Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement
Abstract
:1. Introduction
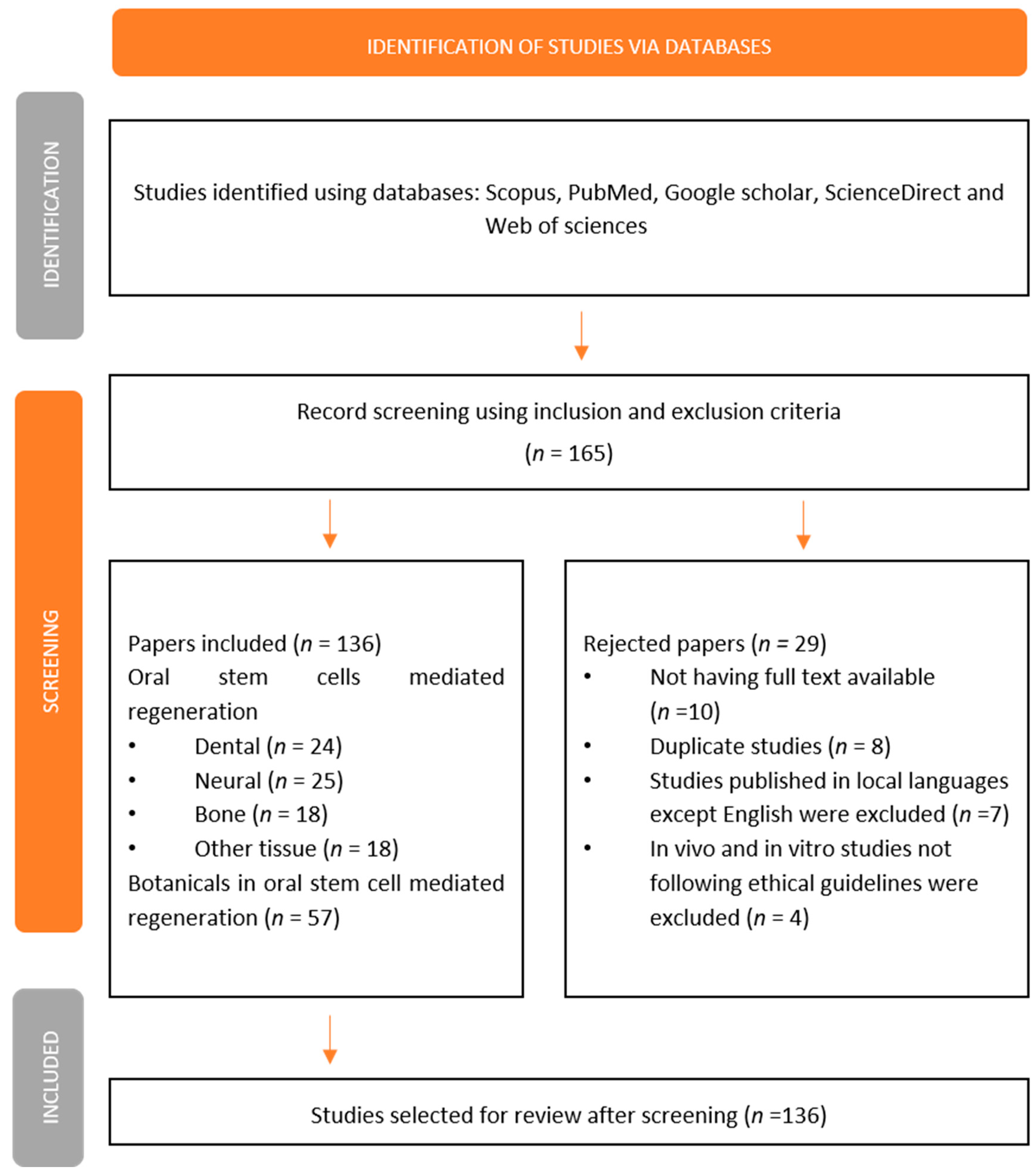
2. Research Methodology
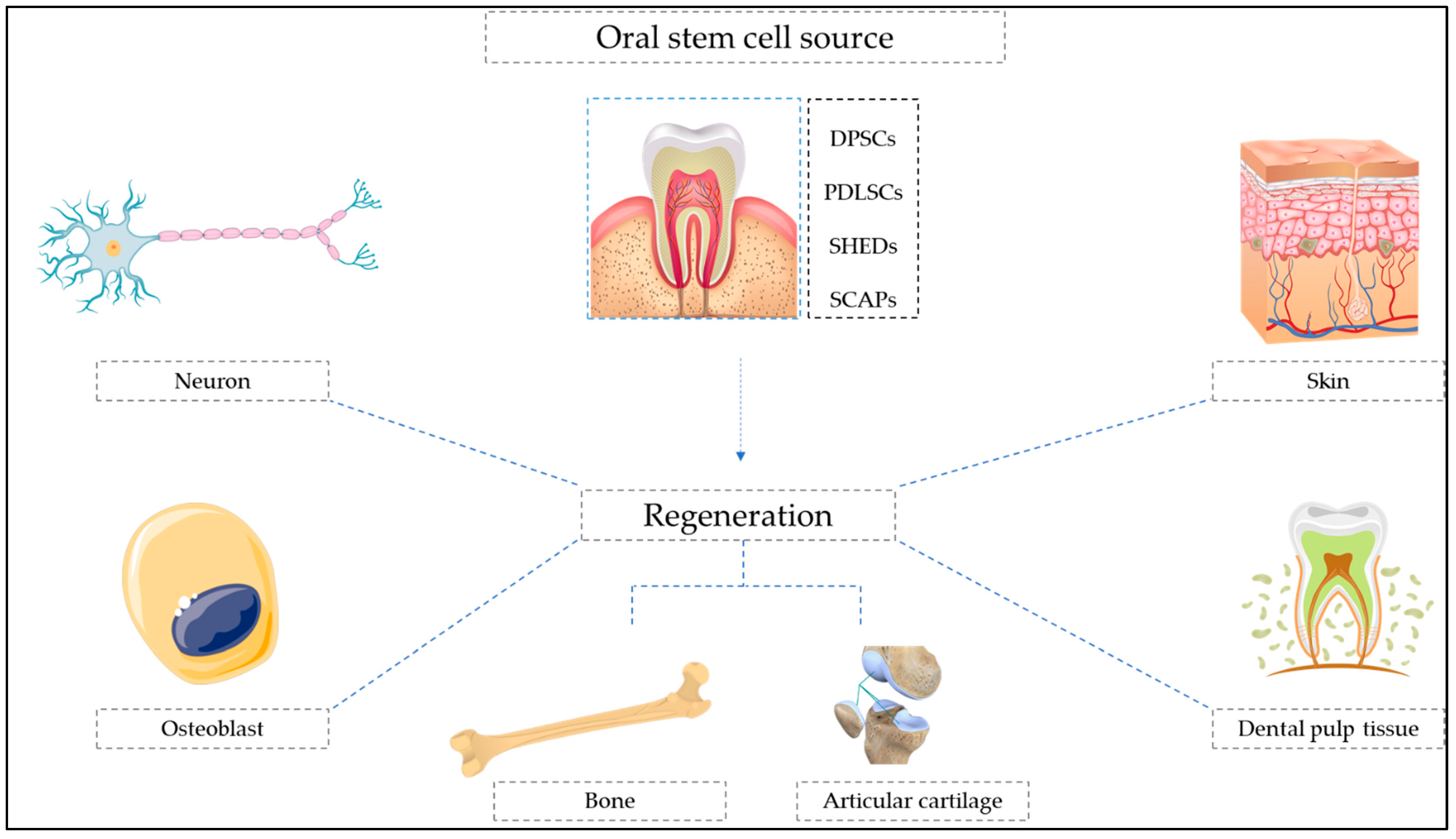
3. Oral Stem Cell-Mediated Dental Regeneration
4. Oral Stem Cell-Mediated Neural Regeneration and Repair
5. Oral Stem Cell-Mediated Bone Regeneration
6. Oral Stem Cell-Mediated Regeneration of Other Tissues
7. Botanicals in Oral Stem Cell-Mediated Regeneration
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Shi, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic stem cells for regenerative dentistry. Clin. Oral Investig. 2008, 12, 113–118. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Mokry, J.; Soukup, T.; Micuda, S.; Karbanova, J.; Visek, B.; Brcakova, E.; Suchanek, J.; Bouchal, J.; Vokurkova, D.; Ivancakova, R. Telomere attrition occurs during ex vivo expansion of human dental pulp stem cells. J. Biomed. Biotechnol. 2010, 2010, 673513. [Google Scholar] [CrossRef]
- Ponnaiyan, D.; Jegadeesan, V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur. J. Dent. 2014, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, P.J. Telomerase and the aging process. Exp. Gerontol. 2007, 42, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Bertani, G.; Bertoni, L.; Di Tinco, R.; De Biasi, S.; Vallarola, A.; Pignatti, E.; Tupler, R.; Salvarani, C.; de Pol, A.; et al. Modulation of Cell Death and Promotion of Chondrogenic Differentiation by Fas/FasL in Human Dental Pulp Stem Cells (hDPSCs). Front. Cell Dev. Biol. 2020, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.M.; Salero, E.; Núñez-Toldrà, R.; Sabater, A.L.; Gil, F.J.; Perez, R.A. Discovering the Potential of Dental Pulp Stem Cells for Corneal Endothelial Cell Production: A Proof of Concept. Front. Bioeng. Biotechnol. 2021, 9, 617724. [Google Scholar] [CrossRef]
- Martínez-Sarrà, E.; Montori, S.; Gil-Recio, C.; Núñez-Toldrà, R.; Costamagna, D.; Rotini, A.; Atari, M.; Luttun, A.; Sampaolesi, M. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res. Ther. 2017, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Sun, L.; Guo, W.; Tian, W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J. Neural Eng. 2017, 14, 026005. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, J.H.; Bae, J.; Bu, Y.; Kim, E.C. Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transplant. 2017, 26, 1001–1016. [Google Scholar] [CrossRef]
- Syed-Picard, F.N.; Du, Y.; Lathrop, K.L.; Mann, M.M.; Funderburgh, M.L.; Funderburgh, J.L. Dental Pulp Stem Cells: A New Cellular Resource for Corneal Stromal Regeneration. Stem Cells Transl. Med. 2015, 4, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Shi, X.; Xiao, F.; Yang, Y.; Zhang, X.; Wang, L.S.; Wu, C.T.; Wang, H. Transplantation of Hepatocyte Growth Factor-Modified Dental Pulp Stem Cells Prevents Bone Loss in the Early Phase of Ovariectomy-Induced Osteoporosis. Hum. Gene Ther. 2018, 29, 271–282. [Google Scholar] [CrossRef]
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, I.; Bhadri, N.; Shahani, P.; Majumdar, D.; Sowmithra, S.; Razdan, R.; Bhonde, R. Functional recovery upon human dental pulp stem cell transplantation in a diabetic neuropathy rat model. Cytotherapy 2017, 19, 1208–1224. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, Y.A.; Lee, Y.M.; Lee, S.Y.; Bae, W.J.; Kim, E.C. PIN1 Suppresses the Hepatic Differentiation of Pulp Stem Cells via Wnt3a. J. Dent. Res. 2016, 95, 1415–1424. [Google Scholar] [CrossRef]
- Adamička, M.; Adamičková, A.; Danišovič, L.; Gažová, A.; Kyselovič, J. Pharmacological Approaches and Regeneration of Bone Defects with Dental Pulp Stem Cells. Stem Cells Int. 2021, 2021, 4593322. [Google Scholar] [CrossRef] [PubMed]
- Kunze, M.; Huber, A.; Krajewski, A.; Lowden, E.; Schuhmann, N.; Buening, H.; Hallek, M.; Noack, M.; Perabo, L. Efficient gene transfer to periodontal ligament cells and human gingival fibroblasts by adeno-associated virus vectors. J. Dent. 2009, 37, 502–508. [Google Scholar] [CrossRef]
- Gronthos, S.; Mrozik, K.; Shi, S.; Bartold, P.M. Ovine periodontal ligament stem cells: Isolation, characterization, and differentiation potential. Calcif. Tissue Int. 2006, 79, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qi, Z.; Qiao, B.; Lv, Z.; Hao, Y.; Li, H. Oxymatrine can attenuate pathological deficits of Alzheimer’s disease mice through regulation of neuroinflammation. J. Neuroimmunol. 2019, 334, 576978. [Google Scholar] [CrossRef]
- Taguchi, T.; Yanagi, Y.; Yoshimaru, K.; Zhang, X.Y.; Matsuura, T.; Nakayama, K.; Kobayashi, E.; Yamaza, H.; Nonaka, K.; Ohga, S.; et al. Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): A promising new treatment in pediatric surgery. Surg. Today 2019, 49, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Liu, S.; Liu, D.; Chen, C.; Xu, X.; Chen, X.; Shi, S. Transplantation of SHED prevents bone loss in the early phase of ovariectomy-induced osteoporosis. J. Dent. Res. 2014, 93, 1124–1132. [Google Scholar] [CrossRef]
- Do Couto Nicola, F.; Marques, M.R.; Odorcyk, F.; Arcego, D.M.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017, 1663, 95–105. [Google Scholar] [CrossRef]
- Yamaza, T.; Alatas, F.S.; Yuniartha, R.; Yamaza, H.; Fujiyoshi, J.K.; Yanagi, Y.; Yoshimaru, K.; Hayashida, M.; Matsuura, T.; Aijima, R.; et al. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res. Ther. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, M.; Yamamoto, A.; Kako, E.; Kaneko, N.; Matsubara, K.; Sakai, K.; Sawamoto, K.; Ueda, M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke 2013, 44, 551–554. [Google Scholar] [CrossRef]
- Yamaza, T.; Sonoda, S.; Tomoda, E.; Tanaka, Y. Properties and Possibilities of Human Dental Pulp-Derived Stem Cells. Arch. Stem Cell Res. 2015, 2, 1012. [Google Scholar]
- Nada, O.A.; El Backly, R.M. Stem Cells From the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Kumar, B.M.; Lee, W.J.; Jeon, R.H.; Jang, S.J.; Lee, Y.M.; Park, B.W.; Byun, J.H.; Ahn, C.S.; Kim, J.W.; et al. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 2014, 320, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yao, R.; Du, J.; Wang, S.; Fan, Z. Depletion of histone demethylase KDM2A enhanced the adipogenic and chondrogenic differentiation potentials of stem cells from apical papilla. Exp. Cell Res. 2013, 319, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 2546367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Xu, Q.; Cullen, K.D.; Le, A.D. Neural Crest Stem-Like Cells Non-genetically Induced from Human Gingiva-Derived Mesenchymal Stem Cells Promote Facial Nerve Regeneration in Rats. Mol. Neurobiol. 2018, 55, 6965–6983. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 6634. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Latif, N.; Abdulrahman, M.; Helal, M.; Grawish, M.E. Regenerative capacity of allogenic gingival margin- derived stem cells with fibrin glue on albino rats’ partially dissected submandibular salivary glands. Arch. Oral Biol. 2017, 82, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Shanti, R.M.; Zhang, Q.; Cannady, S.B.; O’Malley, B.W.; Le, A.D. A Gingiva-Derived Mesenchymal Stem Cell-Laden Porcine Small Intestinal Submucosa Extracellular Matrix Construct Promotes Myomucosal Regeneration of the Tongue. Tissue Eng. Part A 2017, 23, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Xu, Q.; Zhang, Q.; Shanti, R.M.; Le, A.D. SIS-ECM Laden with GMSC-Derived Exosomes Promote Taste Bud Regeneration. J. Dent. Res. 2019, 98, 225–233. [Google Scholar] [CrossRef]
- Hsu, S.H.; Huang, G.S.; Lin, S.Y.F.; Feng, F.; Ho, T.T.; Liao, Y.C. Enhanced chondrogenic differentiation potential of human gingival fibroblasts by spheroid formation on chitosan membranes. Tissue Eng. Part A 2012, 18, 67–79. [Google Scholar] [CrossRef]
- Al-Qadhi, G.; Soliman, M.; Abou-Shady, I.; Rashed, L. Gingival mesenchymal stem cells as an alternative source to bone marrow mesenchymal stem cells in regeneration of bone defects: In vivo study. Tissue Cell 2020, 63, 101325. [Google Scholar] [CrossRef]
