In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Samples Collection and Semen Analysis
2.3. In Vitro Sperm Treatment with Climbazole
2.4. Sperm Vitality and Motility Assessment
2.5. Immunofluorescence and Acrosomal Staining
2.6. Western Blot Analysis
2.7. RNA Extraction, Retrotranscription and qRT-PCR
2.8. ROS Detection
2.9. JC-1 for Assessing Mitochondrial Membrane Potential (MMP)
2.10. DNA Fragmentation Analysis
2.11. Statistical Analysis
3. Results
3.1. CBZ Affects Sperm Vitality and Motility
3.2. Effects of CBZ on Sperm Capacitation
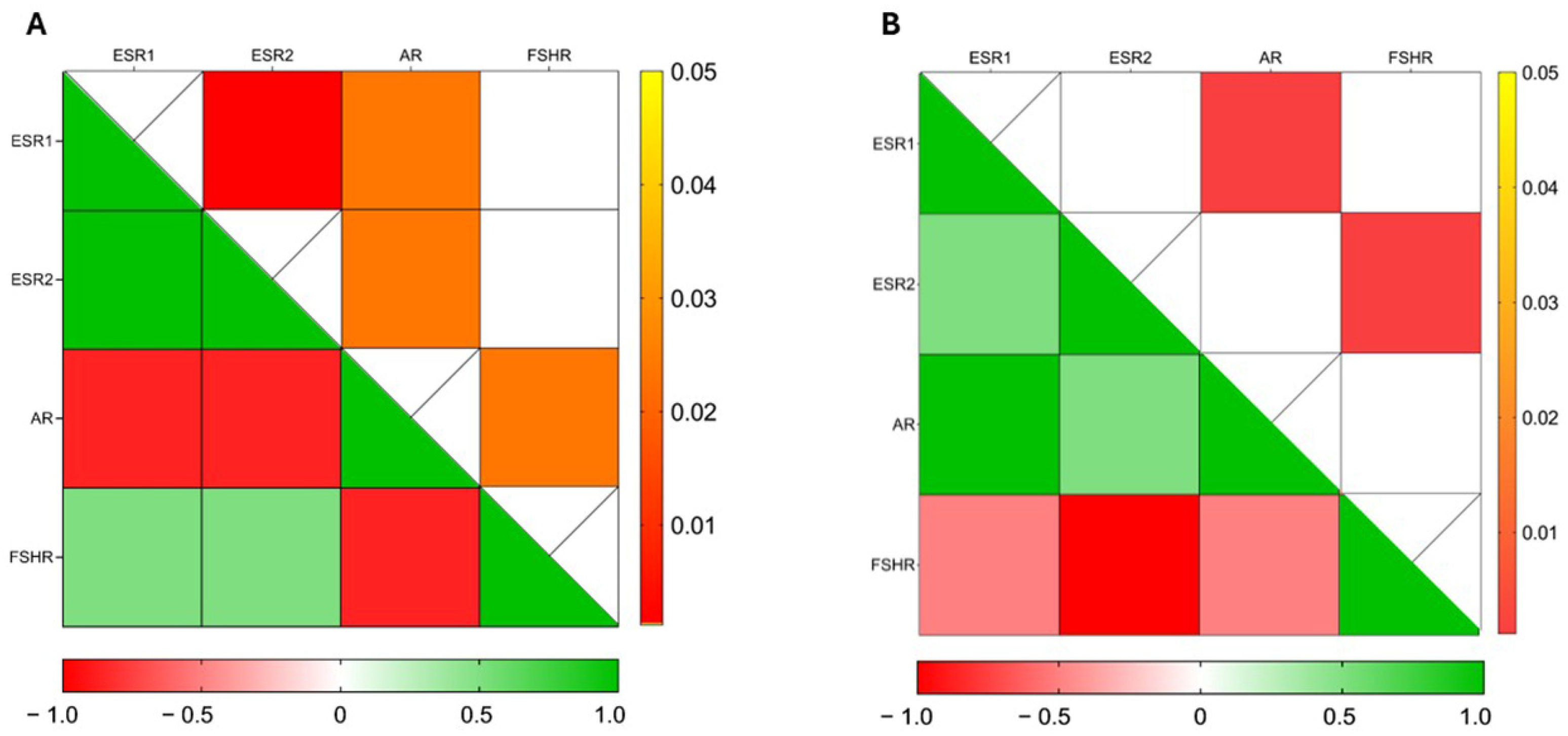
3.3. CBZ Treatment Modulates Hormone Receptor Expression in Sperm
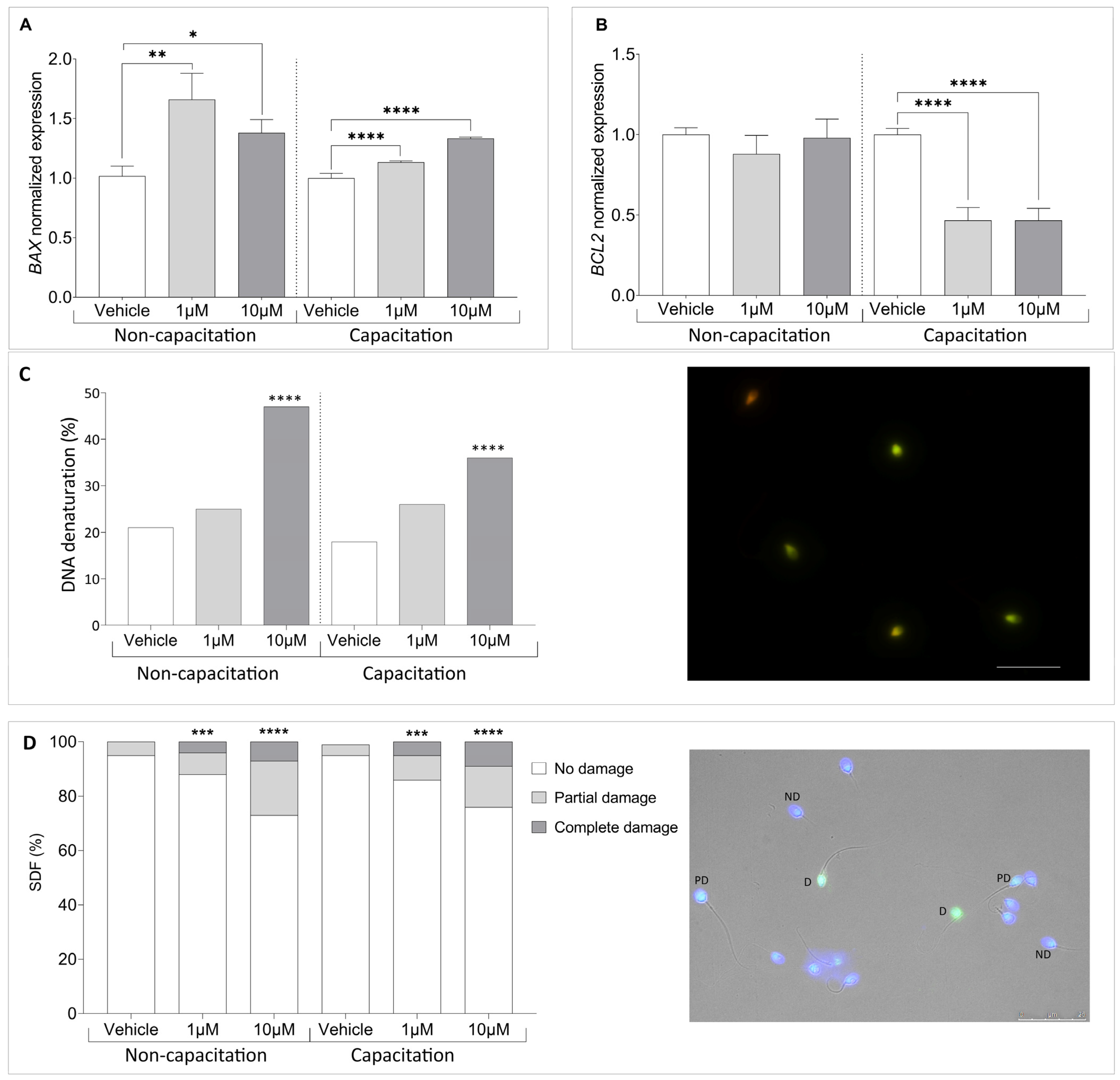
3.4. Climbazole Upregulates Pro-Apoptotic Gene Expression in Human Sperm
3.5. Climbazole Alters Mitochondrial Function in Human Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenardhanan, P.; Panneerselvam, M.; Mathur, P.P. Effect of Environmental Contaminants on Spermatogenesis. Semin. Cell Dev. Biol. 2016, 59, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- Luongo, F.P.; Annunzi, E.; Girolamo, F.; Belmonte, G.; Ponchia, R.; Piomboni, P.; Luddi, A. Assessment of Sperm Chromosomal Abnormalities Using Fluorescence in Situ Hybridization (FISH): Implications for Reproductive Potential. J. Assist. Reprod. Genet. 2024, 41, 2787–2793. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Å.; Heindel, J.J.; Jobling, S.; Kidd, K.A.; Zoeller, R.T. State of the Science of Endocrine Disrupting Chemicals—2012: An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme (UNEP) and WHO; United National Environment Programme, World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150503-1. [Google Scholar]
- Virant-Klun, I.; Imamovic-Kumalic, S.; Pinter, B. From Oxidative Stress to Male Infertility: Review of the Associations of Endocrine-Disrupting Chemicals (Bisphenols, Phthalates, and Parabens) with Human Semen Quality. Antioxidants 2022, 11, 1617. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.J. Reproductive Toxicity and Endocrine Disruption of Potential Chemical Warfare Agents. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 599–613. ISBN 978-0-12-800159-2. [Google Scholar]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Lindh, C.H.; Jönsson, B.A.G.; Giwercman, A. Prenatal Phthalate Exposure and Reproductive Function in Young Men. Environ. Res. 2015, 138, 264–270. [Google Scholar] [CrossRef]
- Turner, G.A.; Matheson, J.R.; Li, G.-Z.; Fei, X.-Q.; Zhu, D.; Baines, F.L. Enhanced Efficacy and Sensory Properties of an Anti-dandruff Shampoo Containing Zinc Pyrithione and Climbazole. Int. J. Cosmet. Sci. 2013, 35, 78–83. [Google Scholar] [CrossRef]
- Wood, A.J.J.; Como, J.A.; Dismukes, W.E. Oral Azole Drugs as Systemic Antifungal Therapy. N. Engl. J. Med. 1994, 330, 263–272. [Google Scholar] [CrossRef]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole Fungicides Affect Mammalian Steroidogenesis by Inhibiting Sterol 14 Alpha-Demethylase and Aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef]
- Kenda, M.; Karas Kuželički, N.; Iida, M.; Kojima, H.; Sollner Dolenc, M. Triclocarban, Triclosan, Bromochlorophene, Chlorophene, and Climbazole Effects on Nuclear Receptors: An in Silico and in Vitro Study. Environ. Health Perspect. 2020, 128, 107005. [Google Scholar] [CrossRef]
- Ndiaye, D.; Perceau, M.; Lorcin, M.; Denis, F.; Gaté, L. Antifungal Climbazole Alters Androgenic Pathways in Mammalian Cells. Toxicol. Vitr. 2024, 99, 105854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z.; Qi, Z.; Yan, S.; Wei, W.; Liu, G.; Cai, Z. Analysis of Transcriptional Response in Zebrafish Eleutheroembryos Exposed to Climbazole: Signaling Pathways and Potential Biomarkers. Environ. Toxic. Chem. 2019, 38, 794–805. [Google Scholar] [CrossRef]
- Trösken, E.R.; Fischer, K.; Völkel, W.; Lutz, W.K. Inhibition of Human CYP19 by Azoles Used as Antifungal Agents and Aromatase Inhibitors, Using a New LC–MS/MS Method for the Analysis of Estradiol Product Formation. Toxicology 2006, 219, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology 2016, 31, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liang, Y.