Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Abiotic Stress Treatments
2.2. Identification of the StGolS and StRFS Genes
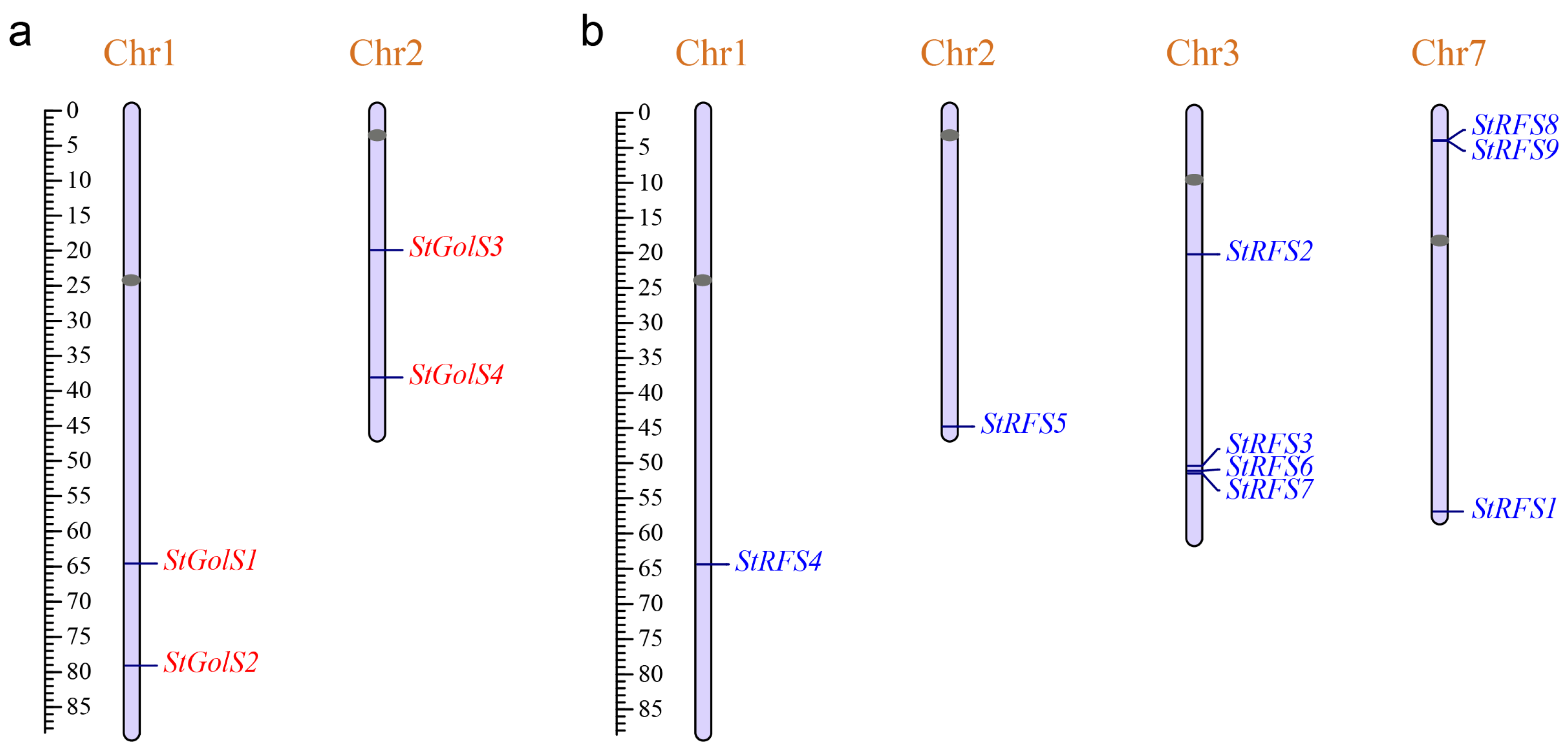
2.3. Chromosome Localization of the StGolS and StRFS Genes
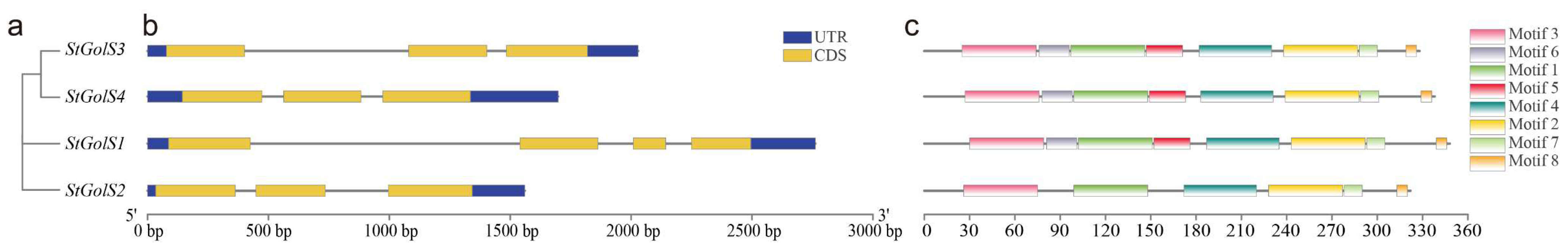
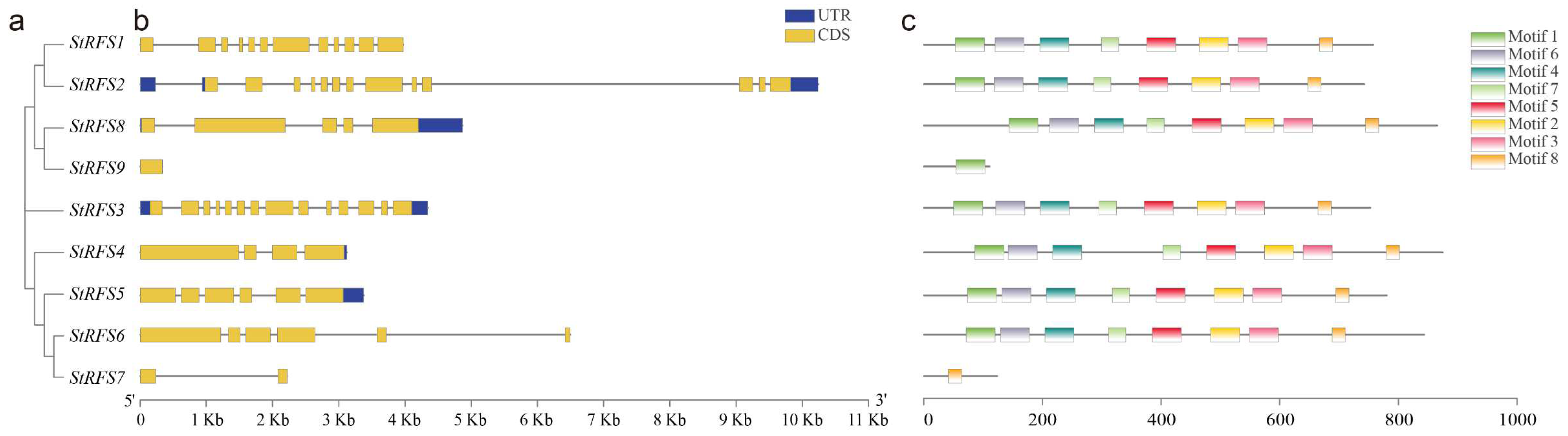
2.4. Phylogenetic Tree Construction of the StGolS and StRFS Genes
2.5. Structure Drawing and Motif Analysis of the StGolS and StRFS Genes
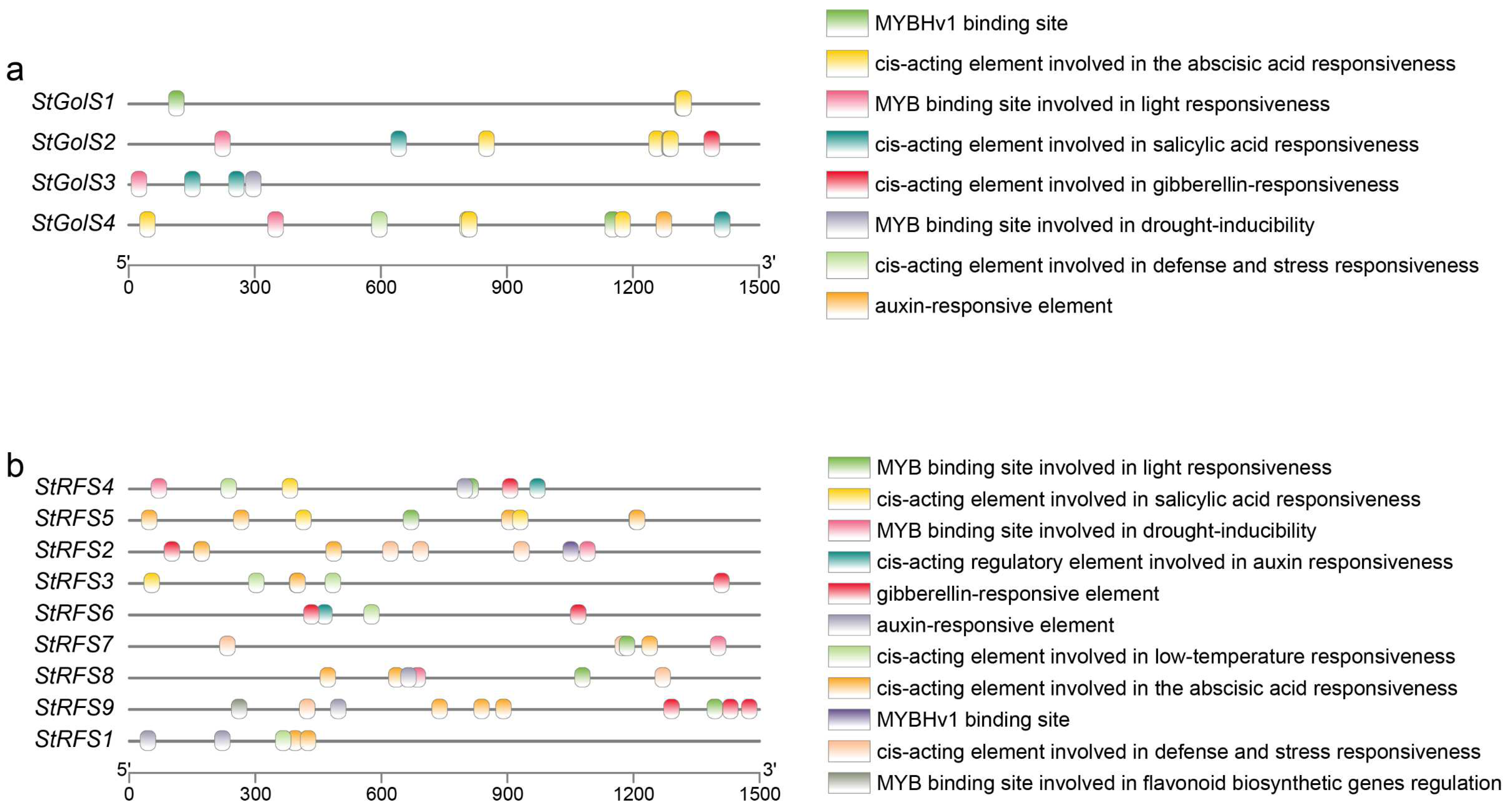
2.6. Cis-Element Analysis in Promoters of the StGolS and StRFS Genes
2.7. Syntenic Analysis of the GolS and RFS Genes
2.8. RNA-seq Analysis
2.9. qRT-PCR Analysis
3. Results
3.1. Identification of the GolS and RFS Gene Families in Potato
3.2. Chromosomal Distribution of the StGolS and StRFS Genes
3.3. Phylogenetic Analysis of the GolS and RFS Genes from Several Differnet Species
3.4. Gene Structure and Motif Composition of the StGolS and StRFS Genes
3.5. Cis-Element Analysis in Promoters of the StGolS and StRFS Genes
3.6. Interspecific Synteny of the GolS and RFS Genes from Several Different Species
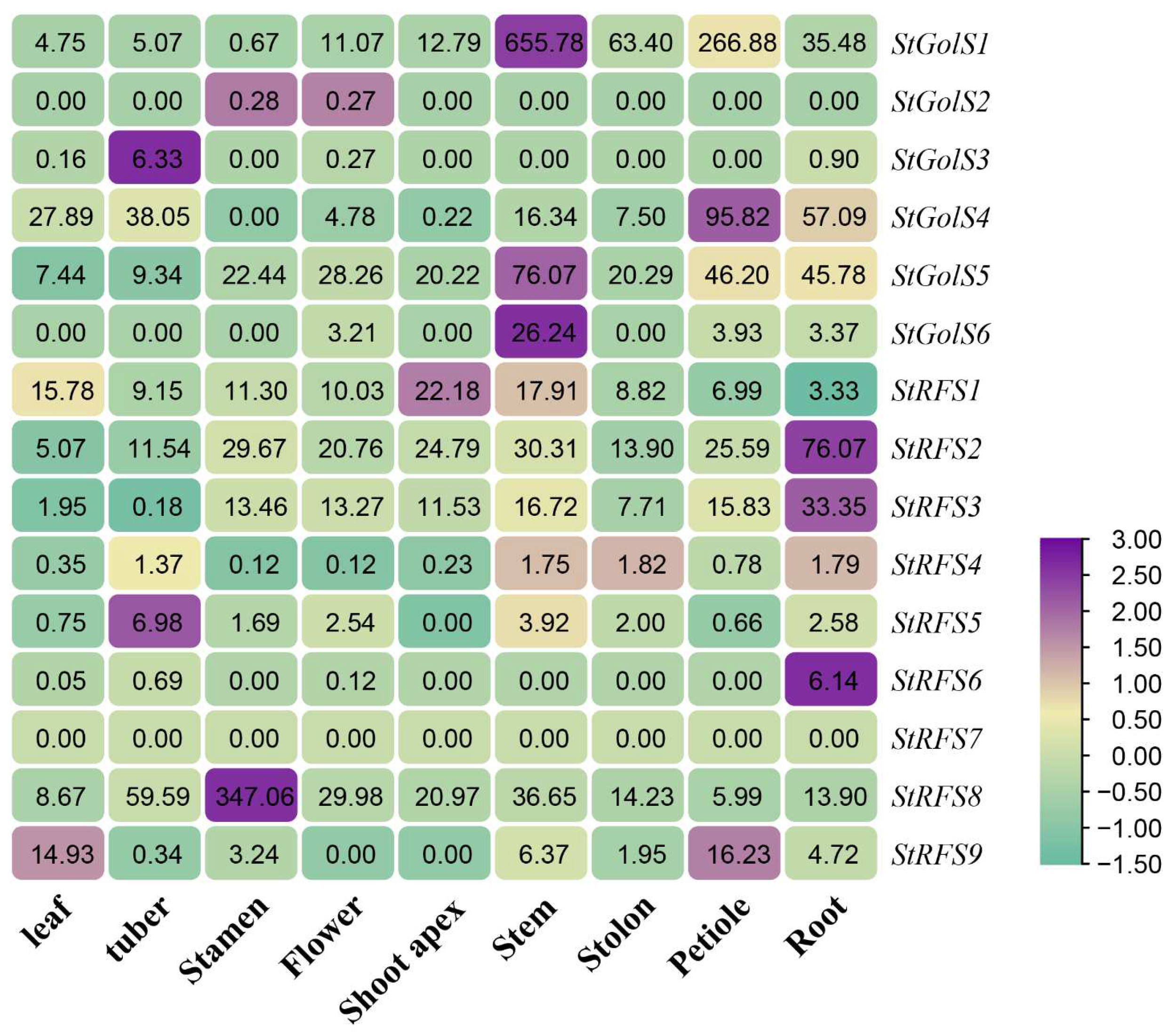
3.7. Expression Characteristics of the StGolS and StRFS Genes in Different Tissues of Potato
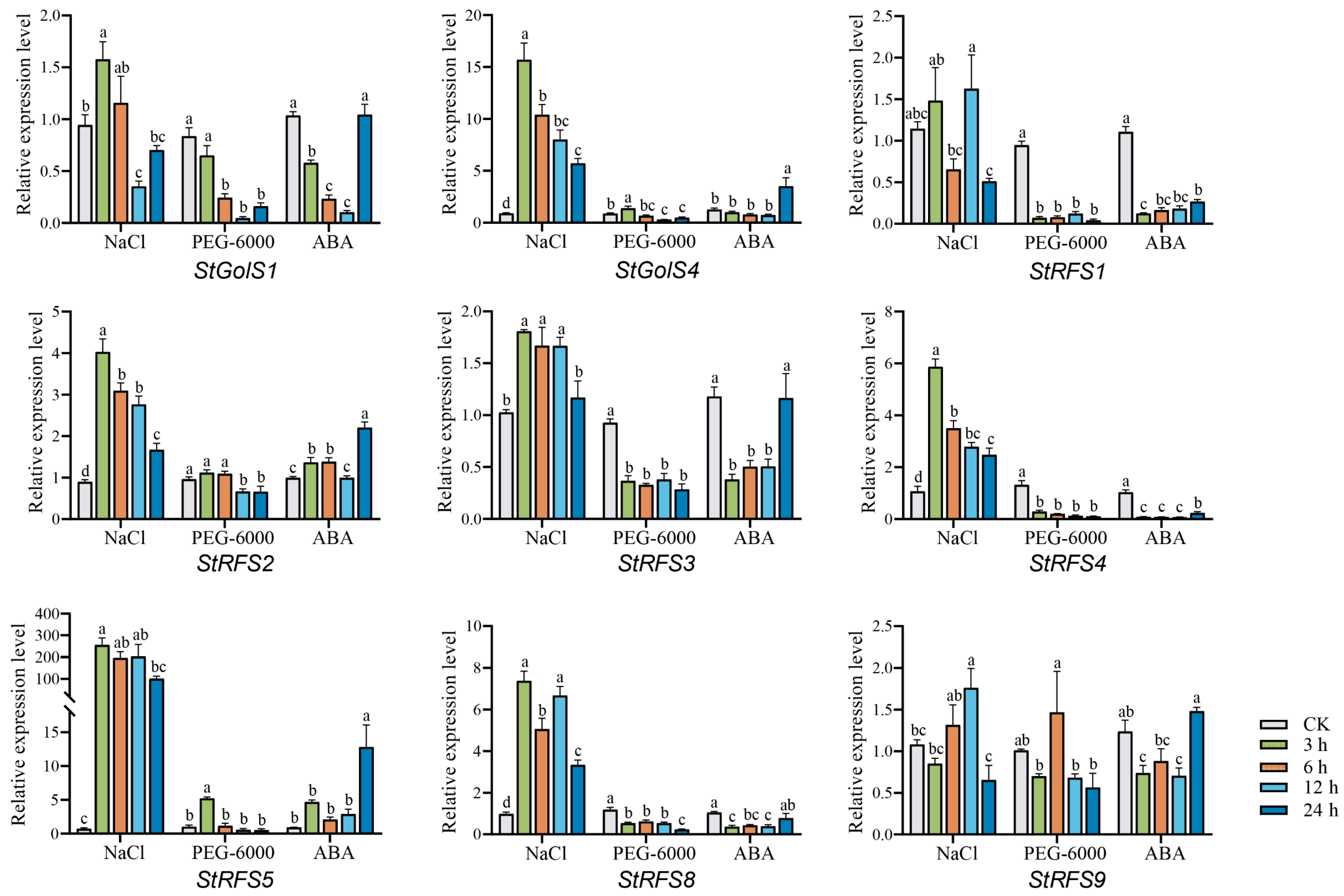
3.8. Expression Patterns of the StGolS and StRFS Genes in Potato under Abiotic Stress
4. Discussion
4.1. Analysis of the Number, Structure, and Evolution of the GolS and RFS Genes in Potato
4.2. StGolS and StRFS Genes Play a Key Role in Potato Growth and Development
