Abstract
Spinal cord injury (SCI) is characterized by a complex and prolonged injury process that exacerbates the damage induced by the primary injury and inhibits the potential for regeneration. SCI frequently results in the devastating loss of neurological functions and thus has serious consequences on patient quality of life. Current treatments are limited and focus on early interventions for the acute management of complications. Therefore, the development of novel treatments targeting ongoing injury processes is required to improve SCI outcomes. We aimed to systematically review studies published in the last 10 years that examined experimental treatments with neuroregenerative and neuroprotective capabilities for the improvement of SCI. We analyzed treatments from 44 studies that were identified through a systematic literature search using three databases: PubMed, Web of Science and EMBASE (searched through Ovid). We performed a meta-analysis for Basso-Beattie-Bresnahan (BBB) locomotion test data and collected immunohistochemistry results to demonstrate neuroregenerative and neuroprotective properties of the treatments, respectively. The two treatments that illustrated the most significant improvements in functional recovery using the BBB test were the combined use of tetrahedral framework nucleic acid (tFNA) with neural stem cells (NSCs) and Fortasyn® Connect (FC) supplementation. Both treatments also attenuated secondary injury processes as demonstrated through immunohistochemistry. Combined tFNA with NSCs and FC supplementation are promising treatments for the improvement of SCI as they both demonstrate neuroregenerative and neuroprotective properties. Further pre-clinical testing is required to validate and determine the long-term efficacies of these treatments for the improvement of SCI.
1. Introduction
Spinal cord injury (SCI) is characterized by damage to the spinal cord resulting in short- or long-term implications to normal sensory, motor, and autonomic functions thus leading to debilitating conditions through impacting an individual’s physical, psychological, and social well-being [1,2,3]. SCI can be due to traumatic or non-traumatic injuries, with traumatic SCI contributing to more than 90% of the total number of affected individuals [1,4]. Common causes for traumatic SCI include road traffic accidents, falls, sports, violence, whilst common causes for non-traumatic injuries include infection, cancer, and disease [1,2]. Globally, it is estimated that SCI affects between 250,000 to 500,000 individuals per year resulting in significant financial burdens for both patients and healthcare services [3,4,5,6,7]. The outcome of SCI depends heavily on the severity and the level of injury, with cervical level injuries, the most common type of SCI, typically resulting in quadriplegia, and thoracic level injures resulting in paraplegia [4]. Ultimately, SCI results in disruption to the complex multicellular interactions present within normal spinal cord physiology which consequently leads to compromised recovery [5,6].
Currently, treatments for SCI are relatively limited and focus on early diagnosis and surgical intervention during the immediate and primary phase of injury to limit the potential loss of neurological functions. However, ongoing injury processes such as the loss of neuronal and glial cells, demyelination, glial scar and cystic cavity formation observed during the secondary phase of injury, result in progressive neurodegeneration and inhibit the potential for regeneration to occur [5,6,7,8]. No reparative treatment exists for SCI with only Lyrica being approved as a palliative treatment for neuropathic pain. Therefore, there is an urgent medical need to devise new therapies that target the underlying pathophysiological processes after SCI.
There have been a number of experimental treatment strategies targeting these processes, for example to promote neuroprotection and neuroregeneration in order to improve the neurological outcomes following SCI. The aim of this study was to systematically review the experimental treatment options which have demonstrated, specifically, neuroregenerative and neuroprotective capabilities for SCI, developed in the last 10 years, and ascertain the best treatment options for further pre-clinical validation. Neuroregeneration through enhanced functional recovery, as observed using the Basso-Beattie-Bresnahan (BBB) locomotion test and neuroprotection through observing improvements in the reduction or inhibition of secondary injury processes using immunohistochemistry were the primary and secondary outcomes of this review and were used to determine the best treatment options for SCI developed in the last 10 years.
2. Materials and Methods
2.1. Review Process
A systematic review of the literature was performed by two authors (F.K. and Z.A.) whilst following the guidelines outlined by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [9]. The protocol for this systematic review is also published in the Open Science Forum registries and can be accessed using the following https://doi.org/10.17605/OSF.IO/PKQZ9.
2.2. Literature Search
A search of published literature that examined the effects of experimental treatments for SCI was carried out on 2nd February 2022 using three electronic databases: PubMed, Web of Science and EMBASE (searched through Ovid). The common search strings used for all three electronic databases were ‘(spinal cord injury) AND (treatments) AND (neuroprotection) AND (axon regeneration)’. Additional search terms included “AND/OR SCI” AND/OR neural regeneration”, “AND/OR neurorehabilitation”, “AND/OR rehabilitation” and “AND/OR neuroprotective”. The literature search was limited to reports written in the English language and only covering reports on neuroregenerative and neuroprotective treatments. Any duplicates generated amongst the three electronic databases were removed and initial shortlisting of manuscripts was performed through screening the titles and abstracts of papers against the outlined inclusion and exclusion criteria. Shortlisted studies were then reviewed and assessed for eligibility after a full text read which resulted in the final selection of 44 studies.
2.3. Inclusion and Exclusion Criteria
To determine eligibility for this systematic review, studies were screened against the following criteria: (1) only manuscripts describing preclinical in vivo animal studies were included (some of these studies also reported in vitro data but this was not analyzed nor included in our systematic review as we were only interested in studies that reported animal data and hence were closer to translation); (2) only studies published in the last 10 years from the years 2012 to 2022 were included; and (3) only studies examining effects of therapeutic treatments on cervical or thoracic spinal cord injuries were included, studies with other central nervous system injuries were not included. Clinical studies, reviews, systematic reviews, books/documents, clinical trials, meta-analyses, and conference articles were all excluded. Assessment of study eligibility was carried out in an unblinded and standardized manner and independently by two reviewers (F.I.K. and Z.A.).
2.4. Risk of Publication Bias
To evaluate the risk of bias in the final selection of the 44 studies, SYRCLE’s risk of bias tool for animal studies, adapted from Cochrane’s risk of bias tool was used [10]. Each study was assessed against 10 different risk parameters to determine the overall risk of bias, and responses to each risk parameter were recorded as the following: (a) yes, if the parameter was positively reported with sufficient supporting methodology; (b) yes, if the parameter was positively reported but had unknown or insufficient methodology; or (c) no, if the parameter was negatively reported in the study or if the parameter was not reported at all. The risk of bias was assessed by two independent reviewers (F.I.K. and Z.A), with any disagreements being settled through discussion.
2.5. Data Extraction and Synthesis
Data collection of the key characteristics of each paper including the author(s), year of publication, location of where the study was conducted, whether an in vitro or in vivo study was performed, the spinal level at which the injury was induced, the treatment used, and the experimental techniques performed were summarized in a pre-designed table. Data for the BBB locomotion test was also collected and summarized in a pre-designed table, studies that did not perform this test were identified and data was recorded as not reported. Data collection for immunohistochemical markers demonstrating neuroprotective or neuroregenerative effects of treatments was also reported and summarized within a pre-designed table, studies that did not perform immunohistochemistry were identified and recorded as not reported.
2.6. Statistical Analysis
Assessment of heterogeneity was performed by examining the differences across studies for methodological heterogeneity. We used Review Manager (RevMan 5.3, Cochrane Informatics & Technology, London, UK) to determine the Q and I2 statistics (in percentage) to establish variation between the studies attributed to heterogeneity. A meta-analysis of sub-groups of studies which reported overall improvement in BBB scores at different time points after injury and treatment was conducted in RevMan 5.3 (Cochrane Informatics & Technology), using the continuous data function, employing a random effects model and selecting mean difference as the effect measure.
3. Results
3.1. Study Selection
Applying the common search strings to all three electronic databases: PubMed, Web of Science and EMBASE, generated a total of 453 search results. After removing duplicates, 431 results remained for initial screening using the inclusion and exclusion criteria as previously outlined. 118 records were then excluded due to reasons not relevant to the study question such as being a non-experimental study which includes reviews, systematic reviews, books or documents, clinical trials, meta-analyses, and conference articles. In addition, any report not written in the English language was also excluded. This left 313 studies that were screened by reading the titles and abstracts following the previously outlined inclusion and exclusion criteria. Papers published outside the 10-year time period from 2012 to 2022 were excluded, papers assessing treatments for other central nervous system injuries other than SCI were also excluded, and papers attempting to enhance delivery of a previous known treatment or attempting to replicate the results of a previous treatment were also excluded, leaving 130 articles that required a full text read to assess for eligibility. Following full text reads of these articles, the final selection of 44 studies were identified and included for analysis in this systematic review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The literature search process and screening for eligibility is presented in the PRISMA diagram below (Figure 1).
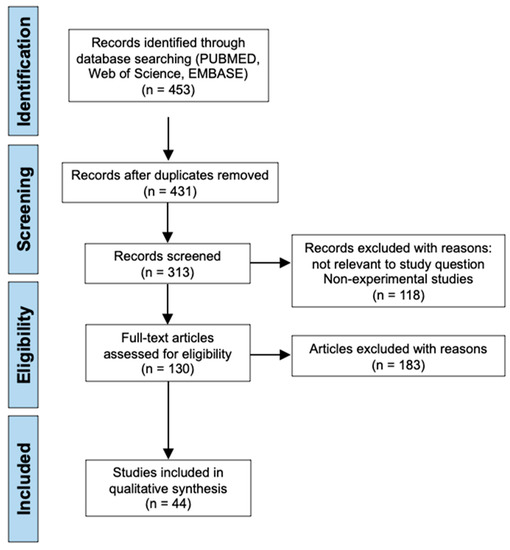
Figure 1.
PRISMA flow chart illustrating the literature search process and screening of papers included in this systematic review.
3.2. Study Characteristics
A summary of the key characteristics of each paper is presented in Table 1. The studies included in this review were conducted in a range of 16 different countries with a large proportion of the studies being based in China (47.7%) followed by the United States of America (USA) (9.1%), and Spain (6.8%). The effects of potential therapeutic treatments for SCI can be observed using both in vitro and in vivo methods. In this review, all of the 44 studies used in vivo methodology with a significant proportion of studies only using in vivo methodology (63.6%) and a smaller proportion utilizing both in vivo and in vitro.

Table 1.
Summary of the key characteristics of all 44 included studies.
The severity of the outcome of SCI can be influenced by the location of where the injury was obtained. The spinal level at which the injury was induced for each of the studies was therefore reported in Table 1. The majority of the studies used a thoracic level injury (93.2%) leaving only two studies by Karalija et al. [23] and Chen et al. [49] that performed cervical level injuries at the spinal levels C3 and C5, respectively. There was also a single study performed by Karalija et al. [24] that induced a SCI in the lumbar region of the spinal cord at L5. The most common injury was at the level T10 (25.0%) closely followed by T9 (22.7%) and then T8 (15.9%).
