Microglia-Derived Brain Macrophages Associate with Glioblastoma Stem Cells: A Potential Mechanism for Tumor Progression Revealed by AI-Assisted Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wet Laboratory Work
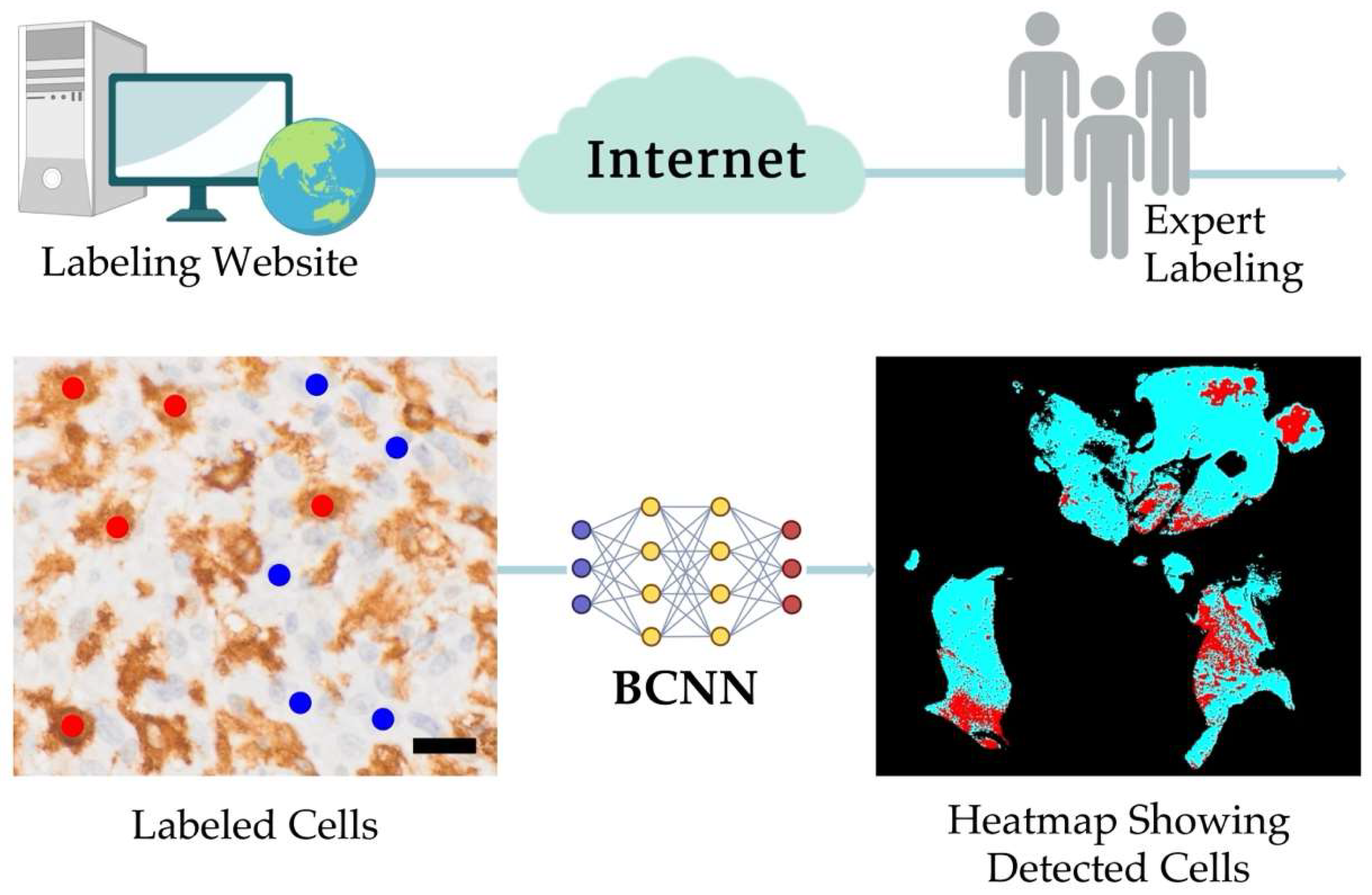
2.2. The PathoFusion Framework
2.3. Dataset Preparation, Partitioning, Filtering, and Thresholding for CD276, Iba1, and CD163 Immunoreactive Cell Analysis
2.4. Post-Processing Steps
3. Results
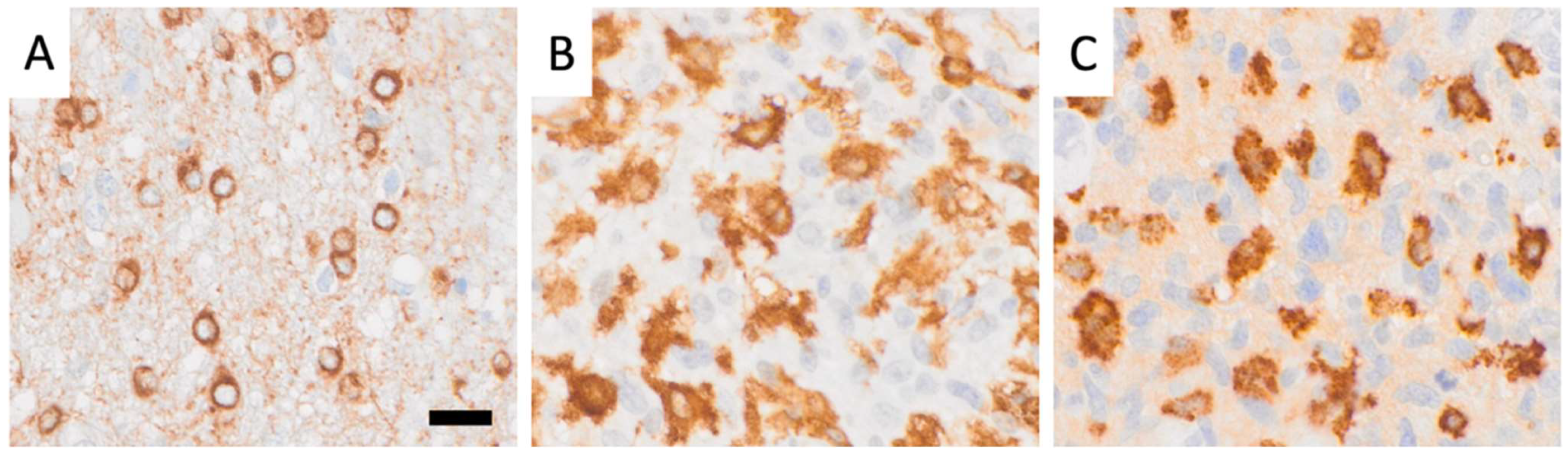
3.1. Morphological Tissue Analysis Is Greatly Aided by AI Assistance
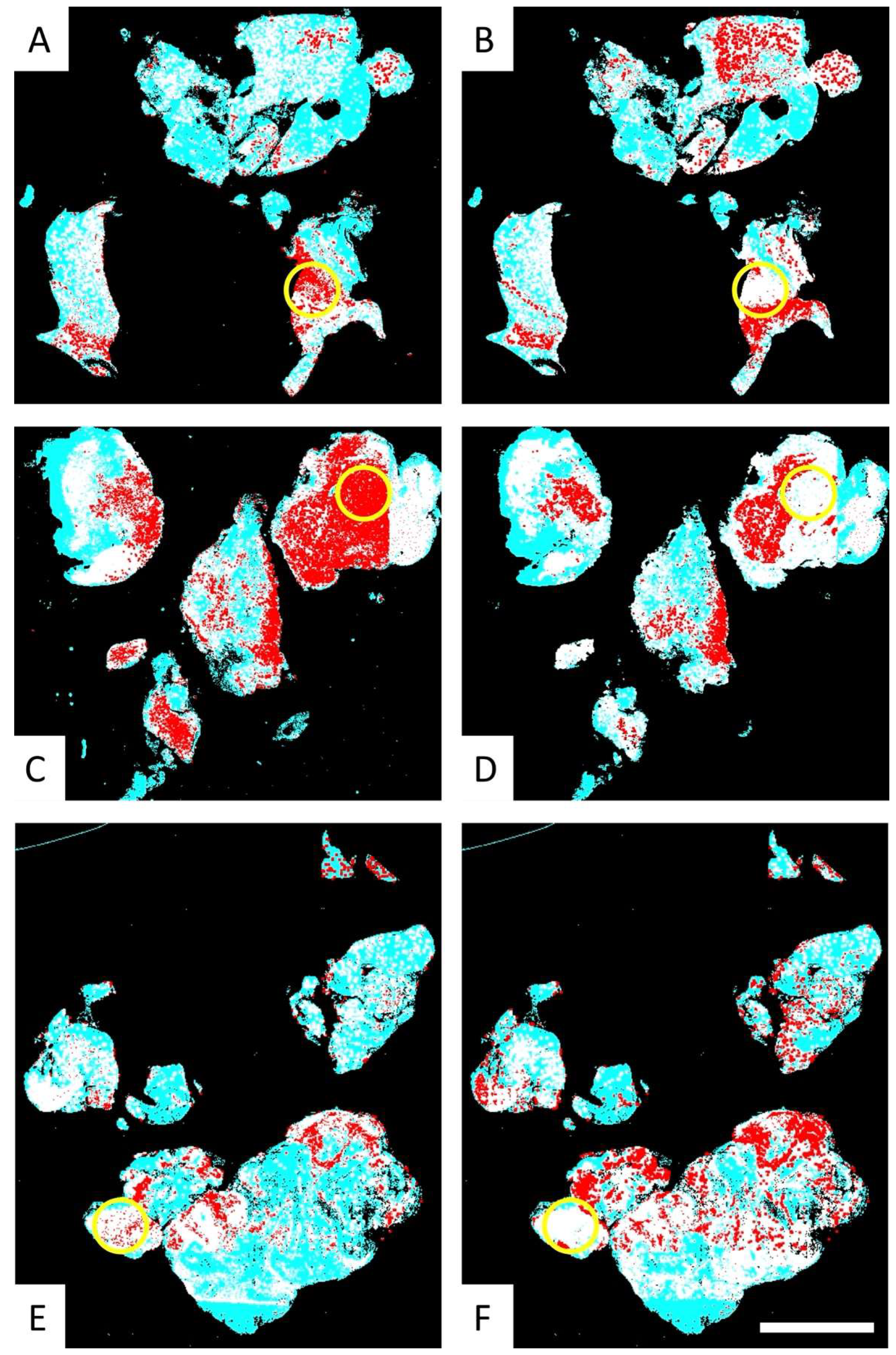
3.2. The PathoFusion Framework Allows Visualization of the Locations of GSCs and Microglia/Macrophages on the Basis of Multimodal Heatmaps
3.3. Fusion Heatmaps Reveal Regional Differences Between the Localization of CD276+ GSCs and Iba1+ and CD163+ Macrophages
3.4. Comparative Analysis of the Multimodal Heatmaps Indicates That Microglia-Derived Brain Macrophages Are More Frequently Associated with Glioblastoma Stem Cells than Myeloid Cells
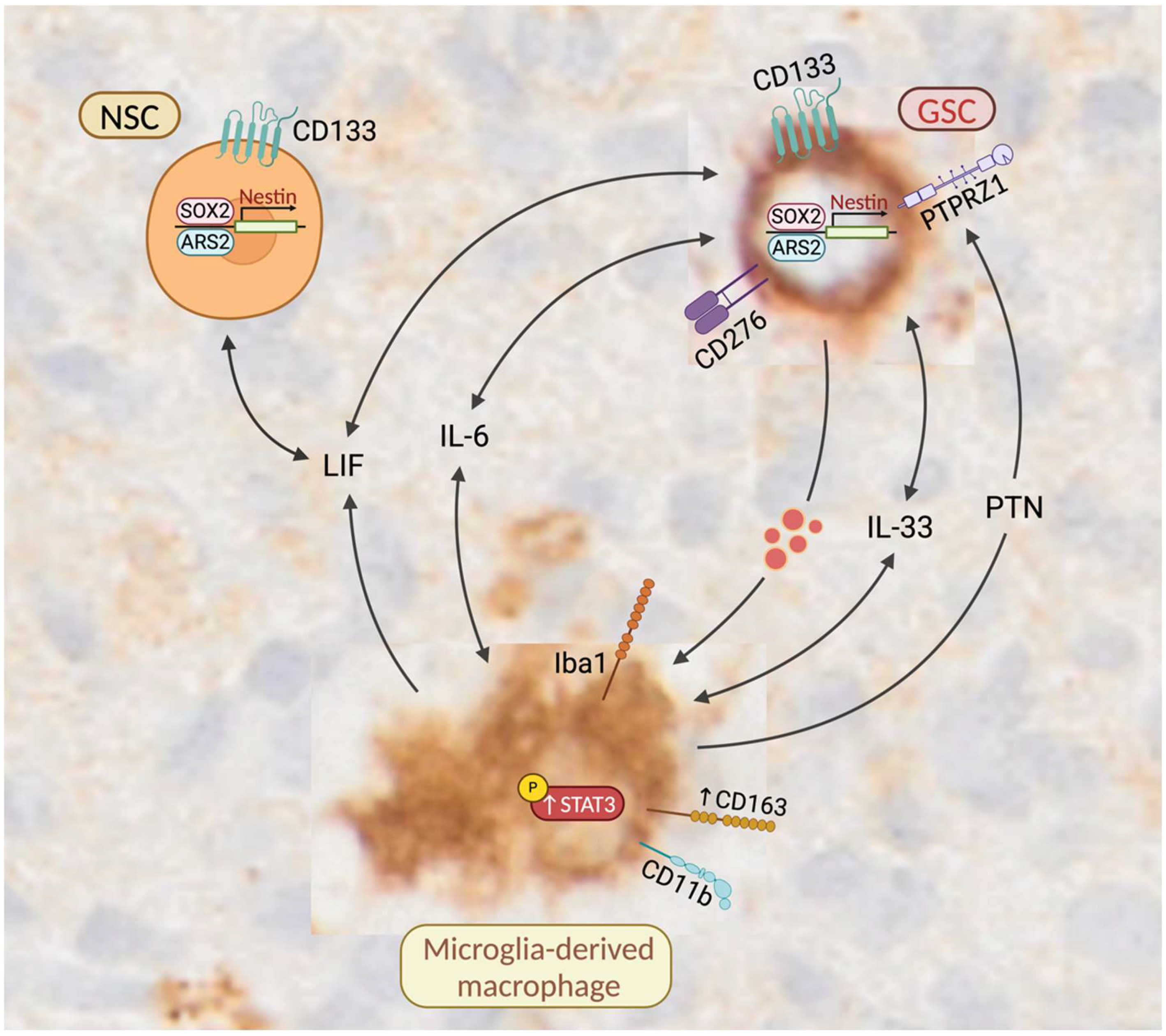
4. Discussion
4.1. Molecular Similarities Between GSCs and NSCs
4.2. Co-Localization of Microglia-Derived Brain Macrophages and NSCs Is a Normal Feature of the SVZ
4.3. Microglia That Reside in Close Proximity to NSCs in the SVZ Are Characterized by a More Amoeboid Morphology
4.4. GSCs May Enhance the Expression of CD163 in Tumor-Associated Macrophages
4.5. CD163-Expressing Macrophages Promote the Self-Renewal and Maintenance of GSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poon, C.C.; Sarkar, S.; Yong, V.W.; Kelly, J.J. Glioblastoma-associated microglia and macrophages: Targets for therapies to improve prognosis. Brain 2017, 140, 1548–1560. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Hambardzumyan, D. Macrophages and microglia: The cerberus of glioblastoma. Acta Neuropathol. Commun. 2021, 9, 54. [Google Scholar] [CrossRef]
- Loh, C.; Zheng, Y.; Alzoubi, I.; Alexander, K.-L.; Lee, M.; Cai, W.-D.; Song, Y.; Mcdonald, K.; Nowak, A.-K.; Banati, R.-B.; et al. Microglia and brain macrophages are differentially associated with tumor necrosis in glioblastoma: A link to tumor progression. Oncol. Res. 2024. [Google Scholar] [CrossRef]