- Ansari, S.; Sarrion, P.; Hasani-Sadrabadi, M.M.; Aghaloo, T.; Wu, B.M.; Moshaverinia, A. Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels. J. Biomed. Mater. Res. A 2017, 105, 2957–2967. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, Z.; Song, W.; Sun, W.; Hong, R.; Pothukuchi, A.; Xu, Q. Systematically transplanted human gingiva-derived mesenchymal stem cells regulate lipid metabolism and inflammation in hyperlipidemic mice with periodontitis. Exp. Ther. Med. 2020, 19, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, R.; Barathidasan, R.; Raja, S.T.K.; Arumugam, G.; Kuruvilla, S.; Shanthi, P.; Ranga Rao, S. Human gingival derived neuronal cells in the optimized caffeic acid hydrogel for hemitransection spinal cord injury model. J. Cell. Biochem. 2020, 121, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef]
- Paganelli, A.; Tarentini, E.; Benassi, L.; Kaleci, S.; Magnoni, C. Mesenchymal stem cells for the treatment of psoriasis: A comprehensive review. Clin. Exp. Dermatol. 2020, 45, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Hsu, N.C.; Wang, S.M.; Wang, F.N. Successful Treatment of Plaque Psoriasis with Allogeneic Gingival Mesenchymal Stem Cells: A Case Study. Case Rep. Dermatol. Med. 2020, 2020, 4617520. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Akiyama, K.; Chai, Y.; Le, A.D.; Wang, Z.; Shi, S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J. Dent. Res. 2013, 92, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yan, B.; Li, J.; Zhang, T.; Yang, R.; Wang, X.; Liu, Y.; Liu, D. Acetylsalicylic acid rescues the immunomodulation of inflamed gingiva-derived mesenchymal stem cells via upregulating FasL in mice. Stem Cell Res. Ther. 2019, 10, 368. [Google Scholar] [CrossRef]
- Yang, R.; Yu, T.; Liu, D.; Shi, S.; Zhou, Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.; Zhang, S.; Gao, J.; Gan, X.; Zheng, J.; Lu, L.; Zeng, W.; Gu, J. Human gingiva-derived mesenchymal stem cells alleviate inflammatory bowel disease via IL-10 signalling-dependent modulation of immune cells. Scand. J. Immunol. 2019, 90, e12751. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z.; et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013, 65, 1181–1193. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Shi, S. Transplantation of gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis. Arthritis Res. Ther. 2016, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine 2019, 43, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, Z.X.; Zeng, D.; Huang, F.; Wang, J.; Liu, Y.; Bellanti, J.A.; Olsen, N.; Zheng, S.G. B7-H1 Promotes the Functional Effect of Human Gingiva-Derived Mesenchymal Stem Cells on Collagen-Induced Arthritis Murine Model. Mol. Ther. 2020, 28, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Xu, Z.; Xu, A.; Liu, Y.; Fu, Q.; Wang, J.; Huang, F.; Zheng, Y.; Qi, G.; Sun, B.; et al. Human gingiva-derived mesenchymal stem cells are therapeutic in lupus nephritis through targeting of CD39–CD73 signaling pathway. J. Autoimmun. 2020, 113, 102491. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, Z.; Chen, Y.; Deng, Y.; Zeng, D.; Liu, Y.; Huang, F.; Wang, J.; Liu, Y.; Bellanti, J.A.; et al. CD39 Produced from Human GMSCs Regulates the Balance of Osteoclasts and Osteoblasts through the Wnt/β-Catenin Pathway in Osteoporosis. Mol. Ther. 2020, 28, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.L.; Sun, C.X.; Liu, Y.Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.F.; Mazzon, E.; Trubiani, O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Int. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, M.; Yan, X.; Wen, Y.; Zeng, Q.; Yue, W.; Yang, P.; Pei, X. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011, 20, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.C.; Wang, Z.G.; Ji, Q.X.; Yu, X.B.; Xu, X.Y.; Yuan, C.Q.; Deng, J.; Yang, P.S. Systemically transplanted human gingiva-derived mesenchymal stem cells contributing to bone tissue regeneration. Int. J. Clin. Exp. Pathol. 2014, 7, 4922–4929. [Google Scholar] [PubMed]
- Kandalam, U.; Kawai, T.; Ravindran, G.; Brockman, R.; Romero, J.; Munro, M.; Ortiz, J.; Heidari, A.; Thomas, R.; Kuriakose, S.; et al. Predifferentiated Gingival Stem Cell-Induced Bone Regeneration in Rat Alveolar Bone Defect Model. Tissue Eng. Part A 2021, 27, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Heinayati, A.; Bao, D.; Liu, H.; Ding, X.; Tong, X.; Wang, L.; Wang, B.; Qin, H. Small molecule inhibitor of TGF-β signaling enables robust osteogenesis of autologous GMSCs to successfully repair minipig severe maxillofacial bone defects. Stem Cell Res. Ther. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10, eaai8524. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Zhang, K.; Zhang, W.; Liu, H.; Xu, Z.; Li, H.; Lou, J.; Ge, L.; Xu, B. Treatment of gingival defects with gingival mesenchymal stem cells derived from human fetal gingival tissue in a rat model. Stem Cell Res. Ther. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, C.; Guo, L.; Du, J.; Li, X.; Liu, Y. Ecological Balance of Oral Microbiota Is Required to Maintain Oral Mesenchymal Stem Cell Homeostasis. Stem Cells 2018, 36, 551–561. [Google Scholar] [CrossRef]
- Isaac, J.; Nassif, A.; Asselin, A.; Taïhi, I.; Fohrer-Ting, H.; Klein, C.; Gogly, B.; Berdal, A.; Robert, B.; Fournier, B.P. Involvement of neural crest and paraxial mesoderm in oral mucosal development and healing. Biomaterials 2018, 172, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, A.L.; Shi, S.; Hill, C.; Wilder-Smith, P.; Krasieva, T.B.; Le, A.D. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Rahimi, B.; Rahimi, G.; Yew Low, T.; Saraygord-Afshari, N.; Alizadeh, E. Cytoprotective effects of antioxidant supplementation on mesenchymal stem cell therapy. J. Cell. Physiol. 2020, 235, 6462–6495. [Google Scholar] [CrossRef]
- Chang, W.; Song, B.W.; Moon, J.Y.; Cha, M.J.; Ham, O.; Lee, S.Y.; Choi, E.; Choi, E.; Hwang, K.C. Anti-death strategies against oxidative stress in grafted mesenchymal stem cells. Histol. Histopathol. 2013, 28, 1529–1536. [Google Scholar] [CrossRef]
- Zeng, W.; Xiao, J.; Zheng, G.; Xing, F.; Tipoe, G.L.; Wang, X.; He, C.; Chen, Z.Y.; Liu, Y. Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci. Rep. 2015, 5, 11100. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of Antioxidant Supplements on the Survival and Differentiation of Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017, 5032102. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chang, W.C.; Hung, K.H.; Yang, D.M.; Cheng, Y.H.; Liao, Y.W.; Woung, L.C.; Tsai, C.Y.; Hsu, C.C.; Lin, T.C.; et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front. Aging Neurosci. 2014, 6, 191. [Google Scholar] [CrossRef]
- Udalamaththa, V.L.; Jayasinghe, C.D.; Udagama, P.V. Potential role of herbal remedies in stem cell therapy: Proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Pirmoradi, S.; Fathi, E.; Farahzadi, R.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Curcumin Affects Adipose Tissue-Derived Mesenchymal Stem Cell Aging Through TERT Gene Expression. Drug Res. 2018, 68, 213–221. [Google Scholar] [CrossRef]
- Fu, X.; Feng, Y.; Shao, B.; Zhang, Y. Taxifolin Protects Dental Pulp Stem Cells under Hypoxia and Inflammation Conditions. Cell Transplant. 2021, 30, 9636897211034452. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2015, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.; Cui, Y.; Zhou, H.; Xu, D.; Shan, T.; Zhang, F.; Guo, Y.; Chen, Y.; Wu, D. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol. Appl. Pharmacol. 2015, 287, 168–177. [Google Scholar] [CrossRef]
- Zeb, S.; Ali1, A.; Zaman, W.; Zeb, S.; Ali, S.; Ullah, F.; Shakoor, A. Pharmacology, taxonomy and phytochemistry of the genus Artemisia specifically from Pakistan: A comprehensive review. Pharm. Biomed. Res. 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Hu, H.M.; Mao, M.H.; Hu, Y.H.; Zhou, X.C.; Li, S.; Chen, C.F.; Li, C.N.; Yuan, Q.L.; Li, W. Artemisinin protects DPSC from hypoxia and TNF-α mediated osteogenesis impairments through CA9 and Wnt signaling pathway. Life Sci. 2021, 277, 119471. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef]
