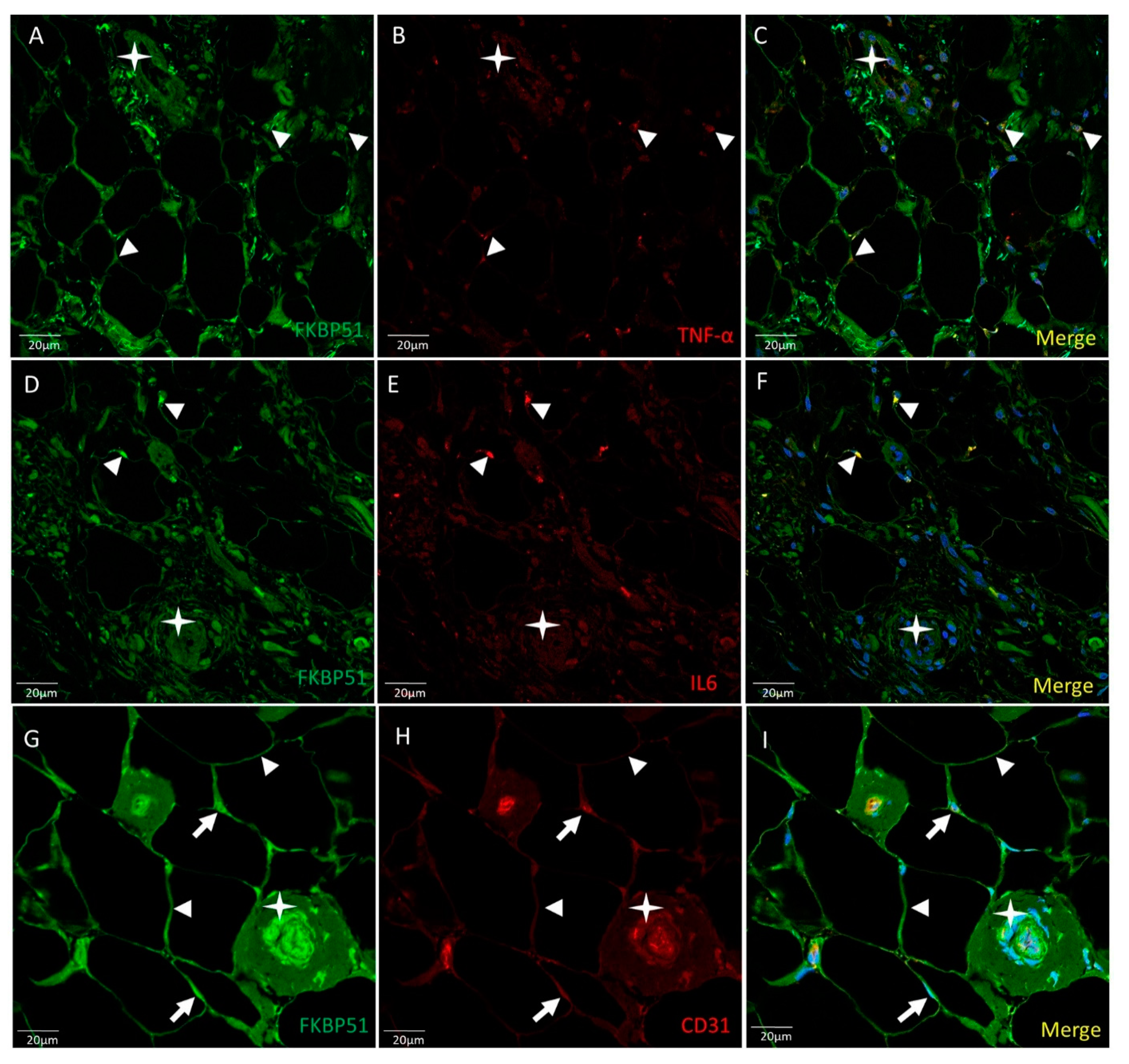
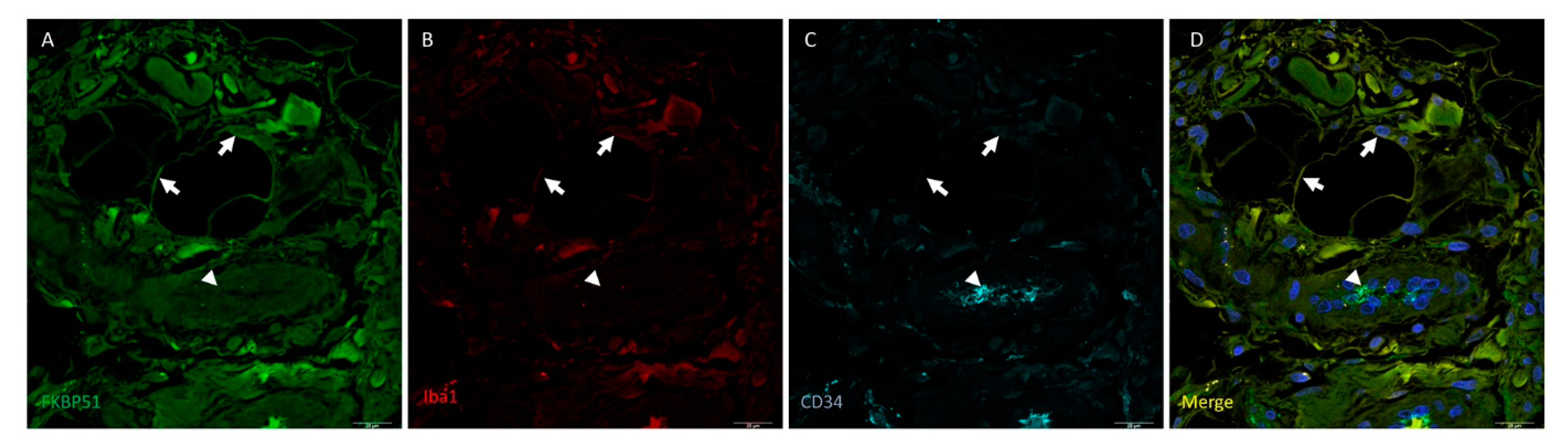
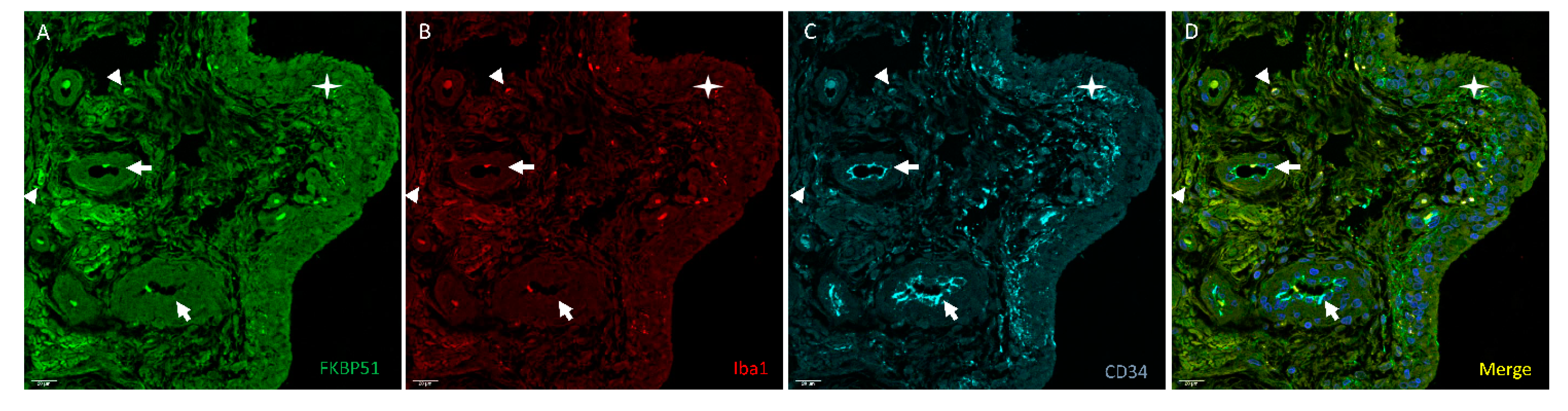
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Suri, P.; Morgenroth, D.C.; Hunter, D.J. Epidemiology of osteoarthritis and associated comorbidities. PM R 2012, 4, S10–S19. [Google Scholar] [CrossRef]
- Duarte, N.; Rodrigues, A.M.; Branco, J.D.C.; Canhão, H.; Hughes, S.L.; Paúl, C. Health and Lifestyles Factors Associated With Osteoarthritis among Older Adults in Portugal. Front. Med. 2017, 4, 192. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [Green Version]
- Bliddal, H.; Christensen, R. The treatment and prevention of knee osteoarthritis: A tool for clinical decision-making. Expert Opin. Pharmacother. 2009, 10, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.-C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Liu, S.-C.; Su, C.-M.; Wang, Y.-H.; Tsai, C.-H.; Tang, C.-H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef]
- Benihoud, K.; Esselin, S.; Descamps, D.; Jullienne, B.; Salone, B.; Bobé, P.; Bonardelle, D.; Connault, E.; Opolon, P.; Saggio, I.; et al. Respective roles of TNF-alpha and IL-6 in the immune response-elicited by adenovirus-mediated gene transfer in mice. Gene Ther. 2007, 14, 533–544. [Google Scholar] [CrossRef]
- Rakocevic, J.; Orlic, D.; Mitrovic-Ajtic, O.; Tomasevic, M.; Dobric, M.; Zlatic, N.; Milasinovic, D.; Stankovic, G.; Ostojić, M.; Labudovic-Borovic, M. Endothelial cell markers from clinician’s perspective. Exp. Mol. Pathol. 2017, 102, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.P.; Díaz-Flores, L.J.; Carrasco, J.L.; Martín-Vasallo, P. CD34+ Stromal Cells/Telocytes as a Source of Cancer-Associated Fibroblasts (CAFs) in Invasive Lobular Carcinoma of the Breast. Int. J. Mol. Sci. 2021, 22, 3686. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.J.; To, B.; White, I.; Millecamps, M.; Beier, F.; Grol, M.W.; Stone, L.S.; Séguin, C.A. Diet-induced obesity leads to behavioral indicators of pain preceding structural joint damage in wild-type mice. Arthritis Res. Ther. 2021, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [Green Version]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Porzionato, A.; Sarasin, G.; Petrelli, L.; Guidolin, D.; Rossato, M.; Fontanella, C.G.; Natali, A.; De Caro, R. The Infrapatellar Adipose Body: A Histotopographic Study. Cells. Tissues. Organs 2016, 201, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Macchi, V.; Carniel, E.L.; Frigo, A.; Porzionato, A.; Picardi, E.E.E.; Favero, M.; Ruggieri, P.; de Caro, R.; Natali, A.N. Biomechanical behavior of Hoffa’s fat pad in healthy and osteoarthritic conditions: Histological and mechanical investigations. Australas. Phys. Eng. Sci. Med. 2018, 41, 657–667. [Google Scholar] [CrossRef]