-Q.; Liao, X.; Chen, X.-F.; Wang, T.; Song, Y.; Lin, Z.-C.; Qi, Z.; Chen, Z.-F.; Cai, Z. Metabolomics Reveals the Reproductive Abnormality in Female Zebrafish Exposed to Environmentally Relevant Levels of Climbazole. Environ. Pollut. 2021, 275, 116665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Luongo, F.P.; Perez Casasus, S.; Haxhiu, A.; Barbarulo, F.; Scarcella, M.; Governini, L.; Piomboni, P.; Scarica, C.; Luddi, A. Exposure to Cumulus Cell Secretome Improves Sperm Function: New Perspectives for Sperm Selection In Vitro. Cells 2023, 12, 2349. [Google Scholar] [CrossRef]
- Sati, L.; Cayli, S.; Delpiano, E.; Sakkas, D.; Huszar, G. The Pattern of Tyrosine Phosphorylation in Human Sperm in Response to Binding to Zona Pellucida or Hyaluronic Acid. Reprod. Sci. 2014, 21, 573–581. [Google Scholar] [CrossRef]
- Pérez Casasús, S.; Luongo, F.P.; Haxhiu, A.; Orini, M.; Scupoli, G.; Governini, L.; Piomboni, P.; Buratini, J.; Dal Canto, M.; Luddi, A. Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa. Cells 2024, 13, 625. [Google Scholar] [CrossRef]
- Festucci, F.; Annunzi, E.; Pepe, M.; Curcio, G.; D’Addario, C.; Adriani, W. Dopamine-transporter Heterozygous Rats Carrying Maternal Wild-type Allele Are More Vulnerable to the Development of Compulsive Behavior. Synapse 2022, 76, e22244. [Google Scholar] [CrossRef]
- Baker, A.H.; George, S.J.; Zaltsman, A.B.; Murphy, G.; Newby, A.C. Inhibition of Invasion and Induction of Apoptotic Cell Death of Cancer Cell Lines by Overexpression of TIMP-3. Br. J. Cancer 1999, 79, 1347–1355. [Google Scholar] [CrossRef]
- Piomboni, P.; Gambera, L.; Serafini, F.; Campanella, G.; Morgante, G.; De Leo, V. Sperm Quality Improvement after Natural Anti-Oxidant Treatment of Asthenoteratospermic Men with Leukocytospermia. Asian J. Androl. 2008, 10, 201–206. [Google Scholar] [CrossRef]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of Sperm Capacitation and the Acrosome Reaction: Role of Protein Kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef]