- Hamed, A.A.; Hua, K.; Trinh, Q.M.; Simons, B.D.; Marioni, J.C.; Stein, L.D.; Dirks, P.B. Gliomagenesis mimics an injury response orchestrated by neural crest-like cells. Nature 2025, 638, 499–509. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel geneiba1in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Kanazawa, H.; Ohsawa, K.; Sasaki, Y.; Kohsaka, S.; Imai, Y. Macrophage/microglia-specific protein Iba1 enhances membrane ruffling and Rac activation via phospholipase C-γ-dependent pathway. J. Biol. Chem. 2002, 277, 20026–20032. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Kanazawa, H.; Sasaki, Y.; Kohsaka, S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J. Cell Sci. 2000, 113, 3073–3084. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Garcia-Montero, C.; Fraile-Martinez, O.; Boaru, D.L.; García-Puente, L.; Rios-Parra, A.; Garrido-Gil, M.J.; Casanova-Martín, C.; García-Honduvilla, N.; Bujan, J. AIF1: Function and connection with inflammatory diseases. Biology 2023, 12, 694. [Google Scholar] [CrossRef]
- Sibinga, N.E.; Feinberg, M.W.; Yang, H.; Werner, F.; Jain, M.K. Macrophage-restricted and interferon γ-inducible expression of the allograft inflammatory factor-1 gene requires Pu. 1. J. Biol. Chem. 2002, 277, 16202–16210. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer's Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Yang, Z.; Huang, K.; Liu, W.; Chai, Y. Correlation of AIF-1 Expression with Immune and Clinical Features in 1270 Glioma Samples. J. Mol. Neurosci. 2022, 72, 420–432. [Google Scholar] [CrossRef]
- Ravi, V.M.; Neidert, N.; Will, P.; Joseph, K.; Maier, J.P.; Kückelhaus, J.; Vollmer, L.; Goeldner, J.M.; Behringer, S.P.; Scherer, F. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat. Commun. 2022, 13, 925. [Google Scholar] [CrossRef]
- Philippidis, P.; Mason, J.; Evans, B.; Nadra, I.; Taylor, K.; Haskard, D.; Landis, R. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004, 94, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Etzerodt, A.; Moestrup, S.K. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, Y.; Liu, X.; Sun Zhang, A.; Zhang, H.; Hu, G.; Sun, X.-F. CD163 as a potential biomarker in colorectal cancer for tumor microenvironment and cancer prognosis: A Swedish study from tissue microarrays to big data analyses. Cancers 2022, 14, 6166. [Google Scholar] [CrossRef]
- Yanagawa, N.; Shikanai, S.; Sugai, M.; Koike, Y.; Asai, Y.; Tanji, T.; Sugimoto, R.; Osakabe, M.; Uesugi, N.; Saito, H. Prognostic and predictive value of CD163 expression and the CD163/CD68 expression ratio for response to adjuvant chemotherapy in patients with surgically resected lung squamous cell carcinoma. Thorac. Cancer 2023, 14, 1911–1920. [Google Scholar] [CrossRef]