Eleven of the included studies had reported a range for the level of injury rather than a single spinal level and therefore, the range for all studies reporting thoracic level injuries was calculated to be from the level T6 to T12. The type of experimental treatment that was analyzed by the studies varied significantly as demonstrated in Table 1. Treatments ranged from pharmacological drugs, immunomodulatory treatments, dietary supplementations, stem cell-based transplantations, and even stem cell conditioned mediums. A proportion of the studies had also reported using combined treatment strategies for the synergistic treatment of SCI instead of a single experimental treatment.
A range of many different experimental techniques to analyze the effectiveness of the various treatments were performed in the 44 studies as listed in Table 1. A frequent key aim of these studies was to assess neuronal regeneration, and this was examined using several experimental procedures. An example includes the use of behavioral tests to observe improvements in the functional recovery of animals following SCI which thus indicates neuronal regeneration. A variety of different behavioral methods of testing were used such as the BBB (a 21-point scale based on the Basso 9-point Mouse Scale (BMS) adapted to rats) locomotion test, BMS scoring, inclined plane test, footprint analysis test, grid walking test, CatWalk gait test and sensory tests. The BBB locomotion test was the most commonly used behavioral test (72.7%) followed by BMS scoring (13.6%). As the BBB locomotion test was the most common behavioral test performed, the BBB test results reported in the studies was collected and analyzed in this review using a meta-analysis (see below). Neuronal regeneration was also examined using histological methods, this included the use of haematoxylin eosin (HE) staining to examine alterations/improvements in histology, and Nissl and cresyl violet staining to observe potential changes in neuronal numbers following treatment application.
A number of other experimental procedures were also performed to examine changes induced by the treatments and to therefore determine the effectiveness of the treatments for SCI. In particular, immunohistochemistry and other forms of immunostaining was performed in a large proportion of the studies (95.5%). Immunohistochemistry was also frequently coupled with Western Blotting to analyze changes in protein expression levels. Other experimental techniques carried out by the studies included terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling (TUNEL) assays, enzyme-linked immunosorbent assay (ELISA), quantitative reverse transcription polymerase chain reaction (qRT-PCR), flow cytometry and electrophysiological tests.
3.3. The BBB Locomotion Test
The BBB locomotion test was a common behavioral test that was utilized by 32/44 of the studies. BBB tests were performed on treatment groups that were administered the experimental treatment and underwent SCI, and control groups that only underwent SCI and in some cases were administered a control medium such as phosphate-buffered saline (PBS). How often BBB tests were carried out varied amongst the studies with some studies performing daily BBB tests post SCI and some performing weekly BBB tests post SCI. In order to analyze the BBB test data, the final reported data value for control and treatment groups was collected for the 32 studies and presented in Table 2. The final timepoint for collected BBB test data varied significantly from 7 days (D7) post SCI to 294 days (D297) post SCI. Common timepoints such as D28, D35, D42 and D56 as well as studies analyzing the effects of combined treatments were sub-grouped for five individual meta-analyses, and a final meta-analysis was also performed on all of the 32 studies grouped together (see below).

Table 2.
Summary of the BBB scores for control and treatment groups from the studies that performed the BBB test.
Almost all studies reported an improvement in BBB scores in the treatment groups compared to controls apart from the studies by Liang et al. [34], Mountney et al. [17], and Machova-Urdzikova et al. [37]. Although the BBB score did not surpass control groups that only underwent SCI and no treatment, Liang et al. [34] did report a 5-point improvement in the BBB score for the treatment neural stem cell conditioned medium (NSCM) group compared to the second treatment group which used a control medium. Thus, indicating NSCM treatment to improve functional recovery greater than the control medium [34].
Mountney et al., 2013 [17] reported the same BBB score for the combined chondroitinase ABC (chABC) and sialidase treatment group and the control group. However, there was a 3-point increase in the BBB score for the sialidase alone treatment group compared to the control group [17]. This suggested that sialidase treatment improved functional recovery and that combination treatment of chABC and sialidase failed to do so [17].
In the study by Machova-Urdzikova et al. [37], there was no significant difference reported between the BBB scores of the epigallocatechin gallate (EGCG) treatment group and control group receiving saline treatment, which therefore suggested EGCG to have no significant effect on enhancing functional recovery. However, other behavioural tests such as the flat beam test that assesses balance and coordination skills were also performed in this study [37]. Results from this test reported a significant decrease in the time needed to cross the beam in the EGCG treated group compared to the control group, and therefore suggested an improvement in the behavioural outcomes following SCI with EGCG treatment [37].
BBB Meta-Analysis
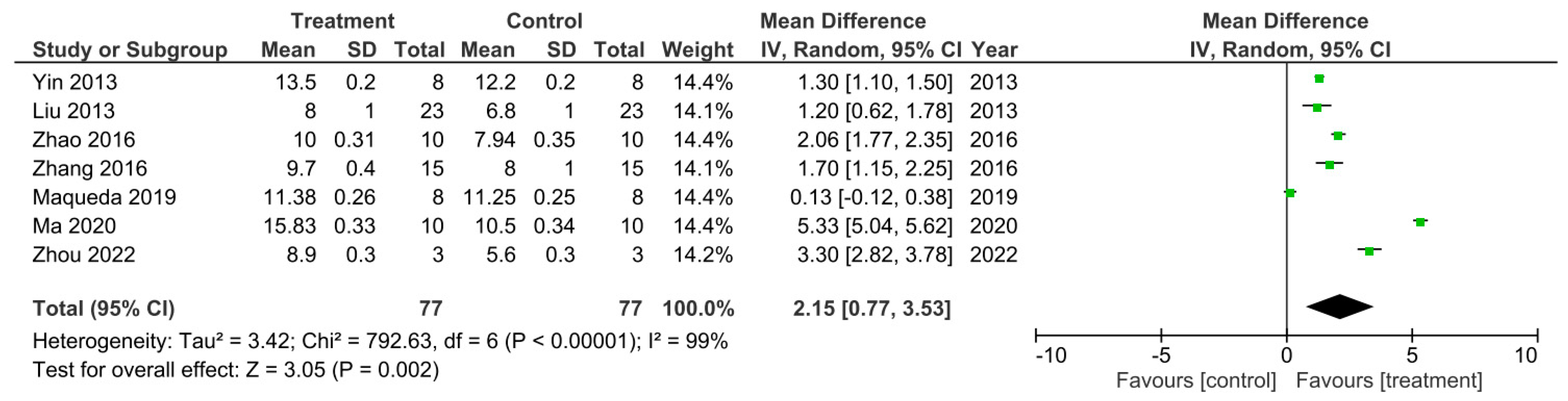
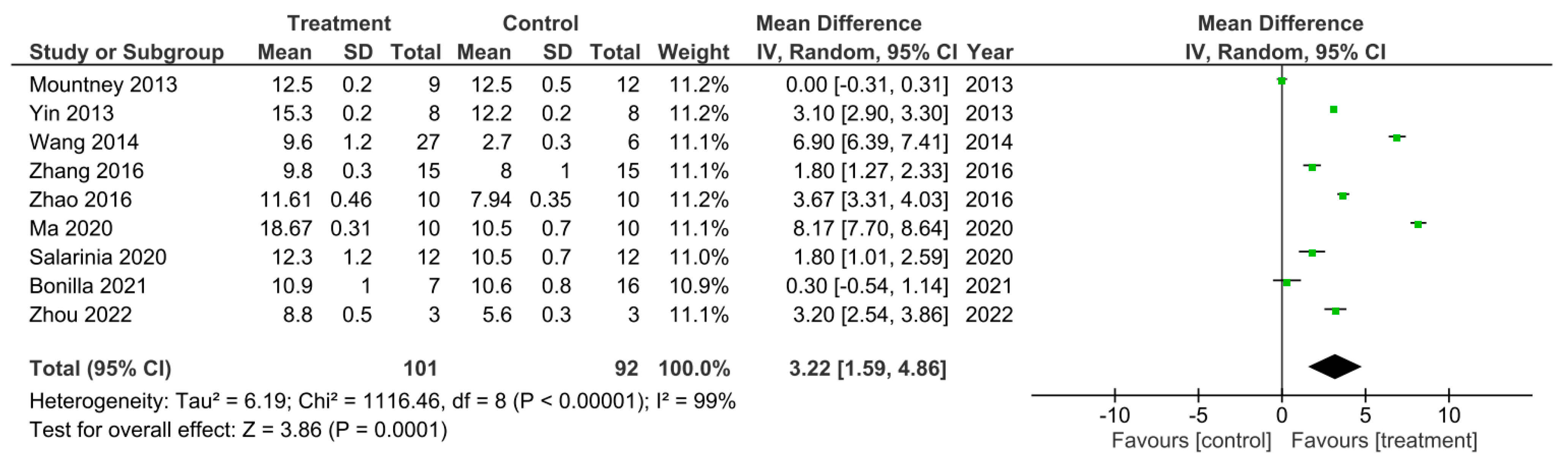
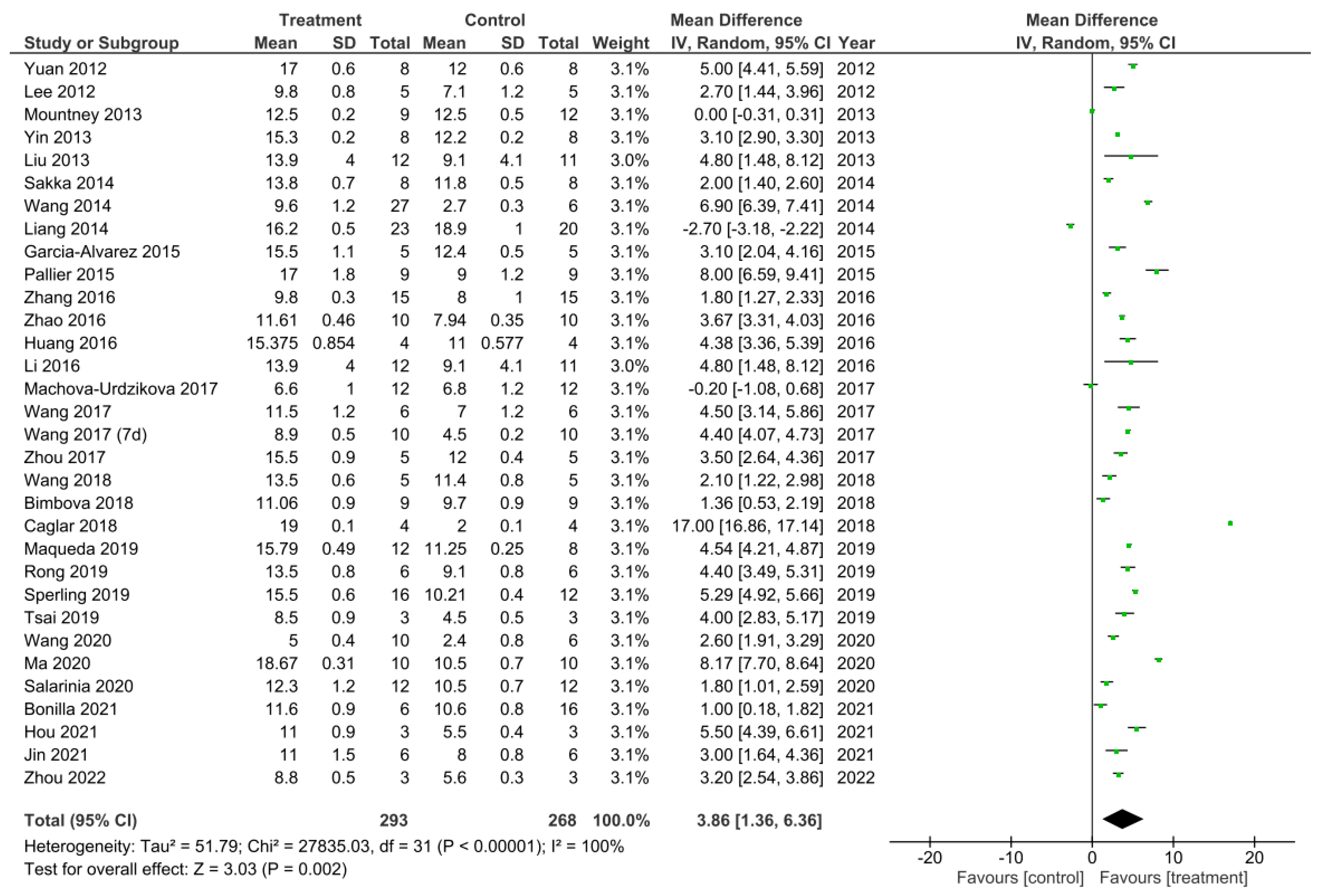
The final experimental timepoint reported for BBB test data varied amongst the 32 studies; therefore, if three or more studies reported the same end timepoint they were sub-grouped together for a meta-analysis. As a result, a meta-analysis was performed on studies with the timepoints: D28, D35, D42 and D56 (Figure 2, Figure 3, Figure 4 and Figure 5). A meta-analysis was also performed on studies that used combined treatments (Figure 6) and a final meta-analysis was carried out on all 32 studies that performed the BBB locomotion test (Figure 7).
