Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Recording of Extracellular Single Unit Activity in Trigeminal Neurons
2.2. Electrophysiological Recordings
2.3. Data Analysis
3. Results
3.1. Characteristics of TG Neurons That Innervate the Skin of the Face
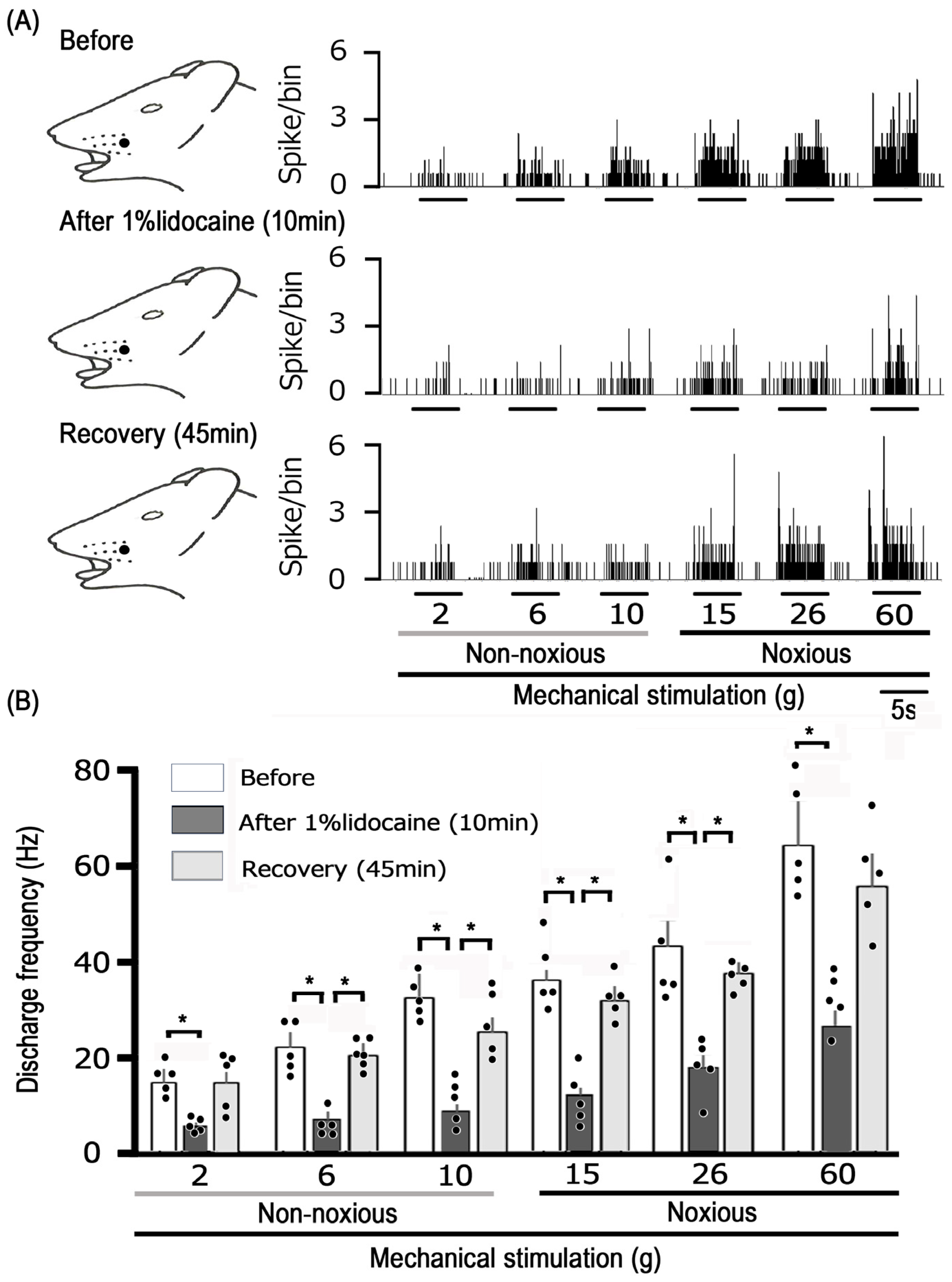
3.2. Impact of Localized EGCG Administration on TG Neuron Excitability in Response to Non-Noxious and Noxious Stimuli
3.3. The Effect of Noxious and Non-Noxious Stimuli on TG Neural Activity Post-EGCG Administration
3.4. Examination of How EGCG and Lidocaine Influence TG Neuronal Response to Mechanical Stimulation
4. Discussion
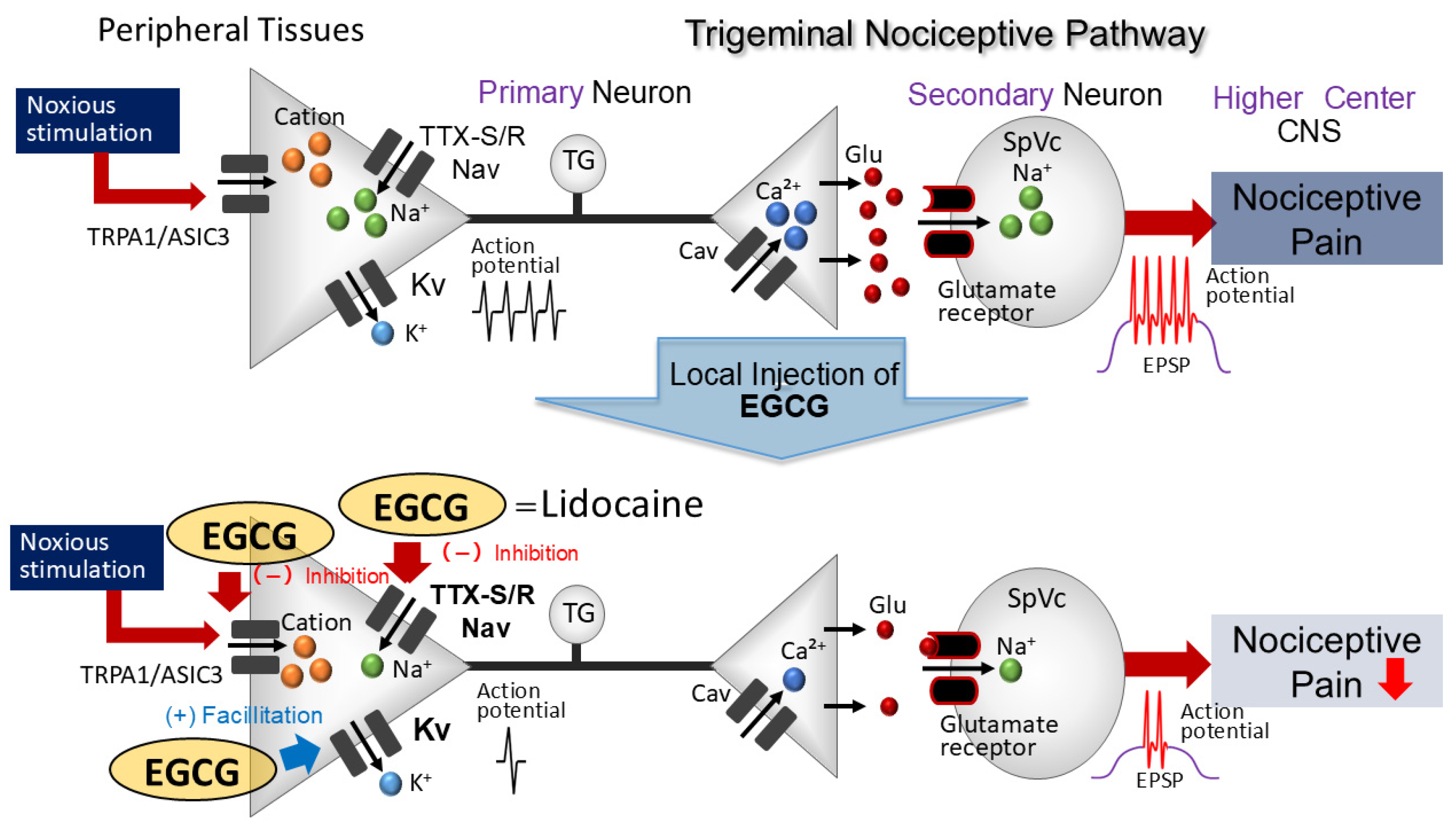
4.1. Topical Application of EGCG Reduces the Excitability of Nociceptive Primary TG Neurons
4.2. Local Administration of EGCG Diminishes TG Neuronal Excitability Through Peripheral Mechanisms
4.3. Importance of EGCG in Reducing Nociceptive Stimulation Effects on TG Neurons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sessle, B.J. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and central mechanisms of trigeminal neuropathic and inflammatory pain. J. Oral Biosci. 2011, 53, 318–329. [Google Scholar] [CrossRef]
- Iwata, K.; Takeda, M.; Oh, S.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Balasubramaniam, R., McCullough, M.J., Eds.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Rao, J.K.; Mihaliak, K.; Kroenke, K.; Bradley, J.; Tierney, W.M.; Weinberger, M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann. Int. Med. 1999, 131, 409–416. [Google Scholar] [CrossRef]
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/alternative medicine use among chronic pain clinic patients. J. Perianesth. Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef]
- Rosenberg, E.I.; Genao, I.; Chen, I.; Mechaber, A.J.; Wood, J.A.; Faselis, C.J.; Kurz, J.; Menon, M.; O’Rorke, J.; Panda, M.; et al. Complementary and alternative medicine use by primary care patients with chronic pain. Pain Med. 2008, 9, 1065–1072. [Google Scholar] [CrossRef]