4.3. Differential Expression of Potato StGolS and StRFS Genes under Salinity, Drought, and Abscisic Acid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.M.; Ghislain, M.; Simon, R.; Jansky, S.H.; Gavrilenko, T. Systematics, Diversity, Genetics, and Evolution of Wild and Cultivated Potatoes. Bot. Rev. 2014, 80, 283–383. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.-Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield Under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; Visser, R.G.F.; van der Linden, C.G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Gul, Z.; Tang, Z.-H.; Arif, M.; Ye, Z. An Insight into Abiotic Stress and Influx Tolerance Mechanisms in Plants to Cope in Saline Environments. Biology 2022, 11, 597. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Yu, J.; Wang, L.; Yu, X.; Ohtani, M.; Kusano, M.; Saito, K.; Demura, T.; Zhuge, Q. Responses of Populus trichocarpa galactinol synthase genes to abiotic stresses. J. Plant Res. 2014, 127, 347–358. [Google Scholar] [CrossRef]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Zhang, C.; Zhang, W.; Deng, N.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Raffinose positively regulates maize drought tolerance by reducing leaf transpiration. Plant J. 2023, 114, 55–67. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, X.; Sun, W.; Yu, X.; Demura, T.; Li, D.; Zhuge, Q. Galactinol synthase confers salt-stress tolerance by regulating the synthesis of galactinol and raffinose family oligosaccharides in poplar. Ind. Crop. Prod. 2021, 165, 113432. [Google Scholar] [CrossRef]
- Salvi, P.; Kamble, N.U.; Majee, M. Ectopic over-expression of ABA-responsive Chickpea galactinol synthase (CaGolS) gene results in improved tolerance to dehydration stress by modulating ROS scavenging. Environ. Exp. Bot. 2020, 171, 103957. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Ishizaki, T.; Valencia, M.; Ogawa, S.; Dedicova, B.; Ogata, T.; Yoshiwara, K.; Maruyama, K.; Kusano, M.; Saito, K.; et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 2017, 15, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Wang, S.; Li, H.; Xin, Q. Overexpression of a Common Wheat Gene GALACTINOL SYNTHASE3 Enhances Tolerance to Zinc in Arabidopsis and Rice Through the Modulation of Reactive Oxygen Species Production. Plant Mol. Biol. Report. 2016, 34, 794–806. [Google Scholar] [CrossRef]
- Zhao, T.-Y.; Thacker, R.; Corum III, J.W.; Snyder, J.C.; Meeley, R.B.; Obendorf, R.L.; Downie, B. Expression of the maize GALACTINOL SYNTHASE gene family: (I) Expression of two different genes during seed development and germination. Physiol. Plant. 2004, 121, 634–646. [Google Scholar] [CrossRef]
- Downie, B.; Gurusinghe, S.; Dahal, P.; Thacker, R.R.; Snyder, J.C.; Nonogaki, H.; Yim, K.; Fukanaga, K.; Alvarado, V.; Bradford, K.J. Expression of a GALACTINOL SYNTHASE Gene in Tomato Seeds Is Up-Regulated before Maturation Desiccation and Again after Imbibition whenever Radicle Protrusion Is Prevented. Plant Physiol. 2003, 131, 1347–1359. [Google Scholar] [CrossRef]
- Egert, A.; Keller, F.; Peters, S. Abiotic stress-induced accumulation of raffinose in Arabidopsis leaves is mediated by a single raffinose synthase (RS5, At5g40390). BMC Plant Biol. 2013, 13, 218. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Liu, Y.; Li, X.; Hao, G.; Han, Q.; Dirk, L.M.A.; Downie, A.B.; Ruan, Y.-L.; Wang, J.; et al. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. J. Biol. Chem. 2020, 295, 8064–8077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, A.; March, G.G.-d.; Sourdioux, M.; Peterbauer, T.; Richter, A. Induction of raffinose oligosaccharide biosynthesis by abscisic acid in somatic embryos of alfalfa (Medicago sativa L.). Plant Sci. 2005, 168, 1075–1082. [Google Scholar] [CrossRef]