- Donà, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (AE1). Int. J. Mol. Sci. 2020, 21, 4063. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Shi, W.-J.; Ma, D.-D.; Zhang, J.-G.; Long, X.-B.; Li, S.-Y.; Gao, F.-Z.; Zhang, Q.-Q.; Ying, G.-G. The Azole Biocide Climbazole Induces Oxidative Stress, Inflammation, and Apoptosis in Fish Gut. Sci. Total Environ. 2024, 923, 171475. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-L.; Chen, Z.-F.; Zou, T.; Lin, Z.-C.; Chen, X.-F.; Wang, Y.; Qi, Z.; Cai, Z. Chronic Exposure to Climbazole Induces Oxidative Stress and Sex Hormone Imbalance in the Testes of Male Zebrafish. Chem. Res. Toxicol. 2021, 34, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.-J.; Shi, W.-J.; Gao, F.-Z.; Ma, D.-D.; Zhang, J.-G.; Li, S.-Y.; Long, X.-B.; Zhang, Q.-Q.; Ying, G.-G. Climbazole Causes Cell Apoptosis and Lipidosis in the Liver of Grass Carp. Aquat. Toxicol. 2023, 263, 106698. [Google Scholar] [CrossRef] [PubMed]
- Adamus, J.; Feng, L.; Hawkins, S.; Kalleberg, K.; Lee, J. Climbazole Boosts Activity of Retinoids in Skin. Int. J. Cosmet. Sci. 2017, 39, 411–418. [Google Scholar] [CrossRef]
- Pople, J.E.; Moore, A.E.; Talbot, D.C.S.; Barrett, K.E.; Jones, D.A.; Lim, F.L. Climbazole Increases Expression of Cornified Envelope Proteins in Primary Keratinocytes. Int. J. Cosmet. Sci. 2014, 36, 419–426. [Google Scholar] [CrossRef]
- Petricca, S.; Carnicelli, V.; Luzi, C.; Cinque, B.; Celenza, G.; Iorio, R. Oxidative Stress, Cytotoxic and Inflammatory Effects of Azoles Combinatorial Mixtures in Sertoli TM4 Cells. Antioxidants 2023, 12, 1142. [Google Scholar] [CrossRef]
- Grasa, P.; Colas, C.; Gallego, M.; Monteagudo, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. Changes in Content and Localization of Proteins Phosphorylated at Tyrosine, Serine and Threonine Residues during Ram Sperm Capacitation and Acrosome Reaction. Reproduction 2009, 137, 655–667. [Google Scholar] [CrossRef]