- Bauer, B.; Tilburt, C.; Sood, A.; Li, G.-X.; Wang, S.-H. Complementary and alternative medicine therapies for chronic pain. Chin. J. Integr. Med. 2016, 6, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Secondary Sensory Neurons following Local Administration of the Phytochemical, (-)-Epigallocatechin-3-gallate. Brain Res. 2023, 1813, 148426. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Kinouchi, R.; Morizumi, S.; Shimazu, Y.; Takeda, M. Local administration of genistein as a local anesthetic agent inhibits the trigeminal nociceptive neuronal activity in rats. Brain Res. Bull. 2012, 172, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Weinrehb, O.; Amit, T.; Youdim, M.B. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implication of neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Komori, A.; Yattsmami, J.; Okabe, S.; Abe, S.; Hara, K.; Suganuma, M.; Kim, S.J.; Fujiki, H. Anticarcinogenic activity of green tea polyphenols. Jpn. J. Clin. Oncol. 1993, 23, 186–190. [Google Scholar] [PubMed]
- Kuroda, Y.; Hara, Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res. 1999, 436, 69–97. [Google Scholar] [CrossRef]
- Shi, X.; Leonard, Y.J.; Ding, M.; Vallyaythan, V.; Castranova, V.; Rojanasakul, Y.; Dong, Z. Antioxidant properties of (-)-epigallocatechin-3-gallate and inhibition of Cr(VI)-induced DNA damage and Cr(IV)- or TPA-stimulated Nf-kappa B activation. Mol. Cell Biochem. 2000, 206, 125–132. [Google Scholar] [CrossRef]
- Valcic, S.; Burr, J.A.; Timmermann, B.N.; Liebler, D.C. Antioxidant chemistry of green tea catechins. New oxidation products of (-)-epigallocatechin-3-gallate and (-)-epigallocatechin from their reactions with peroxyl radicals. Chem. Res. Toxicol. 2000, 13, 801–810. [Google Scholar] [CrossRef]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albni, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef]
- Homma, T.; Hirai, K.; Hara, Y.; Katayama, Y. Tea catechin (-)-epigallocatechin gallate, causes membrane depolarizations of myenteric neurons in the guinea-pig small intestine. Neurosci. Lett. 2001, 309, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Mun, K.C.; Park, W.K.; Lee, S.R.; Suh, S.I.; Baek, W.K.; Yin, M.B.; Kwon, T.K.; Song, D.K. EGCG attenuates AMPA-induced intracellular calcium increase in hippocampal neurons. Biochem. Biophy. Res. Comm. 2002, 290, 1506–1512. [Google Scholar]