- Guo, Q.; Li, X.; Niu, L.; Jameson, P.E.; Zhou, W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021, 186, 677–695. [Google Scholar] [CrossRef]
- Noronha, H.; Silva, A.; Silva, T.; Frusciante, S.; Diretto, G.; Gerós, H. VviRafS5 Is a Raffinose Synthase Involved in Cold Acclimation in Grapevine Woody Tissues. Front. Plant Sci. 2022, 12, 3453. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Crisovan, E.; Hamilton, J.P.; Kim, J.; Laimbeer, P.; Leisner, C.P.; Manrique-Carpintero, N.C.; Newton, L.; Pham, G.M.; Vaillancourt, B.; et al. Genome Reduction Uncovers a Large Dispensable Genome and Adaptive Role for Copy Number Variation in Asexually Propagated Solanum tuberosum. Plant Cell 2016, 28, 388–405. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, W.; Marand, A.P.; Zhu, B.; Buell, C.R.; Jiang, J. Cold stress induces enhanced chromatin accessibility and bivalent histone modifications H3K4me3 and H3K27me3 of active genes in potato. Genome Biol. 2019, 20, 123. [Google Scholar] [CrossRef]
- Jing, Q.; Hou, H.; Meng, X.; Chen, A.; Wang, L.; Zhu, H.; Zheng, S.; Lv, Z.; Zhu, X. Transcriptome analysis reveals the proline metabolic pathway and its potential regulation TF-hub genes in salt-stressed potato. Front. Plant Sci. 2022, 13, 1030138. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, N.; Zhu, X.; Yang, J.; Li, S.; Che, Y.; Liu, W.; Si, H. Identification and expression analysis of StGRAS gene family in potato (Solanum tuberosum L.). Comput. Biol. Chem. 2019, 80, 195–205. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Xu, J.; Duan, S.; Wang, Q.; Li, G.; Jin, L. Transcriptome Profiling Reveals Effects of Drought Stress on Gene Expression in Diploid Potato Genotype P3-198. Int. J. Mol. Sci. 2019, 20, 852. [Google Scholar] [CrossRef] [PubMed]
