The Influence of Cell Isolation and Culturing on Natriuretic Peptide Receptors in Aortic Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. SMC Extraction and—Culture
2.4. RNA Extraction
2.5. cDNA Synthesis
2.6. RT-qPCR
2.7. cGMP ELISA
2.8. Statistical Analysis
3. Results
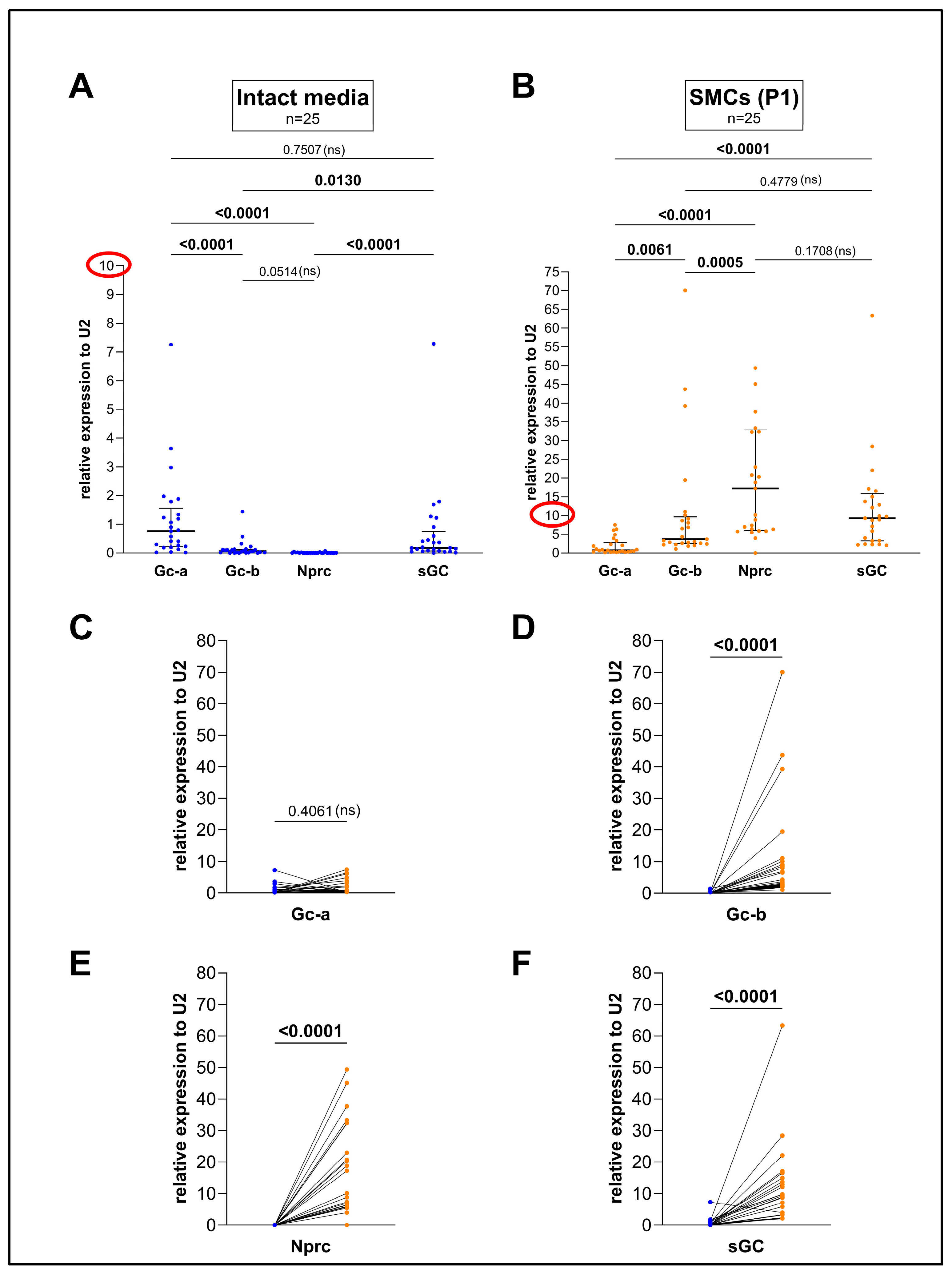
3.1. Culturing Aortic SMCs Dramatically Changes the Expression of Gc-b, sGC, and Nprc but Not of Gc-a in Comparison to the Intact Media
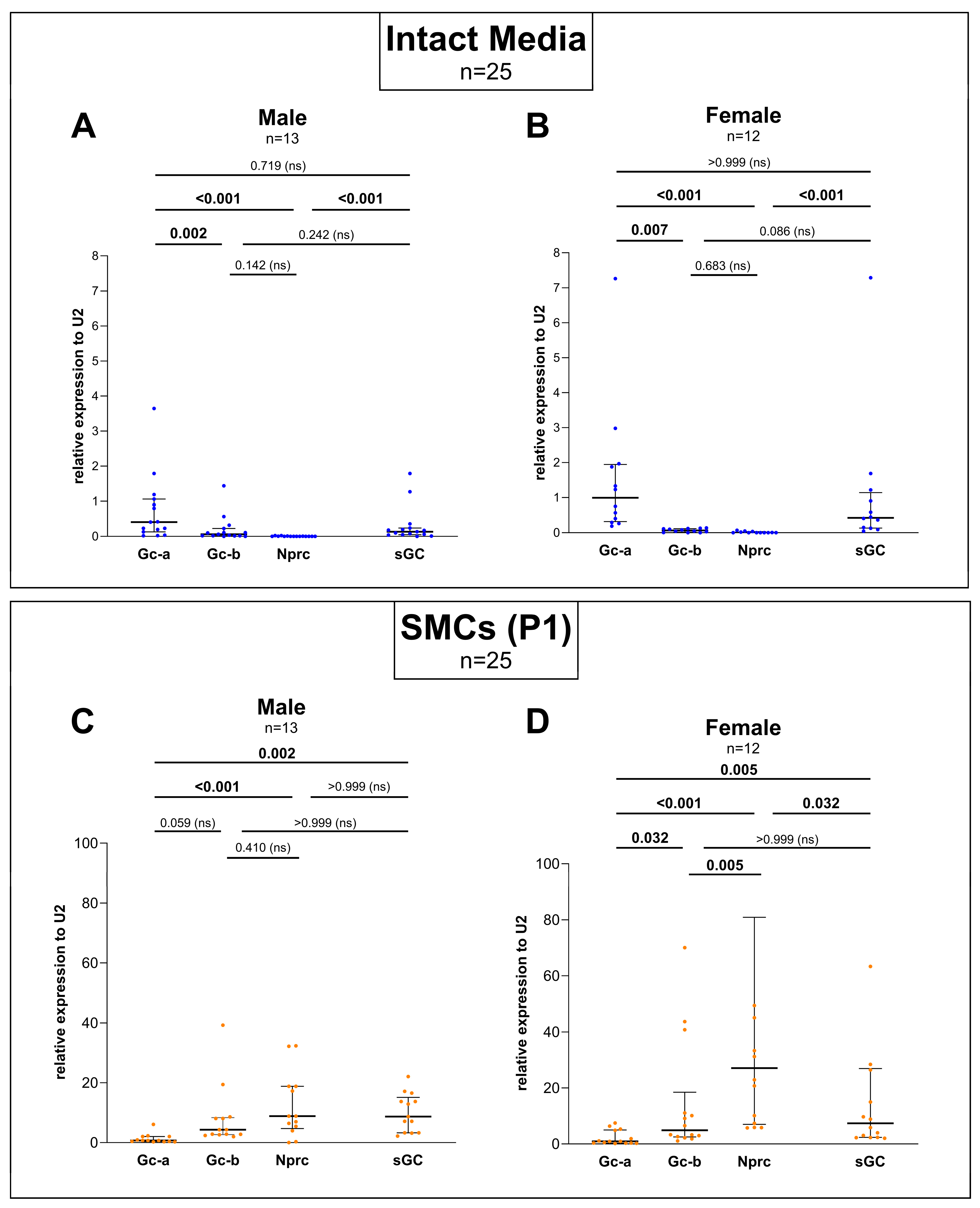
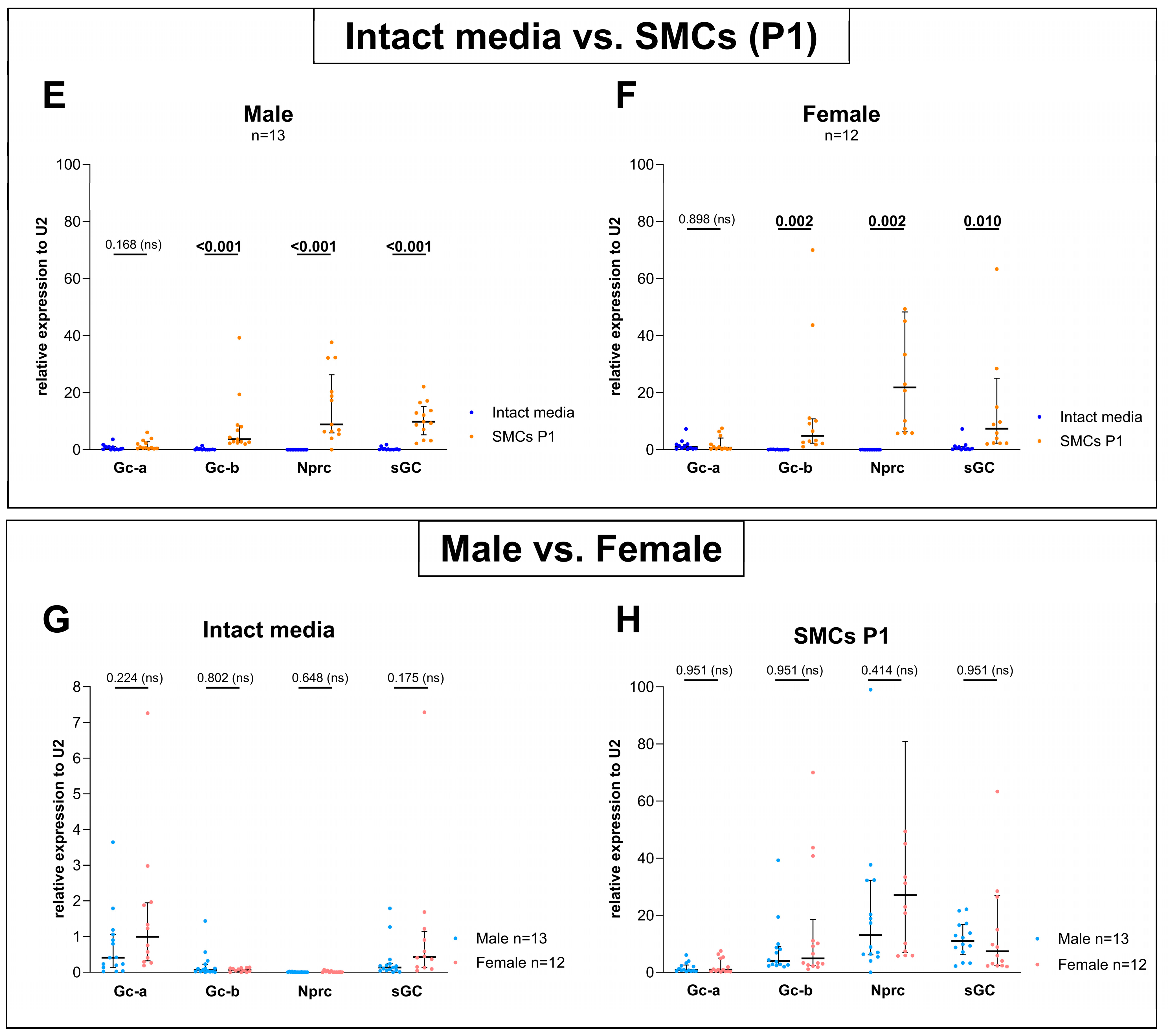
3.2. Sex-Specific Differences Were Found When Comparing the Expression Between Gc-a, Gc-b, Nprc, and sGC but No Differences Could Be Detected Between Male and Female When Comparing Each Gene Separately
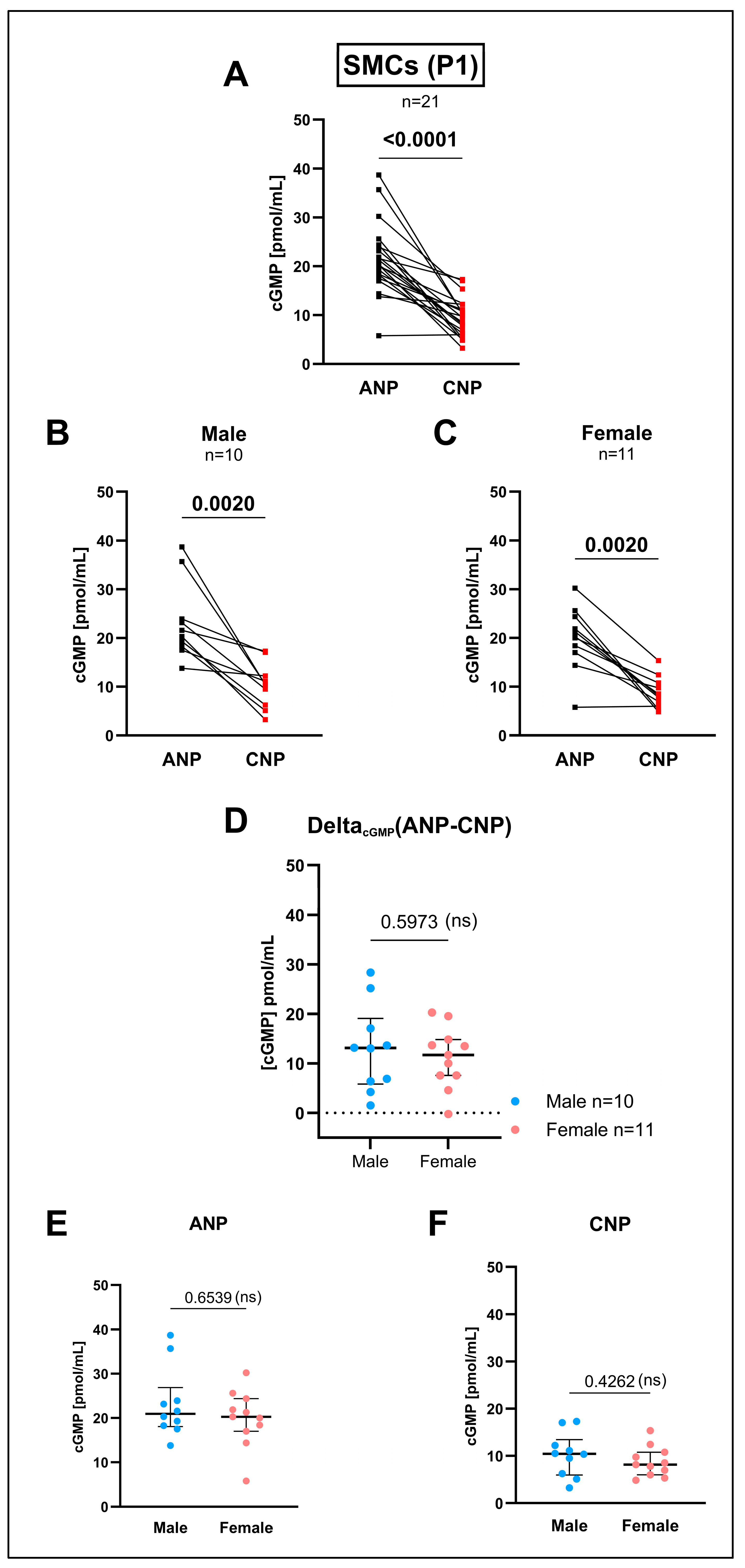
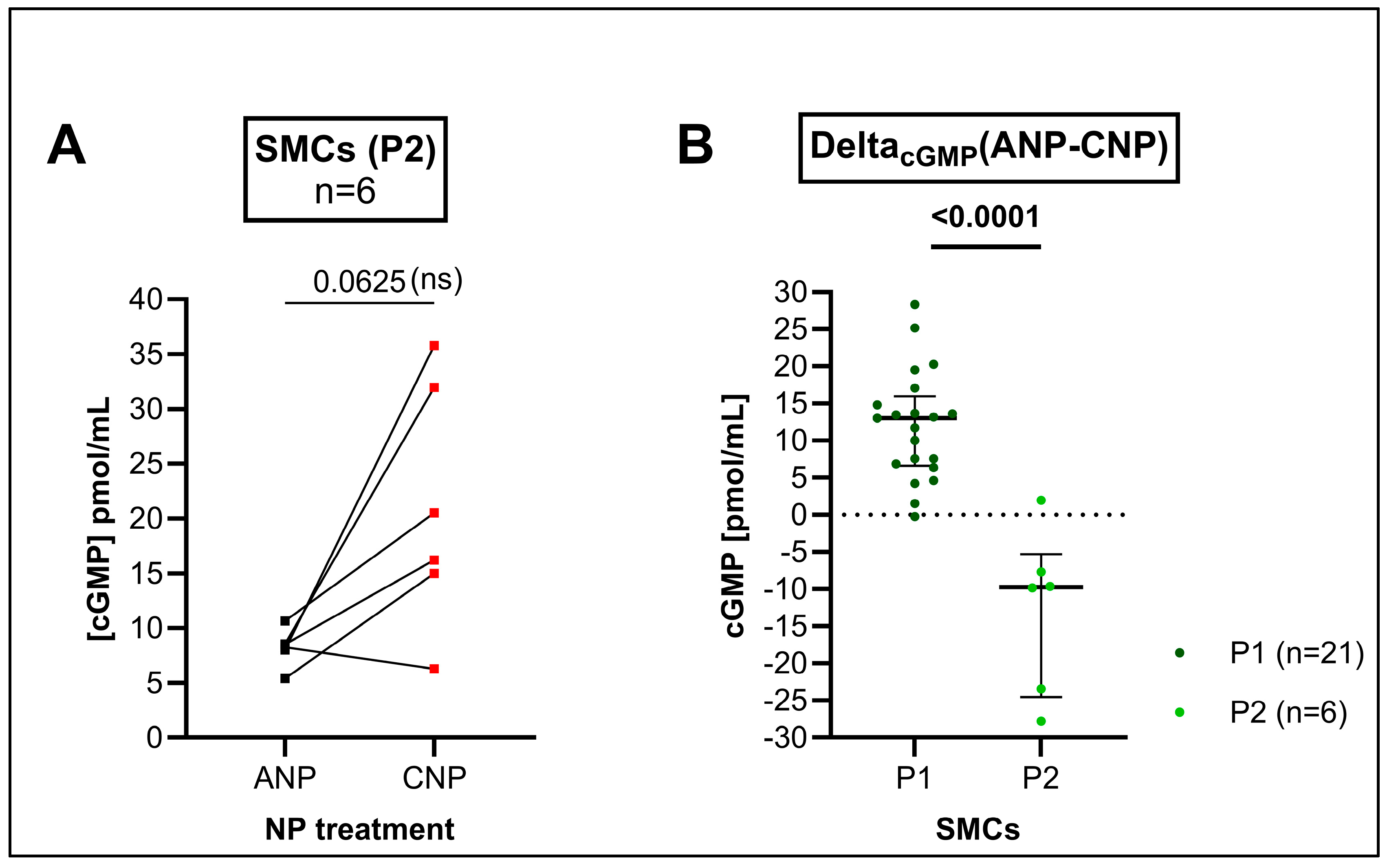
3.3. cGMP Production by the GC-A Ligand ANP and by the GC-B Ligand CNP in Cultured SMCs Differed in Higher Passages in Comparison to the Intact Media but Not Within Early Passages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.R.; Owens, G.K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 2012, 74, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef]
- Nakatsu, K.; Diamond, J. Role of cGMP in relaxation of vascular and other smooth muscle. Can. J. Physiol. Pharmacol. 1989, 67, 251–262. [Google Scholar] [CrossRef]