- Mace, J.; Bhatti, W.; Anand, S. Infrapatellar fat pad syndrome: A review of anatomy, function, treatment and dynamics. Acta Orthop. Belg. 2016, 82, 94–101. [Google Scholar]
- Mohan, R.; John, A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. IUBMB Life 2015, 67, 395–403. [Google Scholar] [CrossRef]
- Eymard, F.; Chevalier, X. Inflammation of the infrapatellar fat pad. Jt. Bone Spine 2016, 83, 389–393. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, J.; Shen, N.; Wang, G.; Zhou, G.; Fang, Y.; Lin, J.; Zhao, J. Identification of pathways and genes associated with synovitis in osteoarthritis using bioinformatics analyses. Sci. Rep. 2018, 8, 10050. [Google Scholar] [CrossRef] [Green Version]
- Bendris, N.; Schmid, S.L. Endocytosis, Metastasis and Beyond: Multiple Facets of SNX9. Trends Cell Biol. 2017, 27, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buday, L.; Tompa, P. Functional classification of scaffold proteins and related molecules. FEBS J. 2010, 277, 4348–4355. [Google Scholar] [CrossRef]
- Garbett, D.; Bretscher, A. The surprising dynamics of scaffolding proteins. Mol. Biol. Cell 2014, 25, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Garcia, E.; O’Bryan, J.P. Intersectin scaffold proteins and their role in cell signaling and endocytosis. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kästle, M.; Kistler, B.; Lamla, T.; Bretschneider, T.; Lamb, D.; Nicklin, P.; Wyatt, D. FKBP51 modulates steroid sensitivity and NFκB signalling: A novel anti-inflammatory drug target. Eur. J. Immunol. 2018, 48, 1904–1914. [Google Scholar] [CrossRef]
- Fries, G.R.; Gassen, N.C.; Rein, T. The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2017, 18, 2614. [Google Scholar] [CrossRef] [Green Version]
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790. [Google Scholar] [CrossRef]
- Barik, S. Immunophilins: For the love of proteins. Cell. Mol. Life Sci. 2006, 63, 2889–2900. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Radanyi, C.; Renoir, J.M.; Housley, P.R.; Pratt, W.B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 2001, 276, 14884–14889. [Google Scholar] [CrossRef] [Green Version]