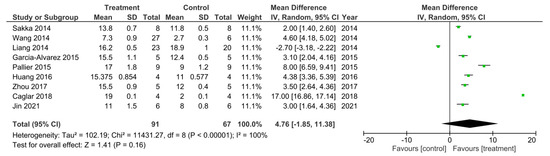
Figure 2.
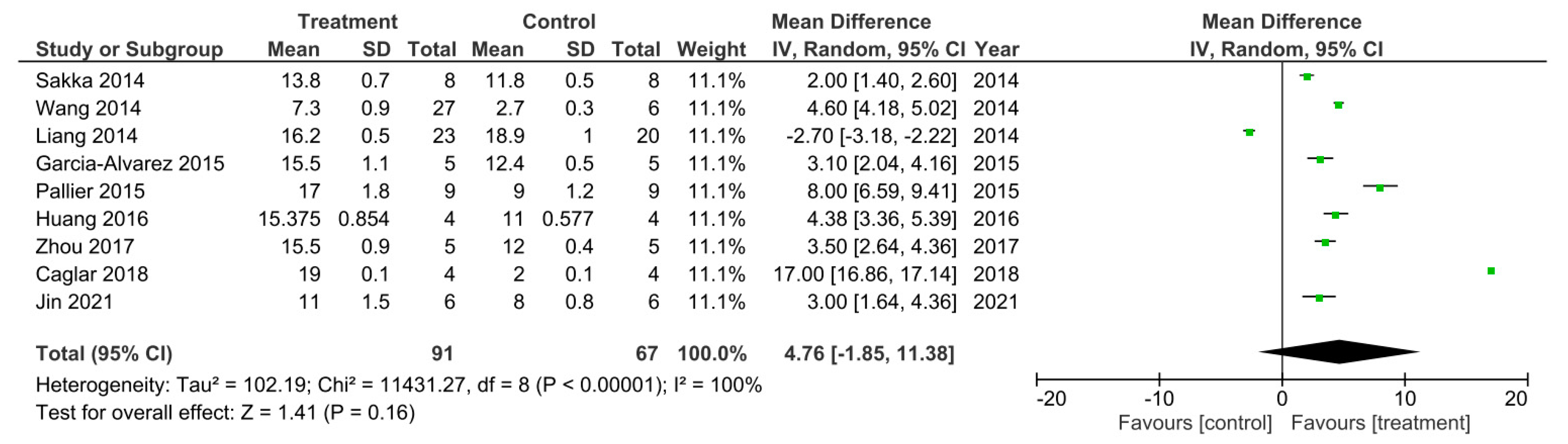
Meta-analysis for the effect of single treatments on the improvement of BBB scores analyzed at 28 days after spinal cord injury in nine studies [11,13,25,27,30,31,33,34,54].
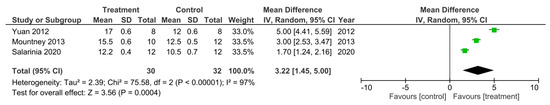
Figure 3.
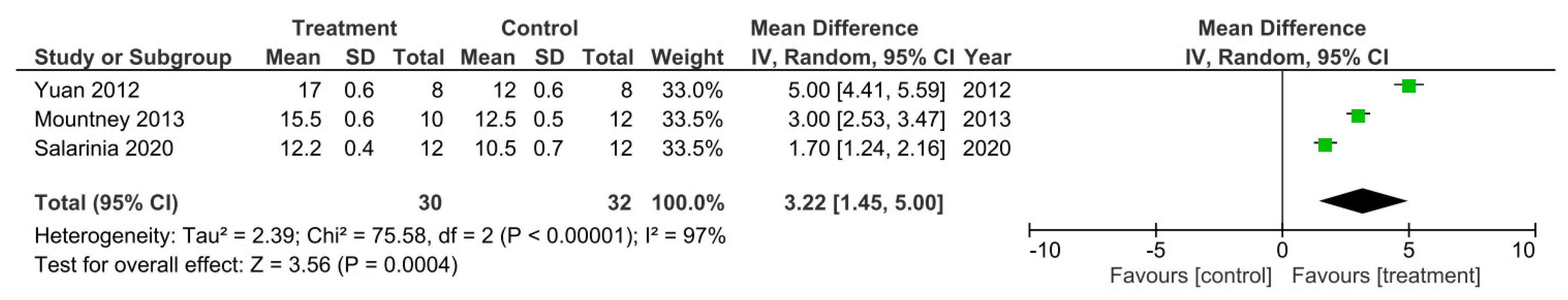
A meta-analysis for the effect of single treatments on the improvement of BBB scores analyzed at 35 days after spinal cord injury in three studies [17,28,41].
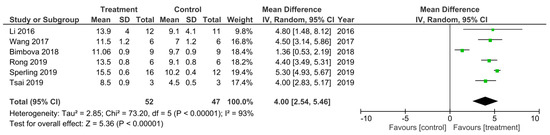
Figure 4.
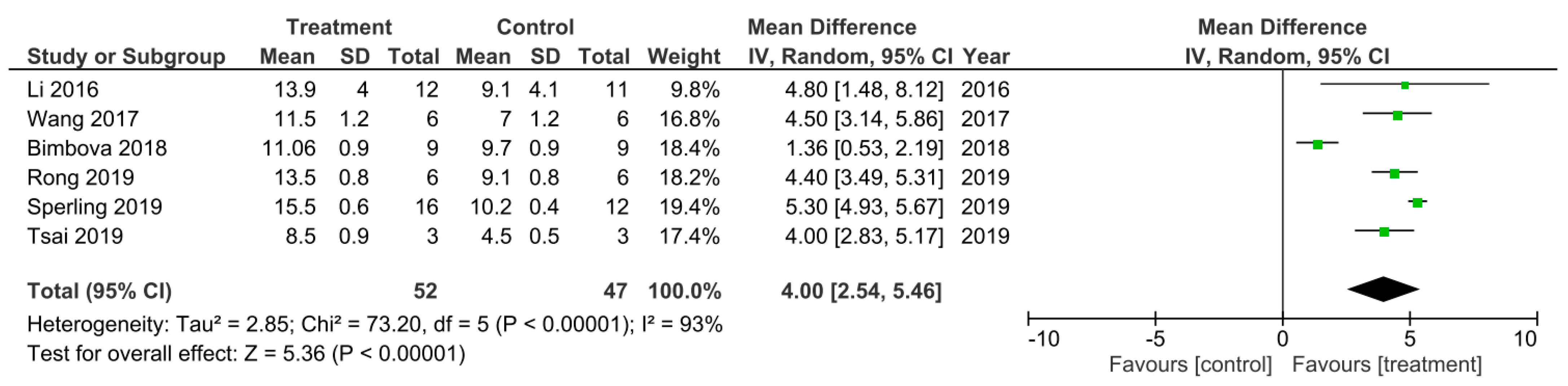
A meta-analysis for the effect of single treatments on the improvement of BBB scores analyzed at 42 days after spinal cord injury in six studies [19,32,46,48,51,53].
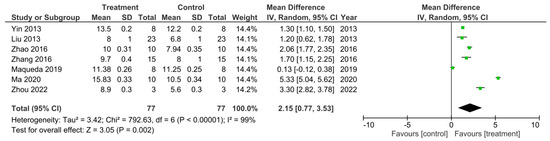
Figure 5.
A meta-analysis for the effect of single treatments on the improvement of BBB scores analyzed at 56 days after spinal cord injury in seven studies [15,18,26,29,40,47,50].
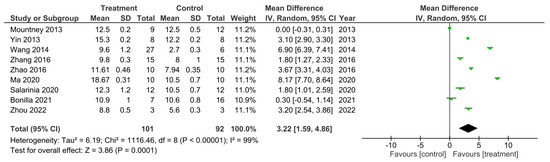
Figure 6.
A meta-analysis for the effect of combined treatments on the improvement of BBB scores analyzed at the final recorded timepoints after spinal cord injury in nine studies [15,17,22,26,27,28,40,47,50].
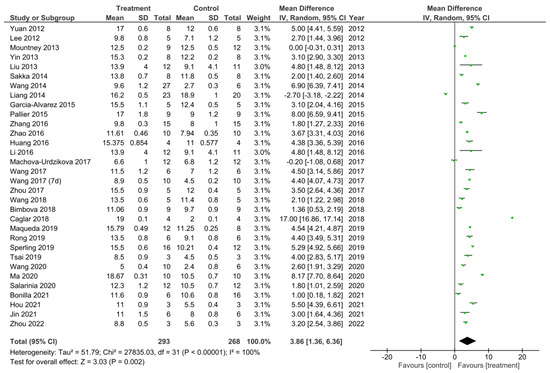
Figure 7.