- Childers, K.C.; Garcin, E.D. Structure/function of the soluble guanylyl cyclase catalytic domain. Nitric Oxide 2018, 77, 53–64. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef]
- Robbins, R.A.; Grisham, M.B. Nitric oxide. Int. J. Biochem. Cell Biol. 1997, 29, 857–860. [Google Scholar] [CrossRef]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell. Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Natriuretic peptide C-receptor: More than a clearance receptor. Am. J. Physiol. 1993, 264 Pt 1, E483–E489. [Google Scholar] [CrossRef]
- Liu, D.; Ceddia, R.P.; Zhang, W.; Shi, F.; Fang, H.; Collins, S. Discovery of another mechanism for the inhibition of particulate guanylyl cyclases by the natriuretic peptide clearance receptor. Proc. Natl. Acad. Sci. USA 2023, 120, e2307882120. [Google Scholar] [CrossRef] [PubMed]
- Rager, C.; Klöpper, T.; Pfeil, U.; Tasch, S.; Whittaker, M.R.; Exintaris, B.; Mietens, A.; Middendorff, R. Reference Gene U2 Enables Direct Comparison between Relative Gene Expression Levels of Vascular Smooth Muscle Cells in Tissue and Culture Using Real-Time Quantitative PCR. Cells 2023, 12, 2135. [Google Scholar] [CrossRef]
- Seeland, U.; Regitz-Zagrosek, V. Sex and gender differences in cardiovascular drug therapy. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 214, pp. 211–236. [Google Scholar] [CrossRef]