- Bakopoulou, A.; About, I. Stem Cells of Dental Origin: Current Research Trends and Key Milestones towards Clinical Application. Stem Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef]
- Hu, L.; Gao, Z.; Xu, J.; Zhu, Z.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration. Biomed Res. Int. 2017, 2017, 9342714. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Hu, L.; Yan, F.; Zhao, B.; Wu, X.; Zhang, C.; Wang, J.; Du, J.; Wang, S. Retinoic Acid Signal Negatively Regulates Osteo/Odontogenic Differentiation of Dental Pulp Stem Cells. Stem Cells Int. 2020, 2020, 5891783. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Chen, B.; Zhu, Q.L.; Kong, H.; Li, Q.H.; Gao, L.N.; Xiao, M.; Chen, F.M.; Yu, Q. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomaterials 2014, 35, 9459–9472. [Google Scholar] [CrossRef] [PubMed]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Gehron Robey, P.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.S.; Choung, H.W.; Shon, W.J.; Seo, B.M.; Lee, E.H.; Cho, J.Y.; Park, J.C. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 2011, 32, 9696–9706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Zhang, Z.; Grande, R.H.M.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the Role of Dental Pulp Stem Cells in Regenerative Therapy. Biology 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef]
- Janebodin, K.; Reyes, M. Neural Crest-Derived Dental Pulp Stem Cells Function as Ectomesenchyme to Support Salivary Gland Tissue Formation. Dentistry 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wu, C.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Li, G.; Wang, T.; Jin, Y.; Lu, W.; Ji, J. Human Periodontal Ligament Stem Cells Transplanted with Nanohydroxyapatite/Chitosan/Gelatin 3D Porous Scaffolds Promote Jaw Bone Regeneration in Swine. Stem Cells Dev. 2021, 30, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Feng, F.; Akiyama, K.; Liu, Y.; Yamaza, T.; Wang, T.M.; Chen, J.H.; Wang, B.B.; Huang, G.T.J.; Wang, S.; Shi, S. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis. 2010, 16, 20–28. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.; Pelissari, C.; Trierveiler, M.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Akazawa, K.; Nagata, M.; Yokoyama, N.; Watabe, T.; Morita, I. Spontaneous differentiation of periodontal ligament stem cells into myofibroblast during ex vivo expansion. J. Cell. Physiol. 2019, 234, 20377–20391. [Google Scholar] [CrossRef]
- Washio, K.; Tsutsumi, Y.; Tsumanuma, Y.; Yano, K.; Srithanyarat, S.S.; Takagi, R.; Ichinose, S.; Meinzer, W.; Yamato, M.; Okano, T.; et al. In vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng. Part A 2018, 24, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Enukashvily, N.I.; Dombrovskaya, J.A.; Kotova, A.V.; Semenova, N.; Karabak, I.; Banashkov, R.E.; Baram, D.; Paderina, T.; Bilyk, S.S.; Grimm, W.D.; et al. Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study. Bioengineering 2021, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Han, N.; Zhang, X.; Yang, H.; Cao, Y.; Wang, S.; Fan, Z. Local Injection of Allogeneic Stem Cells from Apical Papilla Enhanced Periodontal Tissue Regeneration in Minipig Model of Periodontitis. Biomed Res. Int. 2018, 2018, 3960798. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Ruan, J.; Weir, M.D.; Ma, T.; Ren, K.; Schneider, A.; Oates, T.W.; Li, A.; Zhao, L.; et al. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J. Dent. 2020, 92, 103259. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Li, H.; Wang, R.; Yang, D.; Li, B.; Cao, X.; Fu, J. Transplanted Dental Pulp Stem Cells Migrate to Injured Area and Express Neural Markers in a Rat Model of Cerebral Ischemia. Cell. Physiol. Biochem. 2018, 45, 258–266. [Google Scholar] [CrossRef]
- Rafiee, F.; Pourteymourfard-Tabrizi, Z.; Mahmoudian-Sani, M.R.; Mehri-Ghahfarrokhi, A.; Soltani, A.; Hashemzadeh-Chaleshtori, M.; Jami, M.S. Differentiation of dental pulp stem cells into neuron-like cells. Int. J. Neurosci. 2020, 130, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Nito, C.; Sowa, K.; Nakajima, M.; Sakamoto, Y.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Ueda, M.; Okada, T.; et al. Transplantation of human dental pulp stem cells ameliorates brain damage following acute cerebral ischemia. Biomed. Pharmacother. 2018, 108, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Ramirez, C.; Rodríguez-Lozano, F.J.; Tabarés-Seisdedos, R.; Rodenas, M.; Moraleda, J.M.; Jones, J.R.; Martinez, S. Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transplant. 2013, 22, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Martínez-Morga, M.; Martínez, S. Non-proliferative neurogenesis in human periodontal ligament stem cells. Sci. Rep. 2019, 9, 18038. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef]
- Nicola, F.C.; Rodrigues, L.P.; Crestani, T.; Quintiliano, K.; Sanches, E.F.; Willborn, S.; Aristimunha, D.; Boisserand, L.; Pranke, P.; Netto, C.A. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz. J. Med. Biol. Res. 2016, 49, e5319. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Pagella, P.; Miran, S.; Neto, E.; Martin, I.; Lamghari, M.; Mitsiadis, T.A. Human dental pulp stem cells exhibit enhanced properties in comparison to human bone marrow stem cells on neurites outgrowth. FASEB J. 2020, 34, 5499–5511. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.-F.; Peh, G.; Singhal, S.; Goh, B.-T.; Mehta, J.S. Dental stem cells: A future asset of ocular cell therapy. Expert Rev. Mol. Med. 2015, 17, e20. [Google Scholar] [CrossRef]
- Gnanasegaran, N.; Govindasamy, V.; Simon, C.; Gan, Q.F.; Vincent-Chong, V.K.; Mani, V.; Krishnan Selvarajan, K.; Subramaniam, V.; Musa, S.; Abu Kasim, N.H. Effect of dental pulp stem cells in MPTP-induced old-aged mice model. Eur. J. Clin. Investig. 2017, 47, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Lai, P.L.; Chien, Y.; Lee, P.H.; Lai, Y.H.; Ma, H.I.; Shiau, C.Y.; Wang, K.C. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3807. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, N.; Boroujeni, M.E.; Abdollahifar, M.A.; Piryaei, A.; Khodagholi, F.; Mirbehbahani, S.H.; Siroosi, S.; Moghaddam, M.H.; Aliaghaei, A.; Sadeghi, Y. Transplantation of human dental pulp stem cells compensates for striatal atrophy and modulates neuro-inflammation in 3-nitropropionic acid rat model of Huntington’s disease. Neurosci. Res. 2021, 170, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.E.M.B.; Murakami, M.; Hirose, Y.; Nakashima, M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer’s Disease Treatment: An In vitro Study. Stem Cells Int. 2016, 2016, 8102478. [Google Scholar] [CrossRef] [PubMed]
- Mita, T.; Furukawa-Hibi, Y.; Takeuchi, H.; Hattori, H.; Yamada, K.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 293, 189–197. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Pastor-Alonso, O.; Encinas, J.M.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Human Dental Pulp Stem Cells Grown in Neurogenic Media Differentiate into Endothelial Cells and Promote Neovasculogenesis in the Mouse Brain. Front. Physiol. 2019, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Luzuriaga, J.; Irurzun, J.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Vasculogenesis from Human Dental Pulp Stem Cells Grown in Matrigel with Fully Defined Serum-Free Culture Media. Biomedicines 2020, 8, 483. [Google Scholar] [CrossRef]
- Merckx, G.; Hosseinkhani, B.; Kuypers, S.; Deville, S.; Irobi, J.; Nelissen, I.; Michiels, L.; Lambrichts, I.; Bronckaers, A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. Cells 2020, 9, 312. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Yin, Y.; He, X.T.; An, Y.; Tian, B.M.; Hong, Y.L.; Wu, L.A.; Chen, F.M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Miura-Yura, E.; Tsunekawa, S.; Naruse, K.; Nakamura, N.; Motegi, M.; Nakai-Shimoda, H.; Asano, S.; Kato, M.; Yamada, Y.; Izumoto-Akita, T.; et al. Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin-induced diabetic mice. J. Diabetes Investig. 2020, 11, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, P.; Xu, Q.; Park, W.; Lee, S.; Furuhashi, A.; Le, A.D. Neural Progenitor-Like Cells Induced from Human Gingiva-Derived Mesenchymal Stem Cells Regulate Myelination of Schwann Cells in Rat Sciatic Nerve Regeneration. Stem Cells Transl. Med. 2017, 6, 458–470. [Google Scholar] [CrossRef]