- Pham, G.M.; Hamilton, J.P.; Wood, J.C.; Burke, J.T.; Buell, C.R. Construction of a chromosome-scale long-read reference genome assembly for potato. GigaScience 2020, 9, giaa100. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.S.; Eddy, S.R.; Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 2010, 11, 431. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2015, 44, D279–D285. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.e.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Zhu, X.; Richael, C.; Chamberlain, P.; Busse, J.S.; Bussan, A.J.; Jiang, J.; Bethke, P.C. Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves processing quality by decreasing the frequency of sugar-end defects. PLoS ONE 2014, 9, e93381. [Google Scholar] [CrossRef]
- Zhu, X.; Gong, H.; He, Q.; Zeng, Z.; Busse, J.S.; Jin, W.; Bethke, P.C.; Jiang, J. Silencing of vacuolar invertase and asparagine synthetase genes and its impact on acrylamide formation of fried potato products. Plant Biotechnol. J. 2016, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Filiz, E.; Ozyigit, I.I.; Vatansever, R. Genome-wide identification of galactinol synthase (GolS) genes in Solanum lycopersicum and Brachypodium distachyon. Comput. Biol. Chem. 2015, 58, 149–157. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wang, Y.; Zhang, Y.; Dossa, K.; Li, D.; Zhou, R.; Wang, L.; Zhang, X. Genome-wide identification and expression analyses of genes involved in raffinose accumulation in sesame. Sci. Rep. 2018, 8, 4331. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Munir, F.; Gul, A.; Amir, R.; Paracha, R.Z. Genome-wide analysis, identification, evolution and genomic organization of dehydration responsive element-binding (DREB) gene family in Solanum tuberosum. PEERJ 2021, 9, e11647. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yuan, S.; He, Y.; Fan, J.; Zhou, Y.; Qiu, T.; Lin, X.; Yao, Y.; Liu, J.; Fu, S.; et al. Genome-Wide Identification and Expression Profiling Analysis of the Galactinol Synthase Gene Family in Cassava (Manihot esculenta Crantz). Agronomy 2018, 8, 250. [Google Scholar] [CrossRef]
- Yang, J.; Ling, C.; Liu, Y.; Zhang, H.; Hussain, Q.; Lyu, S.; Wang, S.; Liu, Y. Genome-Wide Expression Profiling Analysis of Kiwifruit GolS and RFS Genes and Identification of AcRFS4 Function in Raffinose Accumulation. Int. J. Mol. Sci. 2022, 23, 8836. [Google Scholar] [CrossRef]
- Cui, R.; Wang, X.; Malik, W.A.; Lu, X.; Chen, X.; Wang, D.; Wang, J.; Wang, S.; Chen, C.; Guo, L.; et al. Genome-wide identification and expression analysis of Raffinose synthetase family in cotton. BMC Bioinform. 2021, 22, 356. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Pluskota, W.E.; Stelmaszewska, J.; Szablińska, J. Dehydration induces expression of GALACTINOL SYNTHASE and RAFFINOSE SYNTHASE in seedlings of pea (Pisum sativum L.). J. Plant Physiol. 2014, 171, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, R.; Guo, S. Molecular Cloning and Characterization of GhGolS1, a Novel Gene Encoding Galactinol Synthase from Cotton (Gossypium hirsutum). Plant Mol. Biol. Report. 2012, 30, 699–709. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Zhao, J.; Kong, J.; Zhang, L.; Qi, S.; Chen, J.; Chen, Z.; Zeng, W.; Sun, W. Identification, Characterization and Expression Profiling of the RS Gene Family during the Withering Process of White Tea in the Tea Plant (Camellia sinensis) Reveal the Transcriptional Regulation of CsRS8. Int. J. Mol. Sci. 2023, 24, 202. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. The contribution of carbohydrates including raffinose family oligosaccharides and sugar alcohols to protection of plant cells from oxidative damage. Plant Signal. Behav. 2008, 3, 1016–1018. [Google Scholar] [CrossRef]
- Unda, F.; Canam, T.; Preston, L.; Mansfield, S.D. Isolation and characterization of galactinol synthases from hybrid poplar. J. Exp. Bot. 2011, 63, 2059–2069. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, M.; Liu, M.; Zhang, R.; Sun, W.; Qian, M.; Duan, H.; Chang, W.; Ma, J.; Qu, C.; et al. Genome-Wide Identification, Evolutionary and Expression Analyses of the GALACTINOL SYNTHASE Gene Family in Rapeseed and Tobacco. Int. J. Mol Sci. 2017, 18, 2768. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Górecki, R.J. Raffinose in seedlings of winter vetch (Vicia villosa Roth.) under osmotic stress and followed by recovery. Acta Physiol. Plant. 2011, 33, 725–733. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Y.; Zhang, M.; Li, T.; Dirk, L.M.A.; Downie, B.; Zhao, T. ZmGOLS2, a target of transcription factor ZmDREB2A, offers similar protection against abiotic stress as ZmDREB2A. Plant Mol. Biol. 2016, 90, 157–170. [Google Scholar] [CrossRef]
- McCaskill, A.; Turgeon, R. Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc. Natl. Acad. Sci. USA 2007, 104, 19619–19624. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Chromosome | Start (bp) | End (bp) | Exon Number | Protein Length (aa) | MolWt (kDa) | pI |
|---|---|---|---|---|---|---|---|---|
| StGolS1 | Soltu.DM.01G025230 | chr01 | 64,586,706 | 64,589,468 | 4 | 348 | 39.68 | 6.67 |
| StGolS2 | Soltu.DM.01G040570 | chr01 | 79,121,977 | 79,123,537 | 3 | 322 | 36.74 | 6.40 |