A meta-analysis for the effect of all treatments on the improvement of BBB scores analysed at the final recorded timepoints after spinal cord injury in all 32 studies that performed the BBB test [11,12,13,14,15,16,17,18,19,22,25,26,27,28,29,30,31,32,33,34,37,40,41,42,43,45,46,47,48,50,51,53,54].
There were nine out of the 32 studies that reported their final recorded BBB data value 28 days following SCI [11,13,25,27,30,31,33,34,54]. A meta-analysis was performed on these studies and the data is presented in Figure 2. Study heterogeneity was analyzed using the I2 statistic. For the D28 sub-grouped studies, a 100% heterogeneity between the studies was reported indicating very high heterogeneity. The meta-analysis also demonstrated that in all studies apart from the study performed by Liang et al. [34], the treatment groups had higher BBB scores, recorded at D28 post SCI, that were statistically significant compared to control groups. Therefore, suggesting these treatments to enhance.
Only three out of the 32 studies reported their final BBB test score 35 days after SCI [17,28,41]. These studies were sub-grouped for a meta-analysis as presented in Figure 3. As Mountney et al. [17] reported no difference in the BBB score for the combined chABC and sialidase treatment group, the BBB score for the sialidase alone treatment group was used for this meta-analysis instead. The heterogeneity between the studies was very high at 97% as indicated by the I2 statistic. The meta-analysis also demonstrated that all three studies reported a statistically significant increase in the BBB score for the treatment groups compared to controls. Thus, indicating the treatments to improve functional recovery greater than controls following SCI. The overall effect of these treatments was determined to be statistically significant as indicated by the Z statistic and the black diamond in the forest plot, with a mean difference of 3.22 [1.45, 5.00], p < 0.00001.
There was 6 out of the 32 studies that reported the final BBB test score 42 days following SCI [19,32,46,48,51,53]. These studies were subsequently sub-grouped for a meta-analysis (Figure 4). The heterogeneity between these studies was 93% and thus very high as calculated by the I2 statistic. All of these studies had higher BBB scores that were statistically significant for the treatment groups at 42 days following SCI compared to control groups, which therefore indicated a greater improvement in functional recovery following SCI that was due to the treatments applied. The overall effect of all the treatments was a statistically significant increase in the BBB score compared to control groups as indicated by the Z statistic and black diamond in the forest plot, with a mean difference of 4.00 [2.54, 5.46], p < 0.00001.
There were seven out of the 32 studies that recorded the final BBB test score 56 days after SCI [15,18,26,29,40,47,50]. These studies were sub-grouped for a meta-analysis as presented in Figure 5. The I2 statistic reported a very high heterogeneity between these studies at 99%. All of these studies reported a higher BBB score for the treatment groups compared to control groups which therefore indicated enhanced functional recovery that was due to the treatments administered. However, this was only statistically significant in 6 out of the 7 studies [15,26,29,40,47,50]. The meta-analysis demonstrated that although the treatment resulted in a higher BBB score compared to controls, in the study by Maqueda et al. [18], this increase in the BBB score was not statistically significant as indicated by the 95% confidence interval (CI) in the forest plot. The overall effect of the increase in BBB scores by all the treatments, however, was found to be statistically significant compared to the controls as indicated by the Z statistic and black diamond in the forest plot, with a mean difference of 2.15 [0.77, 3.53], p < 0.002.
From the 32 studies that performed the BBB test, 9 of them analyzed a combined treatment for recovery of SCI [15,17,22,26,27,28,40,47,50]. These studies were sub-grouped for a meta-analysis of their final recorded BBB test score (Figure 6). There was a 99% heterogeneity between these studies as calculated by the I2 statistic. All of the studies, apart from the study by Mountney et al. [17] which investigated the use of combined chABC and sialidase treatment, had an increase in the BBB score in the treatment groups compared to controls, which therefore suggested improved functional recovery. However, only 7 of the studies were statistically significant [15,26,27,28,40,47,50]. The study by Bonilla et al. [22] did not demonstrate a statistically significant increase in the BBB score of the treatment group as indicated by the CI in the forest plot. Despite this, the overall effect of the treatments in all 9 of these studies was a statistically significant increase in BBB score compared to control groups as indicated by the black diamond in the forest plot and Z statistic, with a mean difference of 3.22 [1.59, 4.86], p < 0.0001.
A meta-analysis was performed on the final timepoints for recorded BBB scores from all 32 studies that performed the BBB test [11,12,13,14,15,16,17,18,19,22,25,26,27,28,29,30,31,32,33,34,37,40,41,43,45,46,47,48,50,51,53,54]. The heterogeneity between studies was 100% as calculated by the I2 statistic. Out of the 32 studies, 29 studies demonstrated a statistically significant increase in the BBB scores for the treatment compared to control groups, thus suggesting these treatments to enhance functional recovery [11,12,13,14,15,16,18,19,22,25,26,27,28,29,30,31,32,33,40,41,43,45,46,47,48,50,51,53,54]. The study by Mountney et al. [17] showed no difference in the BBB score for the combined treatment and control group. Despite this, Mountney et al. [17] did demonstrate a statistically significant increase in the BBB score for the sialidase alone treatment compared to the control group as previously described and presented in Figure 3. This therefore indicated sialidase treatment to enhance functional recovery greater than the combined use of chABC and sialidase [17]. The studies by Liang et al. [34] and Machova-Urdzikova et al. [37] also did not result in an increase in the BBB score for the treatment groups, instead the controls were favored. Despite this, the overall effect for all of the treatments in the 32 studies was a statistically significant increase in the BBB score compared to control groups as demonstrated by the black diamond in the forest plot and the Z statistic with a mean difference of 3.86 [1.36, 6.36], p < 0.002.
3.4. Immunohistochemistry
Experimental techniques such as immunohistochemistry and other forms of immunostaining were frequently performed in all of the 44 studies apart from two studies by Caglar et al. [11] and Li et al. [48]. Immunohistochemistry was performed on both control groups and treatment groups in vivo in order to compare and analyze differences in the expression of markers that were due to the treatments administered. These results from immunohistochemical staining would therefore allow for the determination of whether treatments provided neuroprotection and/or whether treatments were able to induce neuroregeneration following SCI. We collected the results for common immunohistochemical markers that were used in the 42 studies that performed immunohistochemistry and presented the results in Table 3.

Table 3.
Immunohistochemical outcomes in studies.
A frequent marker that was observed in many of the studies was glial fibrillary acidic protein (GFAP) as demonstrated in Table 3. GFAP is commonly expressed in astrocytes and is associated with reactive astrogliosis [12]. Reactive astrogliosis induces the formation of the glial scar and therefore inhibits the potential for regeneration to occur [18]. Consequently, a reduction in GFAP staining is a desired outcome as it suggests a reduction or inhibition of reactive astrogliosis [18]. A large proportion of the studies stained for GFAP and in many of these studies GFAP staining was decreased in the treatment groups compared to controls which therefore indicated neuroprotective properties of the treatments due to the reduction or inhibition of reactive astrogliosis [12,15,19,22,25,29,30,35,38,39,40,41,42,50,53].
Neurofilament (NF) or neurofilament 200 (NF-200) are markers for neurons and are common markers used to observe potential neuronal/axonal growth [24,27]. An increase in the staining of this marker therefore suggests neuroregeneration through neuronal or axonal regrowth and spouting [27]. As demonstrated in Table 3, a significant proportion of the studies used NF or NF200 as markers for in vivo immunohistochemical tests on control and treatment groups following SCI. The majority of these studies reported an increase in NF or NF-200 staining in the treatment groups, which thus indicated possible neuroregeneration that was induced by the treatments used [13,14,16,18,19,24,27,28,30,31,35,36,38,47,51,53]. There was only one study by Chen et al. [49] that did not find any difference in NF200 staining in the treatment or control groups however, they did report an increase in NeuN staining. This is another marker for neurons.
Another marker that was frequently stained for using immunohistochemistry was ionized calcium adaptor molecule 1 (Iba-1). This is a microglia marker and staining for this marker allows for the identification of microglia activation [12]. The activation of microglia contributes towards the neuroinflammatory environment and thus further potentiates secondary injury processes such as glial scar formation [41]. Inhibiting or reducing the levels of microglia activation would therefore limit the damaging neuroinflammatory response as well as other secondary injury events [41]. As presented in Table 3, many of the studies stained for the marker Iba-1 and found a decrease in Iba-1 staining in the treatment groups compared to control groups, which suggested that the treatments used provided neuroprotection following SCI through inhibiting microglia activation or reducing the levels of microglia activity [12,18,25,29,35,38,41,43]. Aleksić et al. [44] also found decreased Iba-1 staining in the treatment group however, this was not statistically significant compared to the control group. Furthermore, another study by Bimbova et al. [53] also found decreased Iba-1 staining in the treatment compared to control group at 24 h following SCI, however this difference in staining was no longer observed six weeks following injury, which therefore suggested that the treatment had short term effects on microglia activation.
The survival of neurons and neuronal growth or sprouting were recurrent outcomes assessed by many of the studies by using immunohistochemistry to stain for markers of mature neurons. This allowed for the effects of treatments on changes in neuronal levels or neuronal sprouting/regrowth to be observed. Neuronal nuclear protein (NeuN) and serotonin, which is also known as 5-hydroxytryptamine (5-HT), were two markers that were frequently used in many of the studies in order to assess these outcomes. The majority of the studies that stained for NeuN, found increased NeuN staining in the treatment groups in comparison to controls [15,19,22,25,34,36,42,45,49,50]. This suggested that these treatments promoted the survival of neurons as less NeuN-positive neurons were found in the control groups and thus illustrated the neuroprotective properties of these treatments. However, there was one study by Aleksić et al. [44] that found no difference in NeuN staining in the treatment and control groups, which suggested that the treatment used did not affect the survival of neurons following SCI. Similarly, 5-HT was another useful marker used to observe any possible effects of the treatments on neurons following SCI. The majority of the studies that stained for 5-HT found increased 5-HT staining in the treatment groups compared to control groups which indicated the treatments to induce regeneration of 5-HT positive neurons [16,23,38,42,46,52]. There was only one study by Mountney et al. [17] that found no difference in staining of 5-HT between treatment and control groups which therefore suggested the treatment to have no effect on 5-HT positive neurons.
Caspase-3 is a caspase protein that is involved in the apoptotic pathway and as the apoptotic loss of neuronal and glial cells is frequently observed during SCI, caspase-3, or cleaved caspase-3 (C-caspase-3) was used as a marker for immunohistochemistry in some studies as presented in Table 3 [13,29,34,53]. Studies that used this marker aimed to determine whether the application of treatment would result in changes in the levels of apoptosis observed in mainly neuronal cells, but also in other cells such as oligodendrocytes. All of the studies that examined this marker found a decrease in caspase-3 or C-caspase-3 staining which indicated a reduction in the apoptotic loss of neuronal cells and thus demonstrated the neuroprotective properties of the treatments used [13,29,34,53].