- Asunción-Alvarez, D.; Palacios, J.; Ybañez-Julca, R.O.; Rodriguez-Silva, C.N.; Nwokocha, C.; Cifuentes, F.; Greensmith, D.J. Calcium signaling in endothelial and vascular smooth muscle cells: Sex differences and the influence of estrogens and androgens. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H950–H970. [Google Scholar] [CrossRef]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vascul Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef]
- Karbasion, N.; Xu, Y.; Snider, J.C.; Bersi, M.R. Primary Mouse Aortic Smooth Muscle Cells Exhibit Region- and Sex-Dependent Biological Responses In Vitro. J. Biomech. Eng. 2024, 146, 060904. [Google Scholar] [CrossRef]
- Wagner, B.M.; Robinson, J.W.; Healy, C.L.; Gauthier, M.; Dickey, D.M.; Yee, S.P.; Osborn, J.W.; O’Connell, T.D.; Potter, L.R. Guanylyl cyclase-A phosphorylation decreases cardiac hypertrophy and improves systolic function in male, but not female, mice. FASEB J. 2022, 36, e22069. [Google Scholar] [CrossRef]
- Wong, P.G.; Armstrong, D.W.; Tse, M.Y.; Brander, E.P.; Pang, S.C. Sex-specific differences in natriuretic peptide and nitric oxide synthase expression in ANP gene-disrupted mice. Mol. Cell. Biochem. 2013, 374, 125–135. [Google Scholar] [CrossRef]