- Hähle, A.; Merz, S.; Meyners, C.; Hausch, F. The Many Faces of FKBP51. Biomolecules 2019, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Maiarù, M.; Morgan, O.B.; Mao, T.; Breitsamer, M.; Bamber, H.; Pöhlmann, M.; Schmidt, M.V.; Winter, G.; Hausch, F.; Géranton, S.M. The stress regulator FKBP51: A novel and promising druggable target for the treatment of persistent pain states across sexes. Pain 2018, 159, 1224–1234. [Google Scholar] [CrossRef]
- Maiarù, M.; Tochiki, K.K.; Cox, M.B.; Annan, L.V.; Bell, C.G.; Feng, X.; Hausch, F.; Géranton, S.M. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci. Transl. Med. 2016, 8, 325ra19. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.-F.; Shi, Z.-M.; Zou, J.; Li, X.-L. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed. Pharmacother. 2017, 89, 1252–1261. [Google Scholar] [CrossRef]
- Caratti, G.; Matthews, L.; Poolman, T.; Kershaw, S.; Baxter, M.; Ray, D. Glucocorticoid receptor function in health and disease. Clin. Endocrinol. 2015, 83, 441–448. [Google Scholar] [CrossRef]
- Häusl, A.S.; Balsevich, G.; Gassen, N.C.; Schmidt, M. V Focus on FKBP51: A molecular link between stress and metabolic disorders. Mol. Metab. 2019, 29, 170–181. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Robson, T. FK506 binding proteins and inflammation related signalling pathways; basic biology, current status and future prospects for pharmacological intervention. Pharmacol. Ther. 2020, 215, 107623. [Google Scholar] [CrossRef] [PubMed]
- Bortsov, A.V.; Smith, J.E.; Diatchenko, L.; Soward, A.C.; Ulirsch, J.C.; Rossi, C.; Swor, R.A.; Hauda, W.E.; Peak, D.A.; Jones, J.S.; et al. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain 2013, 154, 1419–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnstaedt, S.D.; Riker, K.D.; Rueckeis, C.A.; Kutchko, K.M.; Lackey, L.; McCarthy, K.R.; Tsai, Y.-H.; Parker, J.S.; Kurz, M.C.; Hendry, P.L.; et al. A Functional riboSNitch in the 3′ Untranslated Region of FKBP5 Alters MicroRNA-320a Binding Efficiency and Mediates Vulnerability to Chronic Post-Traumatic Pain. J. Neurosci. 2018, 38, 8407–8420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltychev, M.; Mattie, R.; McCormick, Z.; Laimi, K. The Magnitude and Duration of the Effect of Intra-articular Corticosteroid Injections on Pain Severity in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020, 99, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiao, Y.-L.; Zhou, R.-F.; Liu, S.; Cui, B.; Wang, L.-C.; Liu, X.-W.; Zhao, Y.-R. FKBP51 acts as a biomarker of early metastasis and is related to carmustine sensitivity in human glioma cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8918–8930. [Google Scholar] [CrossRef]