- Katayama, Y.; Homma, T.; Hara, Y.; Hirai, K. Tea catechin, (-)-epigallocatechin gallate, facilitates cholinergic ganglion transmission in the myenteric plexus of the guinea-pig small intestine. Neurosci. Lett. 2002, 319, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Kim, Y.S.; Park, J.S. Modulation of neuronal activity by EGCG. Brain Res. 2005, 1047, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.H.R.; Baczko, I.; Jones, L.; Fercho, M.; Light, P.E. Inhibition of cardiac voltage-gated sodium channels by grape polyphenols. Br. J. Pharmacol. 2006, 149, 657–665. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Orallo, F. Effect of (-)-epigallocatechin-3-gallate in Ca2+-permeable non-selective cation channels and voltage-operated Ca2+channels in vascular smooth muscle cells. Life Sci. 2007, 80, 2147–2153. [Google Scholar] [CrossRef]
- Kim, T.H.; Lim, J.-M.; Kim, S.S.; Park, M.; Song, J.-H. Effects of (-)-epigallocatechin-3-gallate on Na(+) currents in rat dorsal root ganglion neurons. Eur. J. Pharmacol. 2009, 604, 20–26. [Google Scholar] [CrossRef]
- Kang, J.; Cheng, H.; Ji, J.; Incardona, J.; Rampe, D. In vitro electrocardiographic and cardiac ion channel effects of (-)-epigallocatechin-3-gallate, the main catechin of green tea. J. Pharmacol. Exp. Ther. 2010, 334, 626. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.G.; Li, W.G.; Zhu, J.J.; Huang, C.; Han, S.L.; Jiang, Q.; Xu, T.L.; Liu, J.H. Subtype-selective inhibition of acid-sensing ion channel 3 by a natural flavonoid. CNS Neurosci. Ther. 2019, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Redford, K.E.; Rognant, S.; Jepps, T.A.; Abbott, G.W. KCNQ5 potassium channel activation underlies vasodilation by tea. Cell Physiol. Biochem. 2012, 55, 46–64. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Toyota, R.; Itou, H.; Sashide, Y.; Takeda, M. Suppression of the excitability of rat nociceptive primary sensory neurons following local administration of the phytochemical quercetin. J. Pain 2023, 24, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 97–207. [Google Scholar] [CrossRef]
- Akopian, A.N.; Sivilotti, L.; Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996, 379, 257–262. [Google Scholar] [CrossRef]
- Hille, B. Potassium channels and chloride channels. In Ion Channels of Excitable Membranes, 3rd ed.; Hille, B., Ed.; Sinauer Associates: Sunderland, UK, 2001; pp. 134–167. [Google Scholar]
- Schroeder, J.E.; Fischbach, P.S.; McCleskey, E.W. T-type-calcium channels: Heterogenous expression in rat sensory neurons and selective modulation by phorbol esters. J. Neurosci. 1990, 10, 947–951. [Google Scholar] [CrossRef]