- Chen, Y.Y.; He, S.T.; Yan, F.H.; Zhou, P.F.; Luo, K.; Zhang, Y.D.; Xiao, Y.; Lin, M.K. Dental pulp stem cells express tendon markers under mechanical loading and are a potential cell source for tissue engineering of tendon-like tissue. Int. J. Oral Sci. 2016, 8, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, M.; Bakkar, M.; Jordan, J.; Tran, S.D. Osteogenic Potential of Dental Mesenchymal Stem Cells in Preclinical Studies: A Systematic Review Using Modified ARRIVE and CONSORT Guidelines. Stem Cells Int. 2015, 2015, 378368. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Tvedesøe, C.; Rölfing, J.H.D.; Foldager, C.B.; Lysdahl, H.; Kraft, D.C.E.; Chen, M.; Baas, J.; Le, D.Q.S.; Bünger, C.E. Dental pulp-derived stromal cells exhibit a higher osteogenic potency than bone marrow-derived stromal cells in vitro and in a porcine critical-size bone defect model. SICOT-J 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, F.; Collignon, A.M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; De Llano, J.J.M.; Carda, C. In vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 2017, 8309256. [Google Scholar] [CrossRef]
- Talaat, W.; Smriti Aryal, A.C.; Kawas, S.A.; Samsudin, A.B.R.; Kandile, N.G.; Harding, D.R.K.; Ghoneim, M.M.; Zeiada, W.; Jagal, J.; Aboelnaga, A.; et al. Nanoscale Thermosensitive Hydrogel Scaffolds Promote the Chondrogenic Differentiation of Dental Pulp Stem and Progenitor Cells: A Minimally Invasive Approach for Cartilage Regeneration. Int. J. Nanomed. 2020, 15, 7775–7789. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Lu, A.; De Biasi, S.; Gibellini, L.; La Sala, G.B.; Bruzzesi, G.; Ferrari, A.; Huard, J.; et al. Stem cells isolated from human dental pulp and amniotic fluid improve skeletal muscle histopathology in mdx/SCID mice. Stem Cell Res. Ther. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, G.M.; La Sala, G.B.; Bruzzesi, G.; et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e774–e785. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, F.; Cheng, Y.; Li, Y.; Chen, Y.; Tang, J.; Bei, Y.; Tang, Q.; Zhao, Y.; Huang, Y.; et al. Transforming Growth Factor-β3/Recombinant Human-like Collagen/Chitosan Freeze-Dried Sponge Primed with Human Periodontal Ligament Stem Cells Promotes Bone Regeneration in Calvarial Defect Rats. Front. Pharmacol. 2021, 12, 678322. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, H.A.; Koh, A.E.H.; Lam, C.; Rashid, M.B.A.; Harun, M.H.N.; Saleh, M.F.B.M.; Teh, S.W.; Luu, C.D.; Ng, M.H.; Isa, H.M.; et al. Dental pulp stem cells therapy overcome photoreceptor cell death and protects the retina in a rat model of sodium iodate-induced retinal degeneration. J. Photochem. Photobiol. B Biol. 2019, 198, 111561. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Dental pulp stem cells, a paracrine-mediated therapy for the retina. Neural Regen. Res. 2014, 9, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Lundy, F.; El Karim, I.; Scheven, B. Current and Future Views on Pulpal Pain and Neurogenesis: Current and Emerging Therapeutic Perspectives. In Clinical Approaches in Endodontic Regeneration; Springer: Cham, Switzerland, 2019; pp. 19–36. ISBN 978-3-319-96847-6. [Google Scholar]
- Gomes, J.Á.P.; Monteiro, B.G.; Melo, G.B.; Smith, R.L.; da Silva, M.C.P.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Karamali, F.; Esfahani, M.H.N.; Taleahmad, S.; Satarian, L.; Baharvand, H. Stem cells from apical papilla promote differentiation of human pluripotent stem cells towards retinal cells. Differentiation 2018, 101, 8–15. [Google Scholar] [CrossRef]
- Adriztina, I.; Munir, D.; Sandra, F.; Ichwan, M.; Bashiruddin, J.; Putra, I.B.; Farhat; Sembiring, R.J.; Sartika, C.R.; Chouw, A.; et al. Differentiation capacity of dental pulp stem cell into inner ear hair cell using an in vitro assay: A preliminary step toward treating sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 279, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Bharti, D.; Kang, Y.H.; Lee, S.Y.; Oh, S.J.; Kim, S.B.; Jo, C.H.; Son, J.H.; Sung, I.Y.; Cho, Y.C.; et al. Human Dental Pulp-Derived Mesenchymal Stem Cell Potential to Differentiate into Smooth Muscle-Like Cells In vitro. Biomed Res. Int. 2021, 2021, 8858412. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Jiang, W.; Alraies, A.; Liu, Q.; Gudla, V.; Oni, J.; Wei, X.; Sloan, A.; Ni, L.; Agarwal, M. Bladder Smooth Muscle Cells Differentiation from Dental Pulp Stem Cells: Future Potential for Bladder Tissue Engineering. Stem Cells Int. 2016, 2016, 6979368. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, D.; Alraies, A.; Liu, Q.; Zhu, B.; Sloan, A.J.; Ni, L.; Song, B. Wnt-GSK3 β/β-Catenin Regulates the Differentiation of Dental Pulp Stem Cells into Bladder Smooth Muscle Cells. Stem Cells Int. 2019, 2019, 8907570. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Kang, Y.H.; Shivakumar, S.B.; Bharti, D.; Son, Y.B.; Choi, Y.H.; Park, W.U.; Byun, J.H.; Rho, G.J.; Park, B.W. Stem Cells from Cryopreserved Human Dental Pulp Tissues Sequentially Differentiate into Definitive Endoderm and Hepatocyte-Like Cells in vitro. Int. J. Med. Sci. 2017, 14, 1418–1429. [Google Scholar] [CrossRef]
- Yokoyama, T.; Yagi Mendoza, H.; Tanaka, T.; Ii, H.; Takano, R.; Yaegaki, K.; Ishikawa, H. Regulation of CCl 4-induced liver cirrhosis by hepatically differentiated human dental pulp stem cells. Hum. Cell 2019, 32, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Feng, Z.; Zhang, F.; Liu, Z.; Sun, X.; Ruan, M.; Liu, M.; Jin, S. Therapeutic effect of dental pulp stem cell transplantation on a rat model of radioactivity-induced esophageal injury. Cell Death Dis. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, H.; Sun, C.; Liu, D.; Liu, H.; He, J.; Chen, F.; Wang, W.; Jiang, X.; et al. Dental Pulp Mesenchymal Stem Cells Attenuate Limb Ischemia via Promoting Capillary Proliferation and Collateral Development in a Preclinical Model. Stem Cells Int. 2021, 2021, 5585255. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.; Nepal, N.; Li, G.; Yang, N.; Chen, H.; Lin, Q.; Ji, X.; Zhang, S.; Jin, S. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res. Ther. 2021, 12, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Chen, P.; Wang, L.; Wang, J.; Wang, D.; Guo, W.; Zhou, Y.; Li, Q.; Du, H. Stem Cells from Human Exfoliated Deciduous teeth Promote Hair Regeneration in Mouse. Cell Transplant. 2021, 30, 9636897211042927. [Google Scholar] [CrossRef]
- Shiu, S.T.; Lew, W.Z.; Lee, S.Y.; Feng, S.W.; Huang, H.M. Effects of Sapindus mukorossi Seed Oil on Proliferation, Osteogenetic/Odontogenetic Differentiation and Matrix Vesicle Secretion of Human Dental Pulp Mesenchymal Stem Cells. Materials 2020, 13, 4063. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Kocherova, I.; Marycz, K. The effects of chosen plant extracts and compounds on mesenchymal stem cells-a bridge between molecular nutrition and regenerative medicine- concise review. Phytother. Res. 2017, 31, 947–958. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L.; Jin, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical and Biological Characteristics of Mexican Chia Seed Oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Nien, C.J.; Chen, L.G.; Huang, K.Y.; Chang, W.J.; Huang, H.M. Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In vivo and in vitro Testing. Int. J. Mol. Sci. 2019, 20, 2579. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Ghareghani, M.; Zibara, K.; Tajali Ardakani, M.; Jand, Y.; Azari, H.; Nikbakht, J.; Ghanbari, A. Alyssum homolocarpum seed oil (AHSO), containing natural alpha linolenic acid, stearic acid, myristic acid and β-sitosterol, increases proliferation and differentiation of neural stem cells in vitro. BMC Complement. Altern. Med. 2019, 19, 113. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Radha; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Ayaz, A.; Saqib, S.; Zaman, W.; Butt, M.A.; Ullah, A. Silene conoidea L.: A Review on its Systematic, Ethnobotany and Phytochemical profile. Plant Sci. Today 2019, 6, 373–382. [Google Scholar] [CrossRef]