- Rotoli, D.; Morales, M.; Del Carmen Maeso, M.; Del Pino García, M.; Morales, A.; Ávila, J.; Martín-Vasallo, P. Expression and localization of the immunophilin FKBP51 in colorectal carcinomas and primary metastases, and alterations following oxaliplatin-based chemotherapy. Oncol. Lett. 2016, 12, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Samuels, J.; Pillinger, M.H.; Jevsevar, D.; Felson, D.; Simon, L.S. Critical appraisal of intra-articular glucocorticoid injections for symptomatic osteoarthritis of the knee. Osteoarthr. Cartil. 2021, 29, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.; Maccarrone, G.; Brückl, T.; Scheuer, S.; Hennings, J.; Holsboer, F.; Turck, C.W.; Uhr, M.; Lucae, S. FKBP5 Gene Expression Predicts Antidepressant Treatment Outcome in Depression. Int. J. Mol. Sci. 2019, 20, 485. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, J.J.; Cordova, R.A.; Zheng, D.; Criado-Marrero, M.; Lemus, A.; Li, P.; Baker, J.D.; Nordhues, B.A.; Darling, A.L.; Martinez-Licha, C.; et al. Targeting the FKBP51/GR/Hsp90 Complex to Identify Functionally Relevant Treatments for Depression and PTSD. ACS Chem. Biol. 2018, 13, 2288–2299. [Google Scholar] [CrossRef]
- Toneatto, J.; Guber, S.; Charó, N.L.; Susperreguy, S.; Schwartz, J.; Galigniana, M.D.; Piwien-Pilipuk, G. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J. Cell Sci. 2013, 126, 5357–5368. [Google Scholar] [CrossRef] [Green Version]
- Toneatto, J.; Charó, N.L.; Galigniana, N.M.; Piwien-Pilipuk, G. Adipogenesis is under surveillance of Hsp90 and the high molecular weight Immunophilin FKBP51. Adipocyte 2015, 4, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.J.; Palming, J.; Svensson, M.K.; Rizell, M.; Dalenbäck, J.; Hammar, M.; Fall, T.; Sidibeh, C.O.; Svensson, P.-A.; Eriksson, J.W. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metab.-Clin. Exp. 2014, 63, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Boocock, D.J.; Fellows, C.R.; Miosge, N.; Dixon, J.E.; Liddell, S.; Smith, J.; Mobasheri, A. Molecular phenotyping of the surfaceome of migratory chondroprogenitors and mesenchymal stem cells using biotinylation, glycocapture and quantitative LC-MS/MS proteomic analysis. Sci. Rep. 2019, 9, 9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, S.; Yoshioka, H.; Iwasaki, M.; Senthilkumar, R.; Rajmohan, M.; Karthick, R.; Preethy, S.; Abraham, S.J. A three-dimensional in vitro culture environment of a novel polymer scaffold, yielding chondroprogenitors and mesenchymal stem cells in human chondrocytes derived from osteoarthritis-affected cartilage tissue. J. Orthop. 