- Kinoshita, J.; Fushida, S.; Yamaguchi, T.; Moriyama, H.; Saito, H.; Shimada, M.; Terai, S.; Okamoto, K.; Nakamura, K.; Ninomiya, I. Prognostic value of tumor-infiltrating CD163+ macrophage in patients with metastatic gastric cancer undergoing multidisciplinary treatment. BMC Cancer 2022, 22, 608. [Google Scholar] [CrossRef]
- Garvin, S.; Oda, H.; Arnesson, L.-G.; Lindström, A.; Shabo, I. Tumor cell expression of CD163 is associated to postoperative radiotherapy and poor prognosis in patients with breast cancer treated with breast-conserving surgery. J. Cancer Res. Clin. Oncol. 2018, 144, 1253–1263. [Google Scholar] [CrossRef]
- Kroonen, J.; Nassen, J.; Boulanger, Y.G.; Provenzano, F.; Capraro, V.; Bours, V.; Martin, D.; Deprez, M.; Robe, P.; Rogister, B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int. J. Cancer 2011, 129, 574–585. [Google Scholar] [CrossRef]
- Goffart, N.; Kroonen, J.; Rogister, B. Glioblastoma-initiating cells: Relationship with neural stem cells and the micro-environment. Cancers 2013, 5, 1049–1071. [Google Scholar] [CrossRef]
- Alzoubi, I.; Bao, G.; Zhang, R.; Loh, C.; Zheng, Y.; Cherepanoff, S.; Gracie, G.; Lee, M.; Kuligowski, M.; Alexander, K.L.; et al. An Open-Source AI Framework for the Analysis of Single Cells in Whole-Slide Images with a Note on CD276 in Glioblastoma. Cancers 2022, 14, 3441. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular pathways: Targeting B7-H3 (CD276) for human cancer immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Guo, X.; Xing, Z.; Tao, Y.; Liang, W.; Shi, Z.; Hu, W.; Zhou, S.; Wang, X. Multi-omics analyses of CD276 in pan-cancer reveals its clinical prognostic value in glioblastoma and other major cancer types. BMC Cancer 2023, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lv, X.; Wu, Y.; Xu, J.; Wang, L.; Chen, W.; Zhang, W.; Li, J.; Zhang, S.; Qiu, H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology 2015, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Murakami, R.; Hamanishi, J.; Tanigaki, K.; Hosoe, Y.; Mise, N.; Takamatsu, S.; Mise, Y.; Ukita, M.; Taki, M. B7-H3 suppresses antitumor immunity via the CCL2–CCR2–M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol. Res. 2022, 10, 56–69. [Google Scholar] [CrossRef]
- Zang, X.; Sullivan, P.S.; Soslow, R.A.; Waitz, R.; Reuter, V.E.; Wilton, A.; Thaler, H.T.; Arul, M.; Slovin, S.F.; Wei, J. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 2010, 23, 1104–1112. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol. Ther.-Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef]