- Taki, F.A.; Abdel-Rahman, A.A.; Zhang, B. A comprehensive approach to identify reliable reference gene candidates to investigate the link between alcoholism and endocrinology in Sprague-Dawley rats. PLoS ONE 2014, 9, e94311. [Google Scholar] [CrossRef] [PubMed]
- Wunder, F.; Woermann, A.; Geerts, A.; Milde, M. Pharmacological characterization of receptor guanylyl cyclase reporter cell lines. Eur. J. Pharmacol. 2013, 698, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Cortes-Dericks, L.; Budnik, L.T.; Brunswig-Spickenheier, B.; Pancratius, M.; Speth, R.C.; Mukhopadhyay, A.K.; Middendorff, R. Homologous and lysophosphatidic acid-induced desensitization of the atrial natriuretic peptide receptor, guanylyl cyclase-A, in MA-10 leydig cells. Endocrinology 2006, 147, 2974–2985. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Mukhopadhyay, A.K.; Speth, R.C.; Guidone, G.; Potthast, R.; Potter, L.R.; Middendorff, R. Spatiotemporal regulation of the two atrial natriuretic peptide receptors in testis. Endocrinology 2004, 145, 1392–1401. [Google Scholar] [CrossRef]
- Suga, S.; Nakao, K.; Kishimoto, I.; Hosoda, K.; Mukoyama, M.; Arai, H.; Shirakami, G.; Ogawa, Y.; Komatsu, Y.; Nakagawa, O.; et al. Phenotype-related alteration in expression of natriuretic peptide receptors in aortic smooth muscle cells. Circ. Res. 1992, 71, 34–39. [Google Scholar] [CrossRef]