2021, 23, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Gao, L.; Li, S.; Wei, X.; Du, G.; Wei, D.; Wei, L. Conditional deletion of HDAC4 from collagen type 2α1-expressing cells increases angiogenesis in vivo. Mol. Med. 2020, 26, 36. [Google Scholar] [CrossRef]
- White, W.L. Erratum to: Why I hate the index finger. Hand 2011, 6, 233. [Google Scholar] [CrossRef] [Green Version]





| Number | Gender | Age | BMI | K&L OA Grade |
|---|---|---|---|---|
| 1 | F | 66 | 32 | IV |
| 2 | M | 81 | 32.5 | IV |
| 3 | F | 52 | 24.6 | IV |
| 4 | F | 56 | 34.2 | IV |
| 5 | M | 70 | 35.3 | IV |
| 6 | F | 72 | 31.1 | IV |
| 7 | M | 59 | 31.6 | IV |
| 8 | F | 59 | 43.1 | IV |
| 9 | F | 76 | 29.4 | IV |
| 10 | F | 69 | 30.8 | IV |
| 11 | F | 72 | 30.4 | IV |
| 12 | F | 69 | 27.1 | IV |
| 13 | F | 52 | 33 | IV |
| 14 | M | 75 | 33.5 | IV |
| FKBP51 | TNF-α | IL6 | Iba1 | CD31 | CD34 | MAP2 | |
|---|---|---|---|---|---|---|---|
| Chondrocytes | ++/+++ | +/+++ | +++ | ++ | ++/+++ | +/++ | nc |
| Adipocytes | ++/+++ | +/++ | +/+++ | +/++ | + | + | + |
| Synoviocytes (A type) | ++/+++ | ++ | + | + | + | + | nc |
| Synoviocytes (B type) | ++/+++ | ++ | + | ++ | ++ | +++ | nc |
| Axon fibers | ++ | nc | nc | nc | nc | nc | +++ |
| Endothelial cells | ++/+++ | ++/+++ | + | − | +++ | +++ | nc |
| Vascular endothelial cells | ++/+++ | +/+++ | + | − | +/++ | ++/+++ | nc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poletti, F.; González-Fernández, R.; García, M.-d.-P.; Rotoli, D.; Ávila, J.; Mobasheri, A.; Martín-Vasallo, P. Molecular-Morphological Relationships of the Scaffold Protein FKBP51 and Inflammatory Processes in Knee Osteoarthritis. Cells 2021, 10, 2196. https://doi.org/10.3390/cells10092196
Poletti F, González-Fernández R, García M-d-P, Rotoli D, Ávila J, Mobasheri A, Martín-Vasallo P. Molecular-Morphological Relationships of the Scaffold Protein FKBP51 and Inflammatory Processes in Knee Osteoarthritis. Cells. 2021; 10(9):2196. https://doi.org/10.3390/cells10092196
Chicago/Turabian StylePoletti, Fabián, Rebeca González-Fernández, María-del-Pino García, Deborah Rotoli, Julio Ávila, Ali Mobasheri, and Pablo Martín-Vasallo. 2021. "Molecular-Morphological Relationships of the Scaffold Protein FKBP51 and Inflammatory Processes in Knee Osteoarthritis" Cells 10, no. 9: 2196. https://doi.org/10.3390/cells10092196