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.; Dunn, D.E.; Edwards, D.S. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine 2019, 47, 33–43. [Google Scholar] [CrossRef]
- Sun, F.; Yu, X.; Ju, R.; Wang, Z.; Wang, Y. Antitumor responses in gastric cancer by targeting B7H3 via chimeric antigen receptor T cells. Cancer Cell Int. 2022, 22, 50. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, T.; Liu, F.; Sun, Z.; Shi, H.; Hua, D.; Yang, C. The co-stimulatory molecule B7-H3 promotes the epithelial-mesenchymal transition in colorectal cancer. Oncotarget 2016, 7, 31755–31771. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Jia, L.; koo Kim, J.; Li, J.; Deng, P.; Zhang, W.; Krebsbach, P.H.; Wang, C.-Y. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell 2021, 28, 1597–1613.e1597. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, I.; Zhang, L.; Zheng, Y.; Loh, C.; Wang, X.; Graeber, M.B. PathoGraph: An Attention-Based Graph Neural Network Capable of Prognostication Based on CD276 Labelling of Malignant Glioma Cells. Cancers 2024, 16, 750. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Wang, X.; Xu, R.; Loh, C.; Adeyinka, O.D.; Pieris, D.A.; Cherepanoff, S.; Gracie, G.; Lee, M.; McDonald, K.L. PathoFusion: An open-source AI framework for recognition of pathomorphological features and mapping of immunohistochemical data. Cancers 2021, 13, 617. [Google Scholar] [CrossRef]
- Janowczyk, A.; Basavanhally, A.; Madabhushi, A. Stain normalization using sparse autoencoders (StaNoSA): Application to digital pathology. Comput. Med. Imaging Graph. 2017, 57, 50–61. [Google Scholar] [CrossRef]
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural stem cells as potential glioblastoma cells of origin. Life 2023, 13, 905. [Google Scholar] [CrossRef]
- Zhang, G.-L.; Wang, C.-F.; Qian, C.; Ji, Y.-X.; Wang, Y.-Z. Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J. Stem Cells 2021, 13, 877. [Google Scholar] [CrossRef]
- Sanai, N.; Alvarez-Buylla, A.; Berger, M.S. Neural stem cells and the origin of gliomas. N. Engl. J. Med. 2005, 353, 811–822. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Hira, V.V.; Ploegmakers, K.J.; Grevers, F.; Verbovšek, U.; Silvestre-Roig, C.; Aronica, E.; Tigchelaar, W.; Turnšek, T.L.; Molenaar, R.J.; Van Noorden, C.J. CD133+ and nestin+ glioma stem-like cells reside around CD31+ arterioles in niches that express SDF-1α, CXCR4, osteopontin and cathepsin K. J. Histochem. Cytochem. 2015, 63, 481–493. [Google Scholar] [CrossRef]