- Suga, S.; Nakao, K.; Mukoyama, M.; Arai, H.; Hosoda, K.; Ogawa, Y.; Imura, H. Characterization of natriuretic peptide receptors in cultured cells. Hypertension 1992, 19 Pt 2, 762–765. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Suga, S.I.; Itoh, H.; Komatsu, Y.; Ishida, H.; Igaki, T.; Yamashita, J.; Doi, K.; Chun, T.H.; Yoshimasa, T.; Tanaka, I.; et al. Regulation of endothelial production of C-type natriuretic peptide by interaction between endothelial cells and macrophages. Endocrinology 1998, 139, 1920–1926. [Google Scholar] [CrossRef]
- Potter, L.R. Phosphorylation-Dependent Regulation of Guanylyl Cyclase (GC)-A and Other Membrane GC Receptors. Endocr. Rev. 2024. [CrossRef]
- Jensen, L.F.; Bentzon, J.F.; Albarrán-Juárez, J. The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues. Cells 2021, 10, 2209. [Google Scholar] [CrossRef]
- Lehners, M.; Dobrowinski, H.; Feil, S.; Feil, R. cGMP Signaling and Vascular Smooth Muscle Cell Plasticity. J. Cardiovasc. Dev. Dis. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Grzesik, W.J.; Takahashi, N.; Pandey, K.N.; Pang, S.; Yamauchi, M.; Smithies, O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl. Acad. Sci. USA 1999, 96, 7403–7408. [Google Scholar] [CrossRef]
- Saha, S.; Li, Y.; Lappas, G.; Anand-Srivastava, M.B. Activation of natriuretic peptide receptor-C attenuates the enhanced oxidative stress in vascular smooth muscle cells from spontaneously hypertensive rats: Implication of Gialpha protein. J. Mol. Cell. Cardiol. 2008, 44, 336–344. [Google Scholar] [CrossRef]