- Yu, J.; Duan, Y.; Lu, Q.; Chen, M.; Ning, F.; Ye, Y.; Lu, S.; Ou, D.; Sha, X.; Gan, X.; et al. Cytochrome c Oxidase IV Isoform 1 (COX4-1) Regulates the Proliferation, Migration and Invasion of Trophoblast Cells via Modulating Mitochondrial Function. Placenta 2024, 151, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of Reactive Oxygen Species in Male Infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kwon, J.-S.; Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Their Adverse Effects on the Endoplasmic Reticulum. Int. J. Mol. Sci. 2022, 23, 1581. [Google Scholar] [CrossRef] [PubMed]
- Lahimer, M.; Abou Diwan, M.; Montjean, D.; Cabry, R.; Bach, V.; Ajina, M.; Ben Ali, H.; Benkhalifa, M.; Khorsi-Cauet, H. Endocrine Disrupting Chemicals and Male Fertility: From Physiological to Molecular Effects. Front. Public Health 2023, 11, 1232646. [Google Scholar] [CrossRef]




| Semen Parameters | Mean (SD) |
|---|---|
| Volume (mL) | 3.28 ± 1.71 |
| pH | 7.50 ± 0.16 |
| Sperm Concentration (106/mL) | 79.50 ± 36.90 |
| Total sperm number (106) | 217.1 ± 81.12 |
| Motility (%) | 61.17 ± 3.67 |
| Vitality (%) | 83.50 ± 2.48 |
| Morphology (%) | 8.16 ± 2.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annunzi, E.; Luongo, F.P.; Girolamo, F.; Ponchia, R.; Passaponti, S.; Piomboni, P.; Luddi, A. In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity. Cells 2025, 14, 427. https://doi.org/10.3390/cells14060427
Annunzi E, Luongo FP, Girolamo F, Ponchia R, Passaponti S, Piomboni P, Luddi A. In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity. Cells. 2025; 14(6):427. https://doi.org/10.3390/cells14060427
Chicago/Turabian StyleAnnunzi, Eugenia, Francesca Paola Luongo, Francesca Girolamo, Rosetta Ponchia, Sofia Passaponti, Paola Piomboni, and Alice Luddi. 2025. "In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity" Cells 14, no. 6: 427. https://doi.org/10.3390/cells14060427
APA StyleAnnunzi, E., Luongo, F. P., Girolamo, F., Ponchia, R., Passaponti, S., Piomboni, P., & Luddi, A. (2025). In Vitro Exposure to the Endocrine-Disrupting Chemical Climbazole Impairs Human Sperm Motility, Hormonal Signalling, and Mitochondrial Activity. Cells, 14(6), 427. https://doi.org/10.3390/cells14060427