- Hira, V.V.; Molenaar, R.J.; Breznik, B.; Lah, T.; Aronica, E.; Van Noorden, C.J. Immunohistochemical detection of neural stem cells and glioblastoma stem cells in the subventricular zone of glioblastoma patients. J. Histochem. Cytochem. 2021, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Barazzuol, L.; Ju, L.; Jeggo, P.A. A coordinated DNA damage response promotes adult quiescent neural stem cell activation. PLoS Biol. 2017, 15, e2001264. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Ikota, H.; Sugawara, K.-i.; Nobusawa, S.; Hirato, J.; Nakazato, Y. Nestin expression in brain tumors: Its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol. 2012, 29, 160–167. [Google Scholar] [CrossRef]
- Gruber, J.J.; Zatechka, D.S.; Sabin, L.R.; Yong, J.; Lum, J.J.; Kong, M.; Zong, W.-X.; Zhang, Z.; Lau, C.-K.; Rawlings, J. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 2009, 138, 328–339. [Google Scholar] [CrossRef]
- Andreu-Agullo, C.; Maurin, T.; Thompson, C.B.; Lai, E.C. Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature 2012, 481, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kim, S.S.; Choi, E.; Oh, Y.T.; Lin, W.; Kim, T.-H.; Sa, J.K.; Hong, J.H.; Park, S.H.; Kwon, H.J. ARS2/MAGL signaling in glioblastoma stem cells promotes self-renewal and M2-like polarization of tumor-associated macrophages. Nat. Commun. 2020, 11, 2978. [Google Scholar] [CrossRef]
- Vinel, C.; Rosser, G.; Guglielmi, L.; Constantinou, M.; Pomella, N.; Zhang, X.; Boot, J.R.; Jones, T.A.; Millner, T.O.; Dumas, A.A. Comparative epigenetic analysis of tumour initiating cells and syngeneic EPSC-derived neural stem cells in glioblastoma. Nat. Commun. 2021, 12, 6130. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Sun, G.; Meng, H.; Wang, J.; Guan, Y.; Yin, Y.; Zhao, Z.; Dong, X.; Yin, S. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett. 2019, 466, 1–12. [Google Scholar] [CrossRef]
- Digregorio, M.; Coppieters, N.; Lombard, A.; Lumapat, P.N.; Scholtes, F.; Rogister, B. The expression of B7-H3 isoforms in newly diagnosed glioblastoma and recurrence and their functional role. Acta Neuropathol. Commun. 2021, 9, 59. [Google Scholar] [CrossRef]
- Wilson, C.M.; Ospina, O.E.; Townsend, M.K.; Nguyen, J.; Moran Segura, C.; Schildkraut, J.M.; Tworoger, S.S.; Peres, L.C.; Fridley, B.L. Challenges and opportunities in the statistical analysis of multiplex immunofluorescence data. Cancers 2021, 13, 3031. [Google Scholar] [CrossRef]