- David, M.; Ain, Q.U.; Ahmad, M.; Zaman, W.; Jahan, S. A biochemical and histological approach to study antifertility effects of methanol leaf extract of Asplenium dalhousiae Hook. in adult male rats. Andrologia 2019, 51, e13262. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhao, B.; Zhang, W.; Jia, L.; Zhang, Y.; Xu, X. Curcumin promotes osteogenic differentiation of periodontal ligament stem cells through the PI3K/AKT/Nrf2 signaling pathway. Iran. J. Basic Med. Sci. 2020, 23, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ling, D.; Zhang, F.; Fu, X.; Lai, D.; Zhang, Y. Curcumin promotes osteogenic differentiation of human periodontal ligament stem cells by inducting EGR1 expression. Arch. Oral Biol. 2021, 121, 104958. [Google Scholar] [CrossRef]
- Zhang, L.N.; Wang, X.X.; Wang, Z.; Li, K.Y.; Xu, B.H.; Zhang, J. Berberine improves advanced glycation end products-induced osteogenic differentiation responses in human periodontal ligament stem cells through the canonical Wnt/β-catenin pathway. Mol. Med. Rep. 2019, 19, 5440–5452. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Fu, Y.; Li, C.; Zheng, L.; Huang, D.; Zhou, C.; Sun, J.; Zhou, X. Berberine mediates root remodeling in an immature tooth with apical periodontitis by regulating stem cells from apical papilla differentiation. Int. J. Oral Sci. 2020, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.C.; Wu, Q.S.; Jin, S.; Luo, A.H.; Sun, D.G.; Wang, F. Berberine Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells Through Activating EGFR-MAPK-Runx2 Pathways. Pathol. Oncol. Res. 2020, 26, 1677–1685. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, B.; Luo, Y.; Hu, N.; Chen, D.; Zhang, X.; Xiong, Y. Protective effect of berberine against oxidative stress-induced apoptosis in rat bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2016, 12, 4041–4048. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chin, Y.T.; Kuo, P.J.; Lee, H.W.; Huang, H.M.; Lin, H.Y.; Weng, I.T.; Hsiung, C.N.; Chan, Y.H.; Lee, S.Y. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-glucoside potentiates self-renewal of human dental pulp stem cells via the AMPK/ERK/SIRT1 axis. Int. Endod. J. 2018, 51, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Parveen, Z.; Khan, M.R. Evaluation of antioxidant, anti-inflammatory, analgesic and antipyretic activities of the stem bark of Sapindus mukorossi. BMC Complement. Altern. Med. 2017, 17, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Chen, Y.Y.; Jiao, Q.Y.; Khan, A.; Li, F.; Han, D.F.; Cao, G.D.; Lou, H.X. Triterpenoid saponins from the pulp of Sapindus mukorossi and their antifungal activities. Phytochemistry 2018, 147, 1–8. [Google Scholar] [CrossRef]
- Xing, Y.; Mi, C.; Wang, Z.; Zhang, Z.H.; Li, M.Y.; Zuo, H.X.; Wang, J.Y.; Jin, X.; Ma, J. Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3. Pharmacol. Res. 2018, 135, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xiong, Y.; Zhang, Y.; Jia, L.; Zhang, W.; Xu, X. Rutin promotes osteogenic differentiation of periodontal ligament stem cells through the GPR30-mediated PI3K/AKT/mTOR signaling pathway. Exp. Biol. Med. 2020, 245, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, W.; Xiong, Y.; Zhang, Y.; Jia, L.; Xu, X. Rutin protects human periodontal ligament stem cells from TNF-α induced damage to osteogenic differentiation through suppressing mTOR signaling pathway in inflammatory environment. Arch. Oral Biol. 2020, 109, 104584. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Ma, D.; Gao, H.; Zheng, D.; Zhang, J. Resveratrol rescues TNF-[alpha]-induced inhibition of osteogenesis in human periodontal ligament stem cells via the ERK1/2 pathway. Mol. Med. Rep. 2020, 21, 2085–2094. [Google Scholar]
- Zhang, J.; Li, R.; Man, K.; Yang, X.B. Enhancing osteogenic potential of hDPSCs by resveratrol through reducing oxidative stress via the Sirt1/Nrf2 pathway. Pharm. Biol. 2022, 60, 501–508. [Google Scholar] [CrossRef]
- Sun, J.; Dong, Z.; Zhang, Y.; He, X.; Fei, D.; Jin, F.; Yuan, L.; Li, B.; Jin, Y. Osthole improves function of periodontitis periodontal ligament stem cells via epigenetic modification in cell sheets engineering. Sci. Rep. 2017, 7, 5254. [Google Scholar] [CrossRef]
- Yan, Y.-H.; Li, S.-H.; Li, H.-Y.; Lin, Y.; Yang, J.-X. Osthole Protects Bone Marrow-Derived Neural Stem Cells from Oxidative Damage through PI3K/Akt-1 Pathway. Neurochem. Res. 2016, 42, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, W.; Qin, Z.; Yu, H.; Yu, Z.; Zhong, M.; Sun, K.; Zhang, W. Effects of Naringin on Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells In vitro and In vivo. Stem Cells Int. 2015, 2015, 758706. [Google Scholar] [CrossRef]
- Kajiura, K.; Umemura, N.; Ohkoshi, E.; Ohta, T.; Kondoh, N.; Kawano, S. Shikonin induces odontoblastic differentiation of dental pulp stem cells via AKT-mTOR signaling in the presence of CD44. Connect. Tissue Res. 2021, 62, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Yamano, A.; Kim, J.A.; Lim, J.; Baek, S.H.; Kim, J.E.; Kwon, T.G.; Saito, Y.; Teruya, T.; Choi, S.Y.; et al. Prenylflavonoids isolated from Macaranga tanarius stimulate odontoblast differentiation of human dental pulp stem cells and tooth root formation via the mitogen-activated protein kinase and protein kinase B pathways. Int. Endod. J. 2021, 54, 1142–1154. [Google Scholar] [CrossRef]
- Rolph, D.N.; Deb, M.; Kanji, S.; Greene, C.J.; Das, M.; Joseph, M.; Aggarwal, R.; Leblebicioglu, B.; Das, H. Ferutinin directs dental pulp-derived stem cells towards the osteogenic lineage by epigenetically regulating canonical Wnt signaling. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165314. [Google Scholar] [CrossRef] [PubMed]
- Suardita, K.; Arundina, I.; Tedjosasongko, U.; Yuliati, A.; Peeters, H.H.; Wijaksana, I.K.E.; Surboyo, M.D.C. Concanavalin A Enhanced Proliferation and Osteogenic Differentiation of Dental Pulp Stem Cells. Eur. J. Dent. 2020, 14, 123–127. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yu, C.; Liu, T.; Jia, H. Ginsenoside Rg1 as an effective regulator of mesenchymal stem cells. Front. Pharmacol. 2020, 10, 1565. [Google Scholar] [CrossRef]
- Wang, P.; Wei, X.; Zhou, Y.; Wang, Y.P.; Yang, K.; Zhang, F.J.; Jiang, R. Effect of ginsenoside Rg1 on proliferation and differentiation of human dental pulp cells in vitro. Aust. Dent. J. 2012, 57, 157–165. [Google Scholar] [CrossRef]
- Qiu, J.; Li, W.; Feng, S.H.; Wang, M.; He, Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet. Mol. Res. 2014, 13, 3586–3598. [Google Scholar] [CrossRef]