- Scroggs, R.S.; Fox, A.P. Calcium currents variation between acutely isolated adult dorsal root ganglion neurons of different size. J. Physiol. 1992, 445, 639–658. [Google Scholar] [CrossRef]
- Nelson, M.T.; Todorovic, S.; Perez-Reyes, E. The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des. 2006, 12, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Jevtovic-Todorovic, V.; Todorovic, S.M. The role of peripheral T-type calcium channels in pain transmission. Cell Calcium 2006, 40, 197–203. [Google Scholar] [CrossRef]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol. Pain 2010, 6, 91. [Google Scholar] [CrossRef]
- Boadas-Vaello, P.; Vela, J.M.; Verdú, E. New pharmacological approaches using polyphenols on the physiopathology of neuropathic pain. Curr. Drug Targets 2017, 18, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, L.E.; Chelliboina, N.; Woodman, S.E.; Durham, P.L. Dietary supplementation with grape seed extract prevents development of trigeminal sensitization and inhibits pain signaling in a preclinical chronic temporomandibular disorder model. J. Oral Pathol. Med. 2020, 49, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.D. Local Anesthetics. In Basic and Clinical Pharmacology, 6th ed.; Katzung, B.G., Ed.; McGraw-Hill Medical: New York, NY, USA, 1995; pp. 425–433. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utugi, S.; Chida, R.; Yamaguchi, S.; Sashide, Y.; Takeda, M. Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons. Cells 2025, 14, 52. https://doi.org/10.3390/cells14010052
Utugi S, Chida R, Yamaguchi S, Sashide Y, Takeda M. Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons. Cells. 2025; 14(1):52. https://doi.org/10.3390/cells14010052
Chicago/Turabian StyleUtugi, Syogo, Risako Chida, Sana Yamaguchi, Yukito Sashide, and Mamoru Takeda. 2025. "Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons" Cells 14, no. 1: 52. https://doi.org/10.3390/cells14010052
APA StyleUtugi, S., Chida, R., Yamaguchi, S., Sashide, Y., & Takeda, M. (2025). Local Administration of (−)-Epigallocatechin-3-Gallate as a Local Anesthetic Agent Inhibits the Excitability of Rat Nociceptive Primary Sensory Neurons. Cells, 14(1), 52. https://doi.org/10.3390/cells14010052