3.5. Risk of Bias
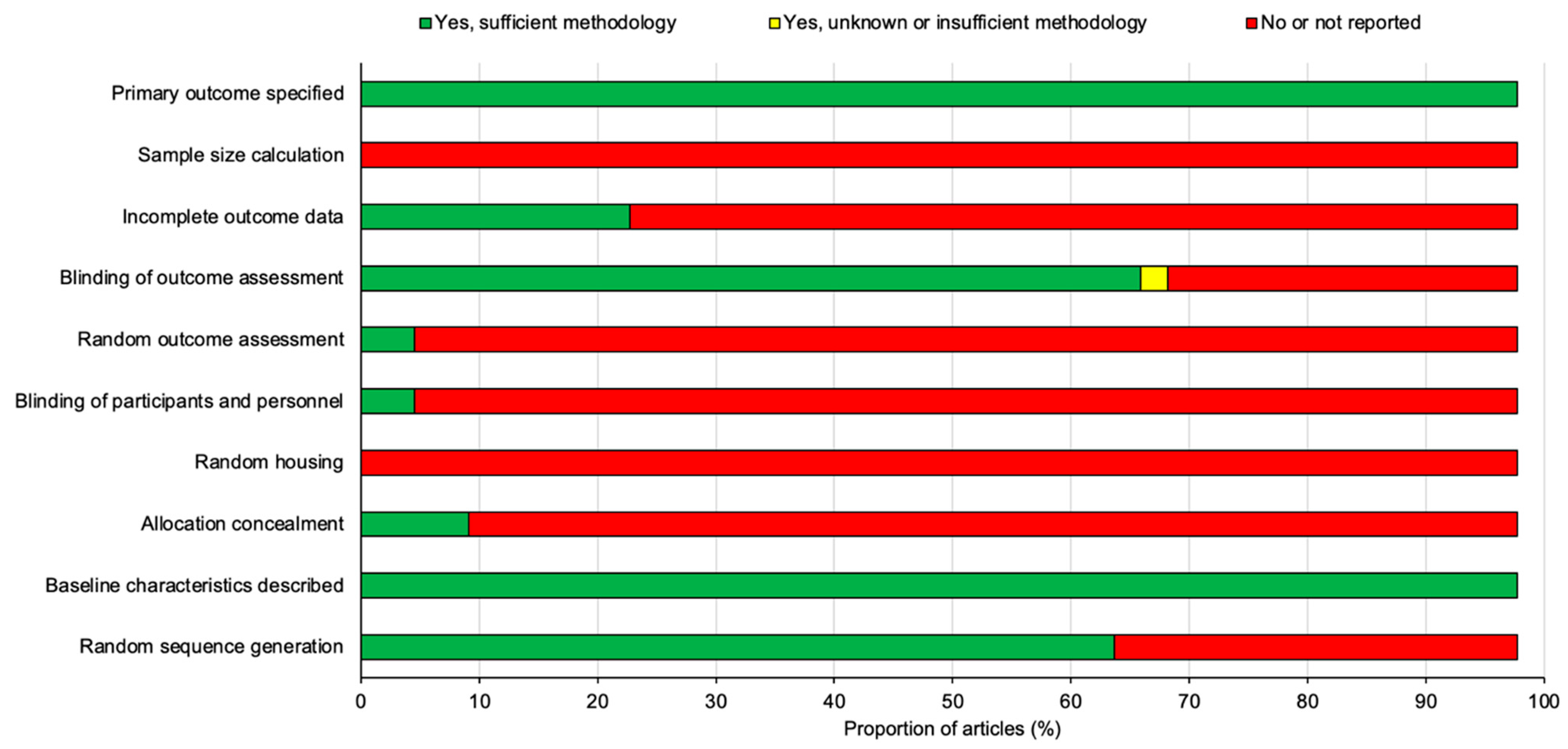
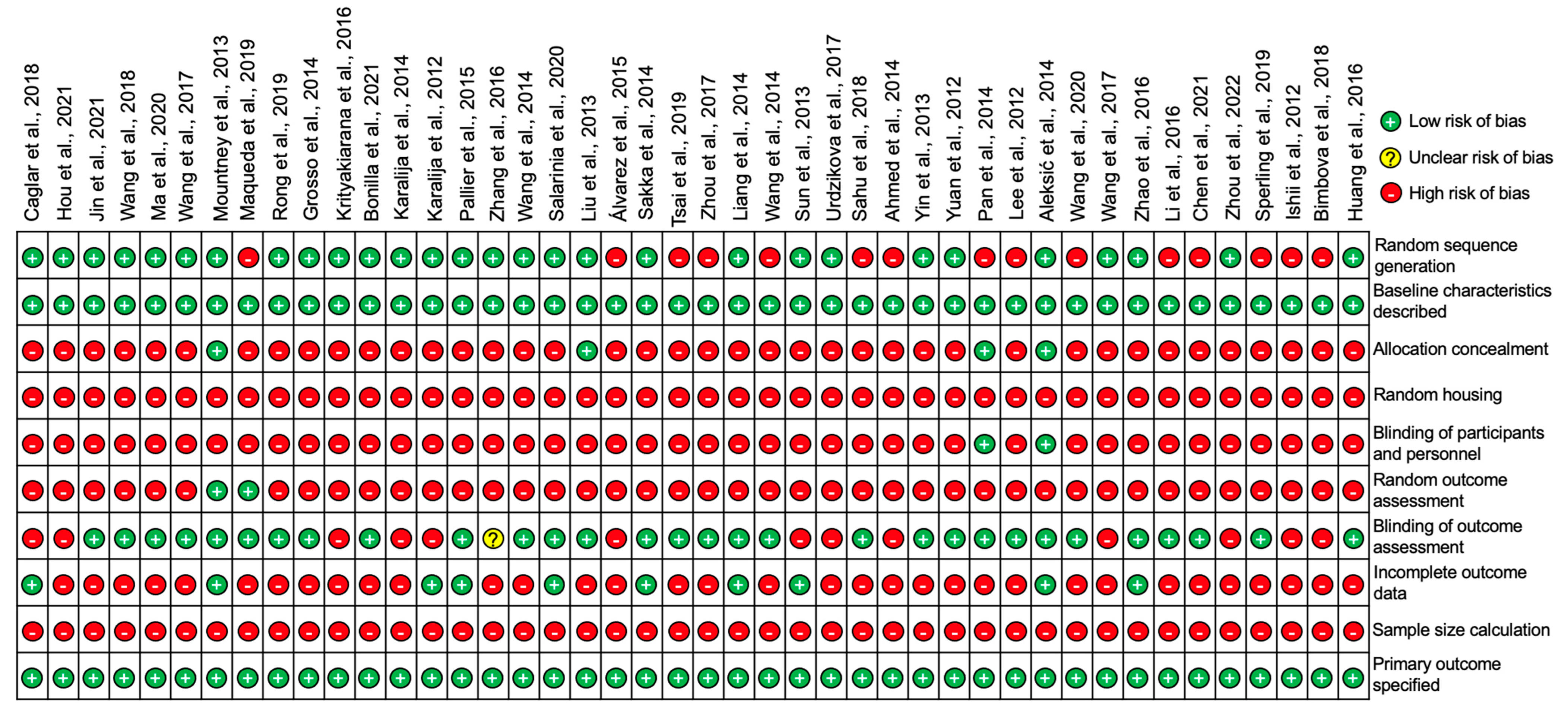
All 44 studies included in this review were assessed for the risk of publication bias using SYRCLE’s risk of bias tool for animal studies adapted from Cochrane’s risk of bias tool [10]. The summary analysis of the risk of bias for all 44 studies is presented below in Figure 8 and the results for each individual studies is presented in Figure 9.
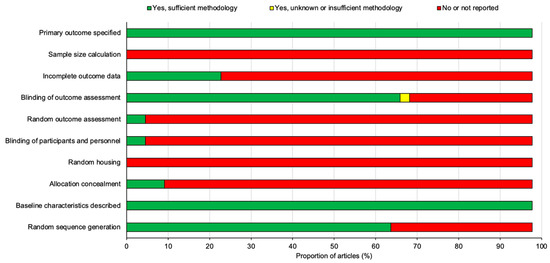
Figure 8.
A summary diagram of the risk of bias for all 44 studies included in this review. All studies were assessed against ten different risk parameters as listed on the y-axis.
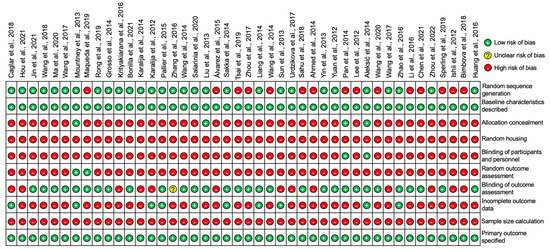
Figure 9.
Diagram to represent the risk of bias in individual studies for all 44 studies included in this review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
All of the studies stated their primary outcomes and described their baseline characteristics however, none of the studies reported using a sample size calculation to determine the appropriate number of animals needed for the study. A proportion of the studies (22.7%) outlined the incomplete outcome data which was largely involving the loss of animals following SCI or in some cases animals that did not demonstrate sufficient functional loss after SCI and thus were excluded from the study. A large proportion of studies (68.2%) blinded the assessors and examiners to the experimental conditions in order to prevent experimental bias however, a significant number of studies (95.5%) failed to randomize outcome assessments leaving only two studies by Mountney et al. [17] and Maqueda et al. [18] that did randomize outcome assessments. The blinding of participants and personnel was also only positively reported in two studies by Pan et al. [42] and Aleksić et al. [44]. There were no studies that reported random housing for the animals used and only a small proportion of studies (9.1%) concealed the allocation of the animals to each experimental group. Despite this, random sequence generation to allocate animals to their experimental group was applied to a large proportion of the studies (65.9%). To summarize, the risk of bias for the studies overall was relatively high as there was a significant proportion of the studies that failed to outline many of the risk parameters listed in Figure 8 and Figure 9.