- Rubattu, S.; Sciarretta, S.; Morriello, A.; Calvieri, C.; Battistoni, A.; Volpe, M. NPR-C: A component of the natriuretic peptide family with implications in human diseases. J. Mol. Med. 2010, 88, 889–897. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhou, Y.; Chen, L.; Wang, Y.Q.; Wang, X.; Pi, Y.; Gao, C.Y.; Li, J.C.; Zhang, L.L. An overview of potential molecular mechanisms involved in VSMC phenotypic modulation. Histochem. Cell Biol. 2016, 145, 119–130. [Google Scholar] [CrossRef]
- Wu, G.; Sharina, I.; Martin, E. Soluble guanylyl cyclase: Molecular basis for ligand selectivity and action in vitro and in vivo. Front. Mol. Biosci. 2022, 9, 1007768. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef]
- Khalil, R.A. Sex hormones as potential modulators of vascular function in hypertension. Hypertension 2005, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Perusquía, M. Androgens and Non-Genomic vascular responses in hypertension. Biochem. Pharmacol. 2022, 203, 115200. [Google Scholar] [CrossRef] [PubMed]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]

| Step | Cycles | Duration | Temperature | |
|---|---|---|---|---|
| 1 | Polymerase activation | 1× | 3 min | 95 °C |
| 2 | Denaturation | 35× | 20 s | 95 °C |