- Wang, L.-C.; Wang, Y.-L.; He, B.; Zheng, Y.-J.; Yu, H.-C.; Liu, Z.-Y.; Fan, R.-r.; Zan, X.; Liang, R.-C.; Wu, Z.-P. Expression and clinical significance of VISTA, B7-H3, and PD-L1 in glioma. Clin. Immunol. 2022, 245, 109178. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal subventricular zone neural stem cells release extracellular vesicles that act as a microglial morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Matarredona, E.R.; Talaverón, R.; Pastor, A.M. Interactions between neural progenitor cells and microglia in the subventricular zone: Physiological implications in the neurogenic niche and after implantation in the injured brain. Front. Cell. Neurosci. 2018, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Goldman, J.E.; Sekino, Y.; Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014, 34, 2231–2243. [Google Scholar] [CrossRef]
- Gonzalez-Perez, O.; Gutierrez-Fernandez, F.; Lopez-Virgen, V.; Collas-Aguilar, J.; Quinones-Hinojosa, A.; Garcia-Verdugo, J.M. Immunological regulation of neurogenic niches in the adult brain. Neuroscience 2012, 226, 270–281. [Google Scholar] [CrossRef]
- Xavier, A.L.R.; Kress, B.T.; Goldman, S.A.; de Menezes, J.R.L.; Nedergaard, M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 2015, 35, 11848–11861. [Google Scholar] [CrossRef]
- Xavier, A.L.; Lima, F.R.; Nedergaard, M.; Menezes, J.R. Ontogeny of CX3CR1-EGFP expressing cells unveil microglia as an integral component of the postnatal subventricular zone. Front. Cell. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Solano Fonseca, R.; Mahesula, S.; Apple, D.M.; Raghunathan, R.; Dugan, A.; Cardona, A.; O'Connor, J.; Kokovay, E. Neurogenic niche microglia undergo positional remodeling and progressive activation contributing to age-associated reductions in neurogenesis. Stem Cells Dev. 2016, 25, 542–555. [Google Scholar] [CrossRef]
- Johnson, D.E.; O'Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- De Boeck, A.; Ahn, B.Y.; D’Mello, C.; Lun, X.; Menon, S.V.; Alshehri, M.M.; Szulzewsky, F.; Shen, Y.; Khan, L.; Dang, N.H. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 2020, 11, 4997. [Google Scholar] [CrossRef]
- Zhu, P.; Hata, R.; Cao, F.; Gu, F.; Hanakawa, Y.; Hashimoto, K.; Sakanaka, M. Ramified microglial cells promote astrogliogenesis and maintenance of neural stem cells through activation of Stat3 function. FASEB J. 2008, 22, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Shingo, T.; Weiss, S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci. 2001, 21, 7642–7653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Z.; Huang, M.; Liu, T.; Wang, Y.; Xu, H.; Duan, H.; Ma, P.; Zhang, L.; Zamvil, S.S. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 2018, 9, 559. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, Z.; Gao, Z.; Qi, Y.; Qiu, W.; Pan, Z.; Guo, Q.; Li, B.; Zhao, S. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021, 12, 373. [Google Scholar] [CrossRef]