4. Discussion
The aim of this report was to systematically review potential experimental treatment options that demonstrated neuroprotective and neuroregenerative capabilities following SCI in order to determine the current best treatment options to undergo further pre-clinical testing for validation. To do this, we searched for papers published in the last 10 years using three scientific databases: PubMed, Web of Science and Ovid Embase. The same relevant search terms were applied to each database resulting in a total of 453 search results. These papers were screened against an inclusion and exclusion criteria and assessed for eligibility to narrow down the results to the final 44 studies that were included in this review [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The details of the key characteristics of all 44 studies were collected and summarized which allowed for the identification of the BBB locomotion test and immunohistochemistry as two common experimental procedures performed amongst the 44 studies. Thus, neuroregeneration through enhancing functional recovery analyzed using the BBB locomotion test, and neuroprotection through observing changes induced by treatments using immunohistochemical markers were the primary and secondary outcomes assessed in this review in order to determine the best treatment options for SCI. Following a meta-analysis of the data from the BBB locomotion test and collection of immunohistochemistry results, we identified multiple studies with experimental treatments that enhanced functional recovery and attenuated secondary injury processes. We also determined a relatively high risk of bias amongst the 44 studies following analysis using SYRCLE’s risk of bias tool for animal studies adapted from Cochrane’s risk of bias tool [10]. The results from this systematic review have therefore highlighted several studies with promising treatment options for the improvement of SCI through illustrating neuroregenerative and/or neuroprotective capabilities.
4.1. Neuroregeneration
The loss of normal sensory, motor, and autonomic functions due to the degenerative injury process is frequently observed following SCI, and therefore the ability to induce regenerative effects in order to improve neurological outcomes is a desirable feature for many experimental treatments [1,2,3]. Neuroregeneration can be demonstrated through the use of behavioral tests that assess the improvement in the functional recovery of animals following SCI. In this review, almost all of the studies (90.9%) used one or more behavioral tests to examine improvements in functional recovery induced by the treatments administered.
The BBB locomotion test was the most common behavioral test utilized amongst the studies (72.7%) and so data from each study was collected and presented in Table 2. The time period for how often BBB tests were performed following SCI varied significantly, with a large proportion of studies performing tests for >28 days following SCI and only three studies by Wang et al. [16], Hou et al. [12] and Wang et al. [45] performing BBB tests for <28 days following SCI. Consequently, only the final reported BBB test score for the treatment and control groups from each study was collected, as this allowed for a fair comparison of the improvement in functional recovery between the studies.
BBB data for the common time periods: D28, D35, D42, D56 and data for combined treatments was then sub-grouped for five different meta-analyses, and a final meta-analysis was also performed for the all the studies that used the BBB test. As illustrated by the meta-analyses, a large proportion of studies had shown their experimental treatments to significantly increase the BBB scores compared to controls and thus demonstrated the use of promising treatments that enhance functional recovery following SCI. There were only two studies by Liang et al. [34] and Machova-Urdzikova et al. [37] that failed to increase the BBB score in the treatment groups.
In the study by Liang et al. [34], neural stem cells were cultured and then removed from the culture medium leaving a neural stem cell conditioned medium (NSCM) that was used to determine whether it would be an effective treatment for SCI. The results from the BBB test and meta-analysis reported the control group achieved a final score of 18.9 and the NSCM group to achieve a final score of 16.2 [34]. However, Liang et al. [34] also investigated the use of a control medium, and this group achieved a final score of 10.1. It was therefore concluded by Liang et al. [34] that NSCM treatment resulted in a five-point improvement in the BBB score when compared to the control medium group. Nevertheless, as illustrated by the meta-analysis in this review, NSCM treatment failed to enhance functional recovery greater than sham controls that only underwent a SCI and thus failed to demonstrate neuroregenerative properties.
The study by Machova-Urdzikova et al. [37] also reported a lower BBB score of 6.6 for the EGCG treated group compared to 6.8 for the control group however, this was not reported to be a significant difference. Instead, neuroregeneration was demonstrated through other behavioral tests such as the flat beam test where EGCG treated animals required significantly less amount of time to cross the beam compared to the control group, which therefore suggested EGCG as a promising treatment for the improvement of SCI [37]. On the other hand, the three studies that resulted in the most significant differences in BBB scores between the treatment and control groups and thus demonstrated the most promising results for the improvement in functional recovery, were the studies by Caglar et al. [11], Ma et al. [15] and Pallier et al. [25] with mean differences of 17.00, 8.17 and 8.00, respectively, as illustrated in the meta-analysis.
Caglar et al. [11] investigated the use of the pharmaceutical drug Riluzole as a treatment for SCI. The results from the BBB tests suggested Riluzole treatment to improve functional recovery significantly, as the BBB score reported at D28 in the treatment group was recorded as 19.0 compared to 2.0 in the control group, producing a mean difference of 17.00 as presented in the meta-analysis [11]. However, this result was produced in an experimental group (Group 8) where Riluzole was administered every 12 h for 5 days before the SCI was induced and so does not reflect normal conditions for the treatment of SCI [11]. Caglar et al. [11] investigated several experimental groups that tested Riluzole treatment at different time points before injury. There was only one experimental group (Group 4) where Riluzole was administered every 12 h for 7 days following injury, and in this group the BBB score at D28 was recorded as 12.0, which is still significantly higher than the control group [11]. Despite this, it was Group 8 that demonstrated the most significant improvement for SCI, and it was concluded that Riluzole treatment is therefore most beneficial prior injury for pre-operative patients at high risk of neurological injury [11]. However, as Riluzole treatment in Group 4 still produced positive results for the improvement of functional recovery following SCI, Riluzole may still prove to be a promising treatment. Additional research into the use of Riluzole after SCI is needed to further investigate potential beneficial effects of Riluzole treatment.
The study by Ma et al. [15] produced the next highest mean difference in BBB scores between the treatment and control groups as illustrated by the meta-analysis. The BBB score at D56 in the treatment group was recorded as 18.67 compared to 10.50 in the control group producing a mean difference if 8.17 [15]. In this study, a combined treatment of tetrahedral framework nucleic acid (tFNA) and neural stem cells (NSCs) was used to investigate the effects on the treatment of SCI [15]. Ma et al. [15] also investigated the use of both treatments, tFNA and NSCs, individually in order to determine whether the co-transplantation of tFNA with NSCs would enhance SCI recovery greater than either treatment alone. The results for the BBB scores at D56 for individual treatments of tFNA and NSCs were 15.33 and 15.83, respectively; thus, proving the synergistic treatment of tFNA and NSCs to enhance functional recovery more than either treatment alone [15]. The combined use of tFNA and NSCs for the treatment of SCI should therefore be investigated further as it has demonstrated promising results for the improvement of functional recovery following SCI.
Pallier et al., 2015 [25] had the next highest mean difference in BBB scores as demonstrated by the meta-analysis. The BBB score for treatment group at D28 was recorded as 17.00 and 9.00 for the control group producing a mean difference of 8.00 [25]. This study investigated the use of dietary supplementation using Fortasyn® Connect (FC) as a treatment for SCI [25]. FC is a combination of multiple nutrients such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), phospholipids, monophosphates, choline, vitamins B12, B6, C and E and selenium [25]. As demonstrated by the BBB data and meta-analysis, FC treatment resulted in a significant improvement in functional recovery compared to the control group thus indicating FC as a promising supplementation treatment for further research into SCI treatments [25].
4.2. Neuroprotection
During the pathogenesis of SCI, there are many processes that exacerbate the outcome of the initial injury, such as the necrosis and apoptosis of neuronal and glial cells that can result in the loss of neurological functions, and the activation of astrocytes that produces the glial scar which in turn restricts the potential for regenerative processes to occur [5,8]. Targeting these injury processes would therefore aid in the recovery of SCI by providing neuroprotection. Consequently, many experimental treatments aim to provide neuroprotection by completely inhibiting or reducing these secondary injury processes. In this systematic review, immunohistochemistry and other forms of immunostaining were identified as common experimental procedures that were frequently performed in a significant proportion (95.5%) of the 44 studies. Thus, the results for common immunohistochemical markers were collected and summarized in Table 3. This allowed for changes in the staining of immunohistochemical markers to be observed, which therefore, demonstrated potential neuroprotective effects that were induced by the treatments administered.
As illustrated from the results in Table 3, there was a significant proportion of the studies that demonstrated treatments with neuroprotective capabilities. Many of these treatments may therefore be potentially effective and promising strategies for the improvement of SCI. However, only 30 of these studies performed both immunohistochemistry and the BBB locomotion test to demonstrate neuroprotective and neuroregenerative properties [12,13,14,15,16,17,18,19,22,25,26,27,28,29,30,31,32,33,34,37,40,41,43,45,46,47,50,51,53,54]. Out of these studies, there was only a few that failed to demonstrate neuroprotection alongside neuroregeneration. For example, in the study by Wang et al. [16] Quercetin was investigated as a potential treatment for SCI. Quercetin treatment successfully managed to enhance functional recovery greater than controls as demonstrated in the meta-analysis however, the treatment also resulted in an increase in GFAP staining which indicated increased astrocyte activation [16]. Wang et al. [16] concluded that this was protective as activation of astrocytes enhances recovery during the early stages following SCI. Despite this, it is important to consider the long-term degenerative consequences of astrocyte activation such as glial scar formation which would ultimately restrict regeneration from occurring. Consequently, although Quercetin treatment may enhance functional recovery, further research to improve the treatment and modulate its effects on astrocyte activation is still required.
On the other hand, out of the three studies that resulted in the greatest improvements in functional recovery using the BBB test, only two of the studies by Ma et al. [15] and Pallier et al. [25] demonstrated neuroprotection using immunohistochemistry. Instead, Caglar et al. [11] demonstrated neuroprotection by examining histopathological changes through the use of haematoxylin eosin (HE) staining. The study found that Riluzole treatment resulted in a greater median number of neurons and a reduction in the number of glial cells compared to controls, which therefore illustrated the neuroprotective properties of Riluzole [11]. However, similar to the BBB test, these results were produced from Riluzole treatment before SCI and thus suggests Riluzole to have greater efficacy when administered prior injury [11].
Immunohistochemical results from Ma et al. [15] successfully demonstrated the neuroprotective properties of the combined treatment of tFNA and NSCs. The markers Nestin and NeuN were increased in the combined treatment group which suggested the treatment to increase the survival and proliferation of NSCs, as well as the differentiation of NSCs into neurons, and thus demonstrated the neuroprotective properties but also neuroregenerative properties of combined tFNA and NSCs treatment [15]. There was also decrease in GFAP staining which indicated the combined treatment to reduce NSC differentiation into astrocytes and thus reduce the formation of the glial scar [15]. Overall, the combined treatment of tFNA and NSCs has shown effective results for the improvement of SCI through demonstrating neuroprotective properties by increasing the survival of neurons and reducing glial scar size, as well as demonstrating neuroregenerative properties through the significant improvements in functional recovery [15]. Further research into the synergistic treatment of tFNA and NSCs would thus be a promising area for the development of SCI treatments.
Similarly, Pallier et al. [25] has also reported positive results for the immunohistochemical tests performed. Increased NeuN staining was observed in the FC treated group which suggested the treatment to reduce neuronal loss [25]. GFAP staining was decreased which indicated FC treatment to reduce reactive astrogliosis and thus reduce the formation of the glial scar [25]. Other neuroprotective properties were also demonstrated through the reduction in Iba-1 staining which indicated a reduction in microglia activation and thus neuroinflammation as well as increased staining for oligodendrocytes in the FC treated group [25]. As a result, FC treatment has demonstrated both neuroprotective and neuroregenerative properties and is therefore another promising area for further research into treatments for SCI.