- Yin, L.-H.; Cheng, W.-X.; Qin, Z.-S.; Sun, K.-M.; Zhong, M.; Wang, J.-K.; Gao, W.-Y.; Yu, Z.-H. Effects of ginsenoside Rg-1 on the proliferation and osteogenic differentiation of human periodontal ligament stem cells. Chin. J. Integr. Med. 2015, 21, 676–681. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Gu, Z.; Lian, M.; Huang, D.; Lu, X.; Feng, X.; Lu, Q. Wedelolactone Enhances Odontoblast Differentiation by Promoting Wnt/β-Catenin Signaling Pathway and Suppressing NF-κB Signaling Pathway. Cell. Reprogram. 2018, 20, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Songsiripradubboon, S.; Kladkaew, S.; Trairatvorakul, C.; Sangvanich, P.; Soontornvipart, K.; Banlunara, W.; Thunyakitpisal, P. Stimulation of Dentin Regeneration by Using Acemannan in Teeth with Lipopolysaccharide-induced Pulp Inflammation. J. Endod. 2017, 43, 1097–1103. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kim, H.J.; Hwang, Y.C.; Rosa, V.; Yu, M.K.; Min, K.S. Effects of Epigallocatechin Gallate, an Antibacterial Cross-linking Agent, on Proliferation and Differentiation of Human Dental Pulp Cells Cultured in Collagen Scaffolds. J. Endod. 2017, 43, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Park, W.J.; Han, Y. Asarylaldehyde enhances osteogenic differentiation of human periodontal ligament stem cells through the ERK/p38 MAPK signaling pathway. Biochem. Biophys. Res. Commun. 2021, 545, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Zhang, W.; Cui, Q.; Fu, Y.; Li, H.; Zhang, J. Kaempferol promotes proliferation and osteogenic differentiation of periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Life Sci. 2020, 258, 118143. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qi, Y.; Zhou, Q.; Huang, X.; Deng, X.; Yu, Y.; Shi, L.E. Betulinic acid promotes the osteogenic differentiation of human periodontal ligament stem cells by upregulating EGR1. Acta Biochim. Biophys. Sin. 2021, 53, 1266–1276. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, L.; Zhao, B.; Xiong, Y.; Wang, Y.N.; Liang, J.; Xu, X. Quercetin reverses TNF-α induced osteogenic damage to human periodontal ligament stem cells by suppressing the NF-κB/NLRP3 inflammasome pathway. Int. J. Mol. Med. 2021, 47, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, L.; Yang, J.; Wang, Z.; Cheng, L. Quercetin promotes osteogenic differentiation and antioxidant responses of mouse bone mesenchymal stem cells through activation of the AMPK/SIRT1 signaling pathway. Phyther. Res. 2021, 35, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.D.; Patel, D.K.; Jin, B.; Choi, S.I.; Lee, O.H.; Lim, K.T. Effects of Cirsium setidens (Dunn) Nakai on the osteogenic differentiation of stem cells. Mol. Med. Rep. 2021, 23, 264. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, J.; Liu, H.; Niu, C.; Chen, D. Salidroside promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells through the BMP signaling pathway. Exp. Ther. Med. 2022, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y. Effect of puerarin on osteogenic differentiation of human periodontal ligament stem cells. J. Int. Med. Res. 2020, 48, 300060519851641. [Google Scholar] [CrossRef]
- Cao, J.; Qiu, X.; Gao, Y.; Cai, L. Puerarin promotes the osteogenic differentiation of rat dental follicle cells by promoting the activation of the nitric oxide pathway. Tissue Cell 2021, 73, 101601. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Zhang, F.; Niu, Z.; Shi, S. Effect of icariin on cell proliferation and the expression of bone resorption/formation-related markers in human periodontal ligament cells. Mol. Med. Rep. 2013, 8, 1499–1504. [Google Scholar] [CrossRef]
- Zhang, X.; Han, N.; Li, G.; Yang, H.; Cao, Y.; Fan, Z.; Zhang, F. Local icariin application enhanced periodontal tissue regeneration and relieved local inflammation in a minipig model of periodontitis. Int. J. Oral Sci. 2018, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Romeo, L.; DIomede, F.; Gugliandolo, A.; Scionti, D.; Lo Giudice, F.; Lanza Cariccio, V.; Iori, R.; Bramanti, P.; Trubiani, O.; Mazzon, E. Moringin Induces Neural Differentiation in the Stem Cell of the Human Periodontal Ligament. Sci. Rep. 2018, 8, 9153. [Google Scholar] [CrossRef]
- Joe, I.S.; Jeong, S.G.; Cho, G.W. Resveratrol-induced SIRT1 activation promotes neuronal differentiation of human bone marrow mesenchymal stem cells. Neurosci. Lett. 2015, 584, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.W.; Zhang, Z.; Liu, M.Y.; Hu, W.P. Differentiation of human dental pulp stem cells into neuronal by resveratrol. Cell Biol. Int. 2017, 41, 1391–1398. [Google Scholar] [CrossRef]
- Jahan, S.; Singh, S.; Srivastava, A.; Kumar, V.; Kumar, D.; Pandey, A.; Rajpurohit, C.; Purohit, A.; Khanna, V.; Pant, A. PKA-GSK3β and β-Catenin Signaling Play a Critical Role in Trans-Resveratrol Mediated Neuronal Differentiation in Human Cord Blood Stem Cells. Mol. Neurobiol. 2018, 55, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Songsaad, A.; Gonmanee, T.; Ruangsawasdi, N.; Phruksaniyom, C.; Thonabulsombat, C. Potential of resveratrol in enrichment of neural progenitor-like cell induction of human stem cells from apical papilla. Stem Cell Res. Ther. 2020, 11, 542. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Meng, N.; Yao, N.; Zhang, K.; Li, Q.; Zhang, Y.; Xing, Q.; Han, K.; Song, J.; et al. Resveratrol Exerts Dosage-Dependent Effects on the Self-Renewal and Neural Differentiation of hUC-MSCs. Mol. Cells 2016, 39, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Namiki, J.; Suzuki, S.; Masuda, T.; Ishihama, Y.; Okano, H. Nestin protein is phosphorylated in adult neural stem/progenitor cells and not endothelial progenitor cells. Stem Cells Int. 2012, 2012, 430138. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, Y.B.; Jaber, M.H.; Ahmed, H.M. Assessment of a novel activity of Acacia nilotica leaf extract for chondrogenesis induction of Mesenchymal stem cells for dental applications. Phytomed. Plus 2021, 1, 100087. [Google Scholar] [CrossRef]
- Girija, D.M.; Kalachaveedu, M.; Rao, S.R.; Subbarayan, R. Transdifferentiation of human gingival mesenchymal stem cells into functional keratinocytes by Acalypha indica in three-dimensional microenvironment. J. Cell. Physiol. 2018, 233, 8450–8457. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, S.Y.; Park, K.R.; Kim, E.C. Baicalein Promotes Angiogenesis and Odontoblastic Differentiation via the BMP and Wnt Pathways in Human Dental Pulp Cells. Am. J. Chin. Med. 2016, 44, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective Mechanism of the Antioxidant Baicalein toward Hydroxyl Radical-Treated Bone Marrow-Derived Mesenchymal Stem Cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef] [PubMed]
- Aryal, Y.P.; Yeon, C.Y.; Kim, T.Y.; Lee, E.S.; Sung, S.; Pokharel, E.; Kim, J.Y.; Choi, S.Y.; Yamamoto, H.; Sohn, W.J.; et al. Facilitating Reparative Dentin Formation Using Apigenin Local Delivery in the Exposed Pulp Cavity. Front. Physiol. 2021, 12, 773878. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, Y.; Xie, W.; Wei, D.; Du, Q. Tanshinone IIA promotes osteogenic differentiation of human periodontal ligament stem cells via ERK1/2-dependent Runx2 induction. Am. J. Transl. Res. 2019, 11, 340–350. [Google Scholar] [PubMed]
- Jang, H.-O.; Ahn, T.-Y.; Ju, J.-M.; Bae, S.-K.; Kim, H.-R.; Kim, D.-S. Odontogenic Differentiation-Induced Tooth Regeneration by Psoralea corylifolia L. Curr. Issues Mol. Biol. 2022, 44, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
| Cell Source | Study Type | Major Findings | References |
|---|---|---|---|
| Oral Stem Cells Mediated Dental Regeneration | |||
| DPSCs | In vivo study to access viability of DPSC constructs for dental pulp regeneration | DPSCs implanted in human tooth root canal were differentiated into odontoblast-like mineralizing cells and human CD31—positive endothelial cells were found at the center of regenerated tissue. | [98] |
| hPDLSCs | In vivo study to examine bone regeneration potential of hPDLSCs with nHA/CG scaffolds in critical sized jawbone defects in minipigs | hPDLSCs with nHA/CG scaffolds increased new bone formation and generated bones larger in size with normal vascularization and architectures. It is also observed that in the bone marrow formed in the hPDLSCs/nHA/CG group, runt-related transcription factor 2 (Runx2) was highly expressed. | [112] |
| DPSCs | Pre-clinical study to develop technology of DPSCs seeded fibrin gel implant formation, with the same size and shape as the bone defect at the site of implantation | In mice, DPSCs seeded fibrin gel implants increased the bone tissue vascularization and volume. | [119] |
| hPDLSCs | In vitro study to examine the hPDLSCs differentiation into cementogenic, osteogenic, and fibrogenic lineages for the cementum-PDL-bone periodontium regeneration | In osteogenic medium, hPDLSCs shows high expressions of osteogenic genes i.e., ALP, RUNX2, COL1, and OPN (14 and 21 days), produced ALP activity and mineral nodules (5 and 10 folds of control). | [121] |
| In fibrogenic medium, hPDLSCs show increased PDL fibrogenic genes expression levels, including FSP-1, PLAP-1, COL1, elastin, and COL3 (28 days) (20–70 folds of control). | |||