| 3 | Primer hybridization | 20 s | 60 °C | |
| 4 | Elongation | 20 s | 72 °C | |
| 5 | Melt curve analysis | 55–95 °C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rager, C.; Klöpper, T.; Tasch, S.; Whittaker, M.R.; Exintaris, B.; Mietens, A.; Middendorff, R. The Influence of Cell Isolation and Culturing on Natriuretic Peptide Receptors in Aortic Vascular Smooth Muscle Cells. Cells 2025, 14, 51. https://doi.org/10.3390/cells14010051
Rager C, Klöpper T, Tasch S, Whittaker MR, Exintaris B, Mietens A, Middendorff R. The Influence of Cell Isolation and Culturing on Natriuretic Peptide Receptors in Aortic Vascular Smooth Muscle Cells. Cells. 2025; 14(1):51. https://doi.org/10.3390/cells14010051
Chicago/Turabian StyleRager, Christine, Tobias Klöpper, Sabine Tasch, Michael Raymond Whittaker, Betty Exintaris, Andrea Mietens, and Ralf Middendorff. 2025. "The Influence of Cell Isolation and Culturing on Natriuretic Peptide Receptors in Aortic Vascular Smooth Muscle Cells" Cells 14, no. 1: 51. https://doi.org/10.3390/cells14010051
APA StyleRager, C., Klöpper, T., Tasch, S., Whittaker, M. R., Exintaris, B., Mietens, A., & Middendorff, R. (2025). The Influence of Cell Isolation and Culturing on Natriuretic Peptide Receptors in Aortic Vascular Smooth Muscle Cells. Cells, 14(1), 51. https://doi.org/10.3390/cells14010051