4.3. Risk of Bias Analysis
In this systematic review, we analyzed the risk of bias for the 44 included studies by assessing each study against 10 different risk parameters using SYRCLE’s risk of bias tool for animal studies adapted from Cochrane’s risk of bias tool [10]. Through this assessment, it was determined that the risk of bias for the 44 studies was relatively high as many studies failed to outline several of the risk parameters. In particular, randomization was identified to be a significant issue in many of the studies.
Out of the 44 studies, 65.9% of them positively reported random sequence generation to allocate animals to the treatment and control groups and only a very small proportion of the studies (9.1%) concealed this allocation process, thus giving rise to a high selection bias despite the baseline characteristics being positively reported in all 44 of the studies [10]. Furthermore, none of the 44 studies reported random housing for animals and only two studies by Pan et al. [42] and Aleksić et al. [44] reported the blinding of participants and personnel. The lack of both of these parameters being positively reported in the studies suggests possible high levels of performance bias within the studies [10]. Blinding of the outcome assessment was positively reported in 68.2% of the studies leaving 13 studies that failed to do so and thus increasing the chances of detection bias within these studies [10]. However, despite 68.2% of the studies blinding the outcome assessors, only two studies by Mountney et al. [17] and Maqueda et al. [18] positively reported randomization for the outcome assessments. Therefore, increasing the chances of a high detection bias in the majority of the 44 studies [10].
A small proportion of the studies (22.7%) described their incomplete outcome data of animals that were excluded from the experiments. This was largely involving animals that did not demonstrate sufficient functional loss after SCI when analyzed using the BBB locomotion test, or animals that were lost due to the SCI procedure itself. There were no studies that reported using sample size calculations to determine the necessary number of animals per study and so the impact of smaller sample sizes should be considered for the validity of each study [10]. The primary outcomes on the other hand, were specified in all of the 44 studies. For future studies, addressing and outlining the risk parameters described in SYRCLE’s risk of bias tool for animal studies adapted from Cochrane’s risk of bias tool is recommended to reduce a high risk of bias [10]. In addition, the high risk of bias in animal studies may be also avoided by stricter adherence to a set of standardized techniques in animal experiments based on the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) [55].
4.4. Limitations
A limitation of this systematic review is the use of only the BBB locomotion test to analyze improvements in functional recovery. The BBB locomotion test was identified as the most frequent behavioral test used amongst the 44 studies (72.7%) and so data was collected for a meta-analysis leaving 12 out of the total 44 studies that were not included in the meta-analysis [20,21,23,24,35,36,38,39,42,44,49,52]. Some of these studies may have utilized other methods to analyze improvements in functional recovery such as the ladder crossing or inclined plane test. Consequently, any significant improvements in functional recovery induced by these treatments were not analyzed in this review. Another limitation regarding the BBB locomotion test involves the range of different end time points for the last recorded BBB score which impacted how well comparisons between studies were made. To resolve this, we sub-grouped common end time points of D28, D35, D42 and D56 to clearly illustrate the differences in improvements in functional recovery between studies that used the same end time point. The use of only immunohistochemistry to demonstrate the neuroprotective capabilities of treatments is also another limitation of this review. There may have been studies that performed other experimental methods such as histology analysis using, HE, cresyl violet or Luxol fast blue staining to assess general and myelin-related changes that indicated neuroprotection and were induced by the treatments administered. Another limitation of this review is the high risk of bias for the 44 included studies that was determined by the SYRCLE’s risk of bias tool for animal studies adapted from Cochrane’s risk of bias tool [10]. Additionally, the literature search process also limits the range of papers included and analyzed in this review, as only three electronic databases were used and only papers that were written in the English language and published in the last 10 years (2012–2022) were included.
4.5. Future Studies
Since the most promising treatments for improving functional recovery after SCI were Riluzole, tFNA + NSC and Fotasyn Connect (FC) [11,15,25], we recommend further studies to evaluate these compounds in preclinical models prior to potential clinical evaluation. In fact, a multi-centre clinical trial was planned with Riluzole in SCI patients, the RISCIS study (NCT01597518), however, the study has been terminated due to slow enrollment of patients. FC is a dietary supplement containing a combination of nutrient precursors and co-factors known to be needed in the synthesis of neuronal membranes. FC includes docosahexaenoic acid, eicosapentaenoic acid, uridine-5’-mono-phosphate, choline, phospholipids, selenium, and B, C, and E vitamins. FC has been tested in clinical trials involving Alzheimer’s disease patients and shown to be beneficial in the early period after disease onset [56]. Tetrahedral framework nucleic acid (tFNA) and NSCs provides a promising future therapy where co-transplantation of biomaterials with NSCs could offer superior healing capacity after SCI. Previous studies have also shown that tFNAs promote NSC proliferation, migration and differentiation into neurons, whilst possessing ani-inflammatory and antioxidative effects on macrophages [57,58,59]. Therefore, this could represent a useful future therapeutic.
5. Conclusions
This systematic review has highlighted several studies, published in the last 10 years, that demonstrated effective experimental treatment options with neuroregenerative and neuroprotective properties for the improvement of SCI. In particular, the combined use of tFNA and NSCs and the use of FC dietary supplementation have shown to be the most promising treatments for SCI. Both treatments have shown neuroregenerative capabilities by significantly enhancing functional recovery greater than controls as demonstrated by the increase in BBB scores achieved in the treatment groups. Both treatments have also demonstrated neuroprotection through attenuating secondary injury processes that exacerbate the overall outcome of SCI. In particular, co-transplantation of tFNA with NSCs has shown to increase neuronal survival and differentiation of NSCs into neurons following SCI, and FC supplementation has demonstrated the ability to reduce degenerative injury processes such as reactive astrogliosis and neuroinflammation from occurring following SCI. However, further pre-clinical testing is still required in order to validate and determine the long-term efficacies of FC supplementation and combined tFNA and NSCs treatment on the improvement of SCI should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
Author Contributions
Conceptualization, Z.A.; methodology, F.I.K. and Z.A.; formal analysis, F.I.K. and Z.A.; data curation, F.I.K. and Z.A.; writing—original draft preparation, F.I.K.; writing—review and editing, F.I.K. and Z.A.; supervision, Z.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as per advice from the NHS Health Research Authority (UK) decision tool, since it is a systematic review of published literature.
Informed Consent Statement
Patient consent was waived because no patients or members of the public were involved in the design, conduct of this study, or reporting of this research.
Data Availability Statement
All data generated as part of this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Ng, A.M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.K.A.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J Clin Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Caglar, Y.S.; Demirel, A.; Dogan, I.; Huseynov, R.; Eroglu, U.; Ozgural, O.; Cansiz, C.; Bahadir, B.; Kilinc, M.C.; Al-Beyati, E.S. Effect of Riluzole on Spinal Cord Regeneration with Hemisection Method Before Injury. World Neurosurg. 2018, 114, e247–e253. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luan, J.; Huang, T.; Deng, T.; Li, X.; Xiao, Z.; Zhan, J.; Luo, D.; Hou, Y.; Xu, L.; et al. Tauroursodeoxycholic acid alleviates secondary injury in spinal cord injury mice by reducing oxidative stress, apoptosis, and inflammatory response. J. Neuroinflammation 2021, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Qi, F.; Chu, F.; Liu, C.; Qian, T.; Zeng, W.; Wang, Q.; Wang, X.; Xiao, J. Morin improves functional recovery after spinal cord injury in rats by enhancing axon regeneration via the Nrf2/HO-1 pathway. Phytother. Res. 2021, 35, 5754–5766. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fang, X.; Yin, Z.S. Endothelial progenitor cell-conditioned medium promotes angiogenesis and is neuroprotective after spinal cord injury. Neural Regen. Res. 2018, 13, 887–895. [Google Scholar]
- Ma, W.; Zhan, Y.; Zhang, Y.; Xie, X.; Mao, C.; Lin, Y. Enhanced Neural Regeneration with a Concomitant Treatment of Framework Nucleic Acid and Stem Cells in Spinal Cord Injury. ACS Appl. Mater. Interfaces 2020, 12, 2095–2106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, M.; Lin, C.; Li, G.; Zhou, X.; Luo, J.; Jin, D. Quercetin reduces neural tissue damage and promotes astrocyte activation after spinal cord injury in rats. J. Cell Biochem. 2017, 119, 2298–2306. [Google Scholar] [CrossRef]
- Mountney, A.; Zahner, M.R.; Sturgill, E.R.; Riley, C.J.; Aston, J.W.; Oudega, M.; Schramm, L.P.; Hurtado, A.; Schnaar, R.L. Sialidase, chondroitinase ABC, and combination therapy after spinal cord contusion injury. J. Neurotrauma 2013, 30, 181–190. [Google Scholar] [CrossRef]
- Maqueda, A.; Rodriguez, F.J. Efficacy of human HC016 cell transplants on neuroprotection and functional recovery in a rat model of acute spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 14, 319–333. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Zhou, Z.; Gong, F.; Bai, J.; Fan, J.; Li, L.; Luo, Y.; Zhou, Z.; Cai, W. Harpagide inhibits neuronal apoptosis and promotes axonal regeneration after spinal cord injury in rats by activating the Wnt/β-catenin signaling pathway. Brain Res. Bull. 2019, 148, 91–99. [Google Scholar] [CrossRef]