| In cementogenic medium, hPDLSCs showed high expressions of cementum genes i.e., BSP, CEMP1, and CAP (21 days) (10–15 folds of control), and synthesized mineralized cementum 40 folds via ALP staining and 50 folds via ABS. | |||
| Oral Stem Cell-mediated Neural Regeneration | |||
| DPSCs | To examine the efficiency of the combination of DPSCs with bioactive hydrogels for repairing large gap peripheral nerve injuries | Direct differentiation of exogeneous DPSCs in CSM-GF conduit resulted in the formation of new nerve tissues at the defect site. This study also demonstrated that bioactive hydrogels combined with DPSCs could regenerate myelinated nerve fibers and Schwann cells. | [122] |
| DPSCs | To examine the efficacy of DPSCs neuronal differentiation induction by EGF and bFGF. | EGF and bFGF-treated DPSCs shows increase in the expression of the neuroprogenitor markers of SRY (sex determining region Y)-box 2 (SOX2) and nestin after 72 h. | [124] |
| A significant increase in transcript levels of nestin, neurogenin 1 (Ngn1), and microtubule-associated protein 2 (MAP2) was observed post treatment as compared to cells maintained in the control media. Treatment also resulted in formation of some neuron-like cells. | |||
| SHEDs | To examine the efficacy of conditioned medium from SHEDs in improving cognitive function in Alzheimer’s disease mouse model | Pro-inflammatory responses induced by β-amyloid plaques is attenuated by SHED-CM and generated a tissue-regenerating/anti-inflammatory environment, accompanied by anti-inflammatory M2-like microglia induction. | [140] |
| SHEDs | In vivo study to examine the beneficial effects on diabetic polyneuropathy in mice by secreted factors in conditioned medium of SHED-CM | In the diabetic mice model, the decline in sensory nerve conduction velocities was significantly prevented in SHED-CM as compared to DMEM. Neurite outgrowth of dorsal root ganglion neurons was also significantly enhanced in SHED-CM. | [145] |
| Oral Stem Cell-mediated Bone Regeneration | |||
| SHEDs | In vivo study to examine the one-time transplantation of SHED may prevent the tail vein ameliorates ovariectomy (OVX) -induced early osteoporotic phenotype | SHED via a FasL/Fas pathway mediated T-cell apoptosis which as result ameliorates the osteopenia phenotype and immune tolerance in OVX mice. | [30] |
| DPSCs | In vivo study to examine DPSCs for possible application in tendon tissue engineering | It is observed that when DPSCs was transplanted in aligned PGA fiber scaffolds, tendon-related markers including tenascin-C, scleraxis, collagens I, eye absent homologue 2 and VI and tenomodulin were significantly enhanced. | [147] |
| DPSC-PGA constructs on transplantation in a mouse model resulted in the formation of mature tendon-like tissue under mechanical loading conditions. | |||
| hDPSCs | A preliminary in vivo study to examine chondrogenic ability of hDPSCs (cultured in an alginate scaffold) to regenerate articular cartilage | It is observed that hDPSCs express collagen II and aggrecan. Significant cartilage regeneration was observed 3 months post implantation of hDPSCs cultured in 3% alginate hydrogels in a rabbit model with cartilage damage. | [154] |
| hPDLSCs | In vivo study to examine effects of hPDLSCs with RHC/CS scaffolds on the repair of critical-size skull injury in rats | It is observed that hPDLSCs proliferate and undergo osteogenic differentiation in TRFS (p < 0.05) accelerated by TGF-β3. | [158] |
| Oral Stem Cells Mediated Regeneration of Other Tissues | |||
| DPSCs | In vitro study to examine the potential utility of DPSCs as an autologous cell source for corneal endothelial therapies by adopting a two-step differentiation protocol for DPSCs | DPSCs were differentiated into neural crest stem-like cells, confirmed by the overexpression of neural crest stem cell markers such as p75, nestin, and AP2 markers. | [14] |
| In second step neural crest stem-like cells were then differentiated into corneal endothelial-like cells, confirmed by the higher expression level of markers such as of COL4A2, ZO-1, COL8A2, ATP1A1 markers. | |||
| DPSCs | In vitro study to examine the ability of DPSCs to differentiate into cochlear hair cell | DPSCs were successfully able to differentiate into neural stem cells with mean 24% nestin-positive cells. | [165] |
| NSCs derived from DPSC were differentiated into inner ear hair cell-like cells with 81% average cells presenting myosin VIIa. | |||
| hDPSCs—cryo | In vitro study to examine ability of long-term cryopreserved dental pulp tissues to differentiation into HLCs and DE cells | It is observed that hDPSCs—cryo were successfully differentiated into DE and functional hepatocytes. Differentiated HLCs (30th day) and DE cells (6th day) significantly increased hepatocyte- and DE-specific markers at the mRNA and protein level. | [169] |
| SHED | In vivo study to examine ability of SHED with C57BL/6 mice skin cells to improve hair regeneration | It is observed that SHED up regulated the expression of Shh and Gli1 pathway. | [174] |
| SHED and skin cells of C57 mice when co-transplanted to nude mice, they were found to promote hair regeneration. | |||
| Source | Bioactive Compounds | Type of Study | Major Findings and Mechanism of Action | References |
|---|---|---|---|---|
| Artemisia annua | Artemisinin (Sigma Aldrich, St. Louis, MO, USA) | In vitro study investigated the effect of Artemisinin on hypoxia and TNF-α mediated osteogenesis impairment in DPSCs | Artemisinin reversed the suppression of cell survival caused by hypoxia or inflammation in DPSCs, along with restoring the osteogenic differentiation potential of DPSCs | [91] |
| Sapindus mukorossi | Seed oil (He He Co., Ltd., Taipei, Taiwan) | In vitro study to examine the effects of S. mukorossi (seed oil) on the differentiation and proliferation of DPSCs | Enhanced the odontogenic/osteogenic differentiation potential of DPSCs by upregulation of ALP gene expression and mineralization-related extracellular vesicle secretion | [175] |
| Curcuma longa | Curcumin (Sigma Aldrich, St. Louis, MO, USA) | In vivo study on effect of curcumin on hPDLSCs osteogenic differentiation | Curcumin increased protein and mRNA levels of COL1, ALP, RUNX2, and activated PI3K/AKT/Nrf2 signaling pathway | [185] |
| Curcuma longa | Curcumin (Solarbio Life Sciences, China) | In vivo study Curcumin displays promoting osteogenic differentiation and its mechanism | Curcumin 10 µmol/L treatment maximal promoting the cells viability, ALP activities, mineralization, and levels of Runx2, OC, OPN, Collagen I, and EGR-1 in hPDLSCs | [186] |
| Berberis vulgaris | Berberine hydrochloride (Wako Pure Chemical Industries, Ltd., USA) | In vitro study to examine effects of AGE and berberine hydrochloride on the hPDLSCs’ osteogenic differentiation ability | Berberine hydrochloride was able to reverse the inhibition of the PDLSCs’ osteogenic potential in an AGEs enriched microenvironment, partly by inhibition of the β-catenin and canonical Wnt pathway | [187] |
| Berberis vulgaris | Berberine (Sigma Aldrich, St. Louis, MO, USA) | In vivo study to examine the effect of berberine on rat root canals of immature teeth with apical periodontitis | Berberine induced β-catenin expression and activated the β-catenin and canonical Wnt pathway in SCAPs which improved root repair in immature teeth with apical periodontitis. | [188] |
| Berberis vulgaris | Berberine (Sigma Aldrich, St. Louis, MO, USA) | In vitro study to examine effects of berberine on the osteogenesis and cell proliferation of DPSCs | Berberine enhanced hDPSC cell proliferation in a dose-dependent pattern and activated MAPK–EGFR–Runx2 signaling pathways. | [189] |
| Reynoutria multiflora (Thunb.) Moldenke | 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D–glucoside (THSG) (Taipei Medical University, Taipei, Taiwan) | In vitro study investigated the effect of THSG on cell proliferation in hDPSCs. | THSG treatment enhance d the renewal ability and proliferative potential of hDPSCs via the AMPK/ERK/SIRT1 axis | [191] |