- Johansson, E.; Grassi, E.S.; Pantazopoulou, V.; Tong, B.; Lindgren, D.; Berg, T.J.; Pietras, E.J.; Axelson, H.; Pietras, A. CD44 interacts with HIF-2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Rep. 2017, 20, 1641–1653. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, Y.; Chen, Y.; Chen, Y.; Hong, W.; Wei, W.; Tu, J. Interaction of tumor-associated microglia/macrophages and cancer stem cells in glioma. Life Sci. 2023, 320, 121558. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, B.; Chen, Y.; Guo, Z.; Yang, X.; Peng, L.; Xia, X.; Chen, L. B7-H3 regulates glioma growth and cell invasion through a JAK2/STAT3/Slug-dependent signaling pathway. OncoTargets Ther. 2020, 13, 2215–2224. [Google Scholar] [CrossRef]
- Kang, F.-B.; Wang, L.; Li, D.; Zhang, Y.-G.; Sun, D.-X. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol. Rep. 2015, 33, 274–282. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, L.; Wang, F.; Zhu, D.; Ge, X.; Hua, D.; Sun, J. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol. Lett. 2017, 14, 6177–6183. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef]
- Graeber, M.; Streit, W.; Kreutzberg, G. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res. 1988, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ping, Y.-F.; Zhou, W.; He, Z.-C.; Chen, C.; Bian, B.-S.-J.; Zhang, L.; Chen, L.; Lan, X.; Zhang, X.-C. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth. Nat. Commun. 2017, 8, 15080. [Google Scholar] [CrossRef] [PubMed]
- Deuel, T.F.; Zhang, N.; Yeh, H.-J.; Silos-Santiago, I.; Wang, Z.-Y. Pleiotrophin: A cytokine with diverse functions and a novel signaling pathway. Arch. Biochem. Biophys. 2002, 397, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Qin, E.Y.; Cooper, D.D.; Abbott, K.L.; Lennon, J.; Nagaraja, S.; Mackay, A.; Jones, C.; Vogel, H.; Jackson, P.K.; Monje, M. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell 2017, 170, 845–859.e819. [Google Scholar] [CrossRef]
- Bhaduri, A.; Di Lullo, E.; Jung, D.; Müller, S.; Crouch, E.E.; Espinosa, C.S.; Ozawa, T.; Alvarado, B.; Spatazza, J.; Cadwell, C.R. Outer radial glia-like cancer stem cells contribute to heterogeneity of glioblastoma. Cell Stem Cell 2020, 26, 48–63.e46. [Google Scholar] [CrossRef]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef]


| Case | Sex | Age at Diagnosis | Survival (Months) | Localization |