- Grosso, M.J.; Matheus, V.; Clark, M.; Van Rooijen, N.; Lannotti, C.A.; Steinmetz, M.P. Effects of an immunomodulatory therapy and chondroitinase after spinal cord hemisection injury. Neurosurgery 2014, 75, 461–471. [Google Scholar] [CrossRef]
- Krityakiarana, W.; Zhao, P.M.; Nguyen, K.; Gomez-Pinilla, F.; Kotchabhakdi, N.; De Vellis, J.; Espinosa-Jeffrey, A. Proof-of Concept that an Acute Trophic Factors Intervention After Spinal Cord Injury Provides an Adequate Niche for Neuroprotection, Recruitment of Nestin-Expressing Progenitors and Regeneration. Neurochem. Res. 2016, 41, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, P.; Hernandez, J.; Giraldo, E.; González-Pérez, M.; Alastrue-Agudo, A.; Elkhenany, H.; Vicent, M.; Navarro, X.; Edel, M.; Moreno-Manzano, V. Human-Induced Neural and Mesenchymal Stem Cell Therapy Combined with a Curcumin Nanoconjugate as a Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2021, 22, 5966. [Google Scholar] [CrossRef] [PubMed]
- Karalija, A.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Novikov, L.N. The effects of N-acetyl-cysteine and acetyl-L-carnitine on neural survival, neuroinflammation and regeneration following spinal cord injury. Neuroscience 2014, 269, 143–151. [Google Scholar] [CrossRef]
- Karalija, A.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Novikov, L.N. Neuroprotective effects of N-acetyl-cysteine and acetyl-L-carnitine after spinal cord injury in adult rats. PLoS ONE 2012, 7, e41086. [Google Scholar] [CrossRef]
- Pallier, P.N.; Poddighe, L.; Zbarsky, V.; Kostusiak, M.; Choudhury, R.; Hart, T. A nutrient combination designed to enhance synapse formation and function improves outcome in experimental spinal cord injury. Neurobiol. Dis. 2015, 82, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lei, F.; Zhou, Q.; Feng, D.; Bai, Y. Combined application of Rho-ROCKII and GSK-3β inhibitors exerts an improved protective effect on axonal regeneration in rats with spinal cord injury. Mol. Med. Rep. 2016, 14, 5180–5188. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Wei, F.-X.; Cen, J.-S.; Ping, S.-N.; Li, Z.-Q.; Chen, N.-N.; Cui, S.-B.; Wan, Y.; Liu, S.-Y. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014, 1575, 87–100. [Google Scholar] [CrossRef]
- Salarinia, R.; Hosseini, M.; Mohamadi, Y.; Ghorbani, A.; Alamdari, D.H.; Mafinezhad, A.; Sadeghnia, H. Combined use of platelet-rich plasma and adipose tissue-derived mesenchymal stem cells shows a synergistic effect in experimental spinal cord injury. J. Chem. Neuroanat. 2020, 110, 101870. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.; Satkunendrarajah, K.; Yao, G.S.; Bayon, Y.; Fehlings, M.G. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013, 9, 8075–8088. [Google Scholar] [CrossRef]
- García-Álvarez, I.; Fernández-Mayoralas, A.; Moreno-Lillo, S.; Sánchez-Sierra, M.; Nieto-Sampedro, M.; Doncel-Pérez, E. Inhibition of glial proliferation, promotion of axonal growth and myelin production by synthetic glycolipid: A new approach for spinal cord injury treatment. Restor. Neurol. Neurosci. 2015, 33, 895–910. [Google Scholar] [CrossRef]
- Sakka, L.; Delétage, N.; Lalloué, F.; Duval, A.; Chazal, J.; Lemaire, J.J.; Meiniel, A.; Monnerie, H.; Gobron, S. SCO-spondin derived peptide NX210 induces neuroprotection in vitro and promotes fiber regrowth and functional recovery after spinal cord injury. PLoS ONE 2014, 9, e93179. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Liou, D.-Y.; Lin, Y.-R.; Weng, C.-F.; Huang, M.-C.; Huang, W.-C.; Tseng, F.-W.; Cheng, H. Attenuating Spinal Cord Injury by Conditioned Medium from Bone Marrow Mesenchymal Stem Cells. J. Clin. Med. 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, C.; Chen, S.; Zhao, H.; Zhou, K.; Wang, W.; Yuan, Y.; Li, Z.; Guo, Y.; Shen, Z.; et al. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget 2017, 8, 52078–52093. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liu, J.; Xiong, J.; Liu, Q.; Zhao, J.; Liang, H.; Zhao, L.; Tang, H. Neural stem cell-conditioned medium protects neurons and promotes propriospinal neurons relay neural circuit reconnection after spinal cord injury. Cell Transplant. 2014, 23, S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zu, J.N.; Li, J.; Chen, C.; Xi, C.Y.; Yan, J.L. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef]
- Sun, C.; Shao, J.; Su, L.; Zhao, J.; Bi, J.; Yang, S.; Zhang, S.; Gao, J.; Miao, J. Cholinergic neuron-like cells derived from bone marrow stromal cells induced by tricyclodecane-9-yl-xanthogenate promote functional recovery and neural protection after spinal cord injury. Cell Transplant. 2013, 22, 961–975. [Google Scholar] [CrossRef]
- Machova-Urdzikova, L.; Ruzicka, J.; Karova, K.; Kloudova, A.; Svobodova, B.; Amin, A.; Dubisova, J.; Schmidt, M.; Kubinova, S.; Jhanwar-Uniyal, M.; et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 2017, 126, 213–223. [Google Scholar] [CrossRef]
- Sahu, S.; Li, R.; Kadeyala, P.K.; Liu, S.; Schachner, M. The human natural killer-1 (HNK-1) glycan mimetic ursolic acid promotes functional recovery after spinal cord injury in mouse. J. Nutr. Biochem. 2018, 55, 219–228. [Google Scholar] [CrossRef]
- Ahmed, Z.; Bansal, D.; Tizzard, K.; Surey, S.; Esmaeili, M.; Gonzalez, A.M.; Berry, M.; Logan, A. Decorin blocks scarring and cystic cavitation in acute and induces scar dissolution in chronic spinal cord wounds. Neurobiol. Dis. 2014, 64, 163–176. [Google Scholar] [CrossRef]
- Yin, Y.; Sun, W.; Li, Z.; Zhang, B.; Cui, H.; Deng, L.; Xie, P.; Xiang, J.; Zou, J. Effects of combining methylprednisolone with rolipram on functional recovery in adult rats following spinal cord injury. Neurochem. Int. 2013, 62, 903–912. [Google Scholar] [CrossRef]
- Yuan, Y.; Su, Z.; Pu, Y.; Liu, X.; Chen, J.; Zhu, F.; Zhu, Y.; Zhang, H.; He, C. Ethyl pyruvate promotes spinal cord repair by ameliorating the glial microenvironment. Br. J. Pharmacol. 2012, 166, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Shen, Y.Q.; Loers, G.; Jakovcevski, I.; Schachner, M. Tegaserod, a small compound mimetic of polysialic acid, promotes functional recovery after spinal cord injury in mice. Neuroscience 2014, 277, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Yang, C.-C.; Kuo, Y.-M.; Sze, C.-I.; Hsu, J.-Y.C.; Huang, Y.-H.; Tzeng, S.-F.; Tsai, C.-L.; Chen, H.-H.; Jou, I.-M. Delayed granulocyte colony-stimulating factor treatment promotes functional recovery in rats with severe contusive spinal cord injury. Spine 2012, 37, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, D.; Aksić, M.; Divac, N.; Radonjić, V.; Filipović, B.; Jakovčevski, I. Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury. Neural. Regen. Res. 2014, 9, 2174–2181. [Google Scholar] [PubMed]
- Wang, H.; Zheng, Z.; Han, W.; Yuan, Y.; Li, Y.; Zhou, K.; Wang, Q.; Xie, L.; Xu, K.; Zhang, H.; et al. Metformin Promotes Axon Regeneration after Spinal Cord Injury through Inhibiting Oxidative Stress and Stabilizing Microtubule. Oxid. Med. Cell. Longev. 2020, 2020, 9741369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, X.; Liu, Z.; Li, Y. Crocetin Potentiates Neurite Growth in Hippocampal Neurons and Facilitates Functional Recovery in Rats with Spinal Cord Injury. Neurosci. Bull. 2017, 33, 695–702. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, Y.; Jiang, J.; Yan, H.; Wang, X.; Huo, H.; Xiao, Y. Neural stem cell transplantation combined with erythropoietin for the treatment of spinal cord injury in rats. Exp. Ther. Med. 2016, 12, 2688–2694. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Zhang, J.; Zhu, P.-Q.; Ma, C.-H.; Yuan, L.-H.; Lu, J.; Luo, Z.-Z.; Xu, G.-H. Intrathecal Administration of Flavopiridol Promotes Regeneration in Experimental Model of Spinal Cord Injury. Turk. Neurosurg. 2016, 26, 922–929. [Google Scholar] [CrossRef]
- Chen, J.; Fu, E.J.; Patel, P.R.; Hostetler, A.J.; Sawan, H.A.; Moss, K.A.; Hocevar, S.E.; Anderson, A.; Chestek, C.A.; Shea, L.D. Lentiviral Interleukin-10 Gene Therapy Preserves Fine Motor Circuitry and Function After a Cervical Spinal Cord Injury in Male and Female Mice. Neurotherapeutics 2021, 18, 503–514. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, X.; Xin, X.; Yang, J.; Pan, Q.; Liu, C.; Liu, Y.; Yu, X.; Li, Z.; Jiao, G.; et al. Click chemistry-conjugated protein-drug micelles with anti-ferroptotic and anti-inflammatory properties promote regeneration in spinal cord injury. Chem. Eng. J. 2022, 428, 132118. [Google Scholar] [CrossRef]
- Sperling, L.E.; Reis, K.P.; Nicola, F.; Teixeira, C.E.; Didó, G.G.; dos Santos, M.G.; Konrath, E.; Netto, C.A.; Pranke, P. Galantamine improves functional recovery and reduces lesion size in a rat model of spinal cord injury. Brain Res. 2019, 1724, 146424. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Jin, X.; Ueno, M.; Tanabe, S.; Kubo, T.; Serada, S.; Naka, T.; Yamashita, T. Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 2012, 3, e363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bimbova, K.; Bacova, M.; Kisucka, A.; Pavel, J.; Galik, J.; Zavacky, P.; Marsala, M.; Stropkovska, A.; Fedorova, J.; Papcunova, S.; et al. A Single Dose of Atorvastatin Applied Acutely after Spinal Cord Injury Suppresses Inflammation, Apoptosis, and Promotes Axon Outgrowth, Which Might Be Essential for Favorable Functional Outcome. Int. J. Mol. Sci. 2018, 19, 1106. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Cao, Y.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.-B.; Hu, J.-Z. Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res. 2016, 1648, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Rasmussen, J. The LipiDiDiet trial: What does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease? Clin. Interv. Aging 2019, 14, 1481–1492. [Google Scholar] [CrossRef]
- Ma, W.; Shao, X.; Zhao, D.; Li, Q.; Liu, M.; Zhou, T.; Xie, X.; Mao, C.; Zhang, Y.; Lin, Y. Self-Assembled Tetrahedral DNA Research Article Nanostructures Promote Neural Stem Cell Proliferation and Neuronal Differentiation. ACS Appl. Mater. Interfaces 2018, 10, 7892–7900. [Google Scholar] [CrossRef]
- Ma, W.; Xie, X.; Shao, X.; Zhang, Y.; Mao, C.; Zhan, Y.; Zhao, D.; Liu, M.; Li, Q.; Lin, Y. Tetrahedral DNA nanostructures facilitate neural stem cell migration via activating RHOA/ROCK2 signaling pathway. Cell Prolif. 2018, 51, e12503. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, S.; Shi, S.; Zhang, T.; Ma, Q.; Tian, T.; Zhou, T.; Cai, X.; Lin, Y. Anti-inflammatory and Antioxidative Effects of Tetrahedral DNA Nanostructures via the Modulation of Macrophage Responses. ACS Appl. Mater. Interfaces 2018, 10, 3421–3430. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).