| Dictamnus dasycarpus | Fraxinellone (Chengdu Herbpurify Co., Ltd., Chengdu, China) | In vitro and in vivo study to examine antitumor effects of fraxinellone on lung cancer cells | Fraxinellone treatment inhibits expression of PD-L1 by HIF-1α and STAT3 signaling pathway downregulation, further inhibiting angiogenesis and proliferation in cancer cells | [194] |
| Fagopyrum esculentum | Rutin (Solarbio Science & Technology Co., Ltd., Beijing, China) | In vitro study to examine the effects of rutin on the PDLSCs’ osteogenic proliferation and differentiation | Rutin increased osteogenic differentiation and proliferation of PDLSCs by GPR30-mediated PI3K/AKT/mTOR signal transduction | [195] |
| Cnidium monnieri | Osthole (National Institutes for Food and Drug Control, Beijing, China) | In vitro study to determine osthole efficiency against defective osteogenic differentiation of P-PDLSCs via epigenetic modification | Osthole (10−7 Mol/L) upregulated MORF, MOZ, and histone acetylases that catalyze acetylation of Histone 3 lisine14 (H3K14) and Histone 3 lisine9 (H3K9) | [199] |
| Osthole treatment enhanced bone formation and cell sheet formation of PDLSC sheets in periodontitis (animal models) | ||||
| Drynaria roosii Nakaike | Naringin (National Institute for the Control of Pharmaceutical and Biological Products, China) | In vitro and in vivo study to examine the effect of naringin on proliferation and osteogenic differentiation of hPDLSCs | Naringin (1 µM) efficiently promoted hPDLSC differentiation and proliferation and increased expression levels was observed in related genes (COL1A2, OPN, RUNX2, and OCN) as compared to the control group | [201] |
| Macaranga tanarius | Isonymphaeol B (Okinawa, Japan) | In vitro study to identify odontogenesis-promoting compounds and examine the molecular mechanism underlying enhanced tooth formation and odontoblast differentiation | Isonymphaeol B shows stimulatory effects on tooth root, dentine formation and odontoblast differentiation via AKT and MAP kinase signaling pathways | [203] |
| Canavalia ensiformis | Concanavalin A (Sigma Aldrich, USA) | In vitro study to determine the effect of concanavalin A on osteogenic and proliferation differentiation of DPSCs | Concanavalin A at concentration of 5 and 10 µg/mL to DPSCs significantly increased the osteogenic and proliferation differentiation of DPSCs (p ≤ 0.05) | [205] |
| Panax ginseng | Ginsenoside Rg1 (Bio-function, Beijing, China) | In vitro study to examine the effects of ginsenoside Rg-1 on osteogenic differentiation and proliferation of hPDLSCs | Ginsenoside Rg-1 enhanced osteogenic differentiation and proliferation of hPDLSCs at an optimum concentration of 10 μmol/L | [209] |
| Eclipta prostrata | Wedelolactone (Dalian, China) | In vitro study investigated effect of wedelolactone on odontoblast differentiation of DPSCs | Wedelolactone induced odontoblast differentiation through NRP1, Sema3A, and NF-κB pathway inhibition and activation of b-catenin pathway | [210] |
| Aloe barbadensis Mill. | Acemannan (Chulalongkorn University, Bangkok, Thailand) | In vitro study to examine effects of acemannan on human deciduous pulp cells and the response after vital pulp therapy in dog deciduous teeth | DPSC proliferation was significantly enhanced by acemannan along with an increase in expression levels of type I collagen, BMP-2, vascular endothelial growth factor, BMP-4, alkaline phosphatase, dentin sialoprotein, and mineralization | [211] |
| Cirsium setidens (Dunn) Nakai | Methanolic extract (Kangwon National University, Republic of Korea) | In vitro study to examine the effects of methanolic extracts of C. setidens on osteogenic differentiation of hPDLSCs | Methanolic extract treatment with concentration of 0.05% significantly increased the viability of PDLSCs and also increased the expression levels of alkaline phosphatase, collagen 1, runt related transcription factor 2, and bone sialoprotein | [218] |
| Rhodiola rosea | Salidroside (Chengdu Must Bio-Technology Co., Shanghai, China) | In vitro study to investigate the effect of salidroside on the odontogenic differentiation and proliferation of hDPSCs | Treatment with salidroside promoted DPSCs cell viability, along with promoting their differentiation into odontogenic and osteogenic linage via activation of the BMP signaling pathway | [219] |
| Moringa oleifera | Moringin (Indena India Pvt. Ltd.; Bangalore, India) | In vitro study to examine efficacy of moringin to induce PDLSCs toward neural progenitor differentiation | Treatment of PDLSCs with moringin resulted in the induction of PDLSC differentiation to neural progenitor cells via increased gene expression levels of genes that were involved in neuron cortical development | [224] |
| Acacia nilotica | Aqueous leaves extract (Hormavu, Bangalore) | In vitro study to investigate the efficacy of Acacia nilotica leaves extract in chondrogenesis induction from mesenchymal stem cells | Treatment of aqueous leaves extract promoted chondrogenesis induction in DPSCs by upregulating the expression of various proteins in the cellular matrix, such as aggrecan, sox9, glycosaminoglycan (GAG), and collagen 2α1 (Col2α1) | [231] |
| Acalypha indica | Methanolic extract (Porur, Chennai, India) | In vitro study on GMSCs highlighted the potential of A. indica (methanolic extract) in increasing the wound healing ability of GMSCs | Treatment of A. indica extract (25 µg/mL) wound closure activity of GMSCs was increased up to 56.91 ± 1.21% in 24 h, while the percentage of wound closure was further enhanced to 89.23 ± 1.09% post 48 h of treatment | [232] |
| Scutellaria baicalensis | Baicalein (Sigma Aldrich, USA) | In vitro study investigated the effect of Baicalein on Angiogenesis and Odontoblastic Differentiation | Baicalein promoted odontoblastic differentiation and angiogenesis of HDPCs by activating the BMP and Wnt/β-catenin signal pathways | [233] |
| Salvia miltiorrhiza | Tanshinone IIA (Sigma Aldrich, USA) | TSA affects the osteogenic differentiation of hPDLSCs. | Tanshinone IIA can induce hPDLSC osteogenesis through the ERK1/2-Runx2 axis | [236] |
| Cullen corylifolium L. | Bakuchiol and C. corylifolium extract (KMD medicinal herbs Co., Yunnan, China) | In vitro study to examine the efficacy of Bakuchiol and C. corylifolium extract as differentiation-inducing substances for tooth regeneration, was determined by monitoring odontogenic differentiation in hDPSCs | Bakuchiol and C. corylifolium extract significantly enhance the odontogenic differentiation potential of hDPSCs via upregulation of odontogenic differentiation marker genes, such as dentin matrix acidic phospho-protein-1, alkaline phosphatase, osteocalcin, and Runt-related transcription factor 2 | [237] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuja, A.; Tyagi, P.K.; Kumar, M.; Sharma, N.; Prakash, S.; Radha; Chandran, D.; Dhumal, S.; Rais, N.; Singh, S.; et al. Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement. Cells 2022, 11, 2792. https://doi.org/10.3390/cells11182792
Ahuja A, Tyagi PK, Kumar M, Sharma N, Prakash S, Radha, Chandran D, Dhumal S, Rais N, Singh S, et al. Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement. Cells. 2022; 11(18):2792. https://doi.org/10.3390/cells11182792
Chicago/Turabian StyleAhuja, Anami, Pankaj Kumar Tyagi, Manoj Kumar, Naveen Sharma, Suraj Prakash, Radha, Deepak Chandran, Sangram Dhumal, Nadeem Rais, Surinder Singh, and et al. 2022. "Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement" Cells 11, no. 18: 2792. https://doi.org/10.3390/cells11182792
APA StyleAhuja, A., Tyagi, P. K., Kumar, M., Sharma, N., Prakash, S., Radha, Chandran, D., Dhumal, S., Rais, N., Singh, S., Dey, A., Senapathy, M., Saleena, L. A. K., Shanavas, A., Mohankumar, P., Rajalingam, S., Murugesan, Y., Vishvanathan, M., Sathyaseelan, S. K., ... Mekhemar, M. (2022). Botanicals and Oral Stem Cell Mediated Regeneration: A Paradigm Shift from Artificial to Biological Replacement. Cells, 11(18), 2792. https://doi.org/10.3390/cells11182792