|---|---|---|---|---|
| 1 * | F | 62 | 16 | Right occipital |
| 2 * | M | 55 | 14 | Left occipital, parietal |
| 3 * | F | 46 | 10 | Left frontal |
| 4 * | M | 70 | 4 | Right frontal |
| 5 | F | 54 | 95 | Left parietal |
| 6 | M | 33 | 15 | Right frontal |
| 7 * | F | 57 | 4 | Right frontotemporal |
| 8 | F | 48 | 27 | Right frontal, occipital, parietal, temporal |
| 9 * | M | 65 | 14 | Right frontal |
| 10 * | M | 69 | 16 | Right frontal |
| 11 | M | 51 | 23 | Left parietal |
| 12 * | F | 55 | 12 | Left frontal |
| 13 | F | 85 | 4 | Left temporal |
| 14 | F | 72 | 13 | Right temporal |
| 15 * | M | 77 | 20 | Right parietal |
| 16 1 | M | 33 | 15 | Right frontal |
| 17 | F | 51 | Unknown | Right frontal |
| 18 | M | 50 | 16 | Left temporal |
| 19 | F | 60 | 20 | Right temporal |
| 20 | F | 75 | 27 | Right frontal |
| 21 * | M | 65 | 10 | Left temporal |
| 22 | M | 33 | 8 | Right frontal |
| 23 | F | 60 | 21 | Left temporal |
| 24 | M | 68 | 10 | Left frontal |
| 25 | F | 59 | 38 | Right occipital |
| 26 * | F | 79 | 12 | Right parietal |
| 27 | M | 73 | 17 | Right frontal |
| 28 * | M | 50 | 20 | Right parietal |
| 29 * | M | 66 | 12 | Right parietal |
| 30 * | M | 50 | 15 | Right temporal |
| 31 | M | 78 | 5 | Right frontal |
| 32 | F | 60 | 15 | Right occipital |
| 33 * | M | 75 | 16 | Right occipital |
| 34 | F | 62 | 13 | Right occipital, parietal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Fuse, H.; Alzoubi, I.; Graeber, M.B. Microglia-Derived Brain Macrophages Associate with Glioblastoma Stem Cells: A Potential Mechanism for Tumor Progression Revealed by AI-Assisted Analysis. Cells 2025, 14, 413. https://doi.org/10.3390/cells14060413
Zheng Y, Fuse H, Alzoubi I, Graeber MB. Microglia-Derived Brain Macrophages Associate with Glioblastoma Stem Cells: A Potential Mechanism for Tumor Progression Revealed by AI-Assisted Analysis. Cells. 2025; 14(6):413. https://doi.org/10.3390/cells14060413
Chicago/Turabian StyleZheng, Yuqi, Haneya Fuse, Islam Alzoubi, and Manuel B. Graeber. 2025. "Microglia-Derived Brain Macrophages Associate with Glioblastoma Stem Cells: A Potential Mechanism for Tumor Progression Revealed by AI-Assisted Analysis" Cells 14, no. 6: 413. https://doi.org/10.3390/cells14060413
APA StyleZheng, Y., Fuse, H., Alzoubi, I., & Graeber, M. B. (2025). Microglia-Derived Brain Macrophages Associate with Glioblastoma Stem Cells: A Potential Mechanism for Tumor Progression Revealed by AI-Assisted Analysis. Cells, 14(6), 413. https://doi.org/10.3390/cells14060413