| StGolS3 | Soltu.DM.02G006360 | chr02 | 19,943,641 | 19,945,670 | 3 | 328 | 37.56 | 5.61 |
| StGolS4 | Soltu.DM.02G024820 | chr02 | 38,094,421 | 38,096,119 | 3 | 338 | 38.65 | 5.44 |
| StRFS1 | Soltu.DM.07G027830 | chr07 | 56,852,708 | 56,856,677 | 12 | 757 | 83.82 | 6.19 |
| StRFS2 | Soltu.DM.03G008410 | chr03 | 20,239,428 | 20,249,663 | 14 | 742 | 81.85 | 6.44 |
| StRFS3 | Soltu.DM.03G025220 | chr03 | 50,422,790 | 50,427,128 | 14 | 752 | 83.26 | 5.59 |
| StRFS4 | Soltu.DM.01G025080 | chr01 | 64,419,085 | 64,422,201 | 4 | 874 | 97.37 | 5.67 |
| StRFS5 | Soltu.DM.02G033230 | chr02 | 44,798,199 | 44,801,571 | 6 | 780 | 86.87 | 5.97 |
| StRFS6 | Soltu.DM.03G026160 | chr03 | 51,135,322 | 51,141,812 | 6 | 843 | 94.30 | 5.97 |
| StRFS7 | Soltu.DM.03G026620 | chr03 | 51,449,668 | 51,451,882 | 2 | 123 | 13.90 | 4.12 |
| StRFS8 | Soltu.DM.07G003310 | chr07 | 3,899,124 | 3,903,987 | 5 | 865 | 94.91 | 5.24 |
| StRFS9 | Soltu.DM.07G003360 | chr07 | 3,954,988 | 3,955,317 | 1 | 110 | 12.51 | 6.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Q.; Chen, A.; Lv, Z.; Dong, Z.; Wang, L.; Meng, X.; Feng, Y.; Wan, Y.; Su, C.; Cui, Y.; et al. Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses. Genes 2023, 14, 1344. https://doi.org/10.3390/genes14071344
Jing Q, Chen A, Lv Z, Dong Z, Wang L, Meng X, Feng Y, Wan Y, Su C, Cui Y, et al. Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses. Genes. 2023; 14(7):1344. https://doi.org/10.3390/genes14071344
Chicago/Turabian StyleJing, Quankai, Airu Chen, Zhaoyan Lv, Zhihao Dong, Lixia Wang, Xiaoke Meng, Yue Feng, Yu Wan, Chengyun Su, Yanjie Cui, and et al. 2023. "Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses" Genes 14, no. 7: 1344. https://doi.org/10.3390/genes14071344
APA StyleJing, Q., Chen, A., Lv, Z., Dong, Z., Wang, L., Meng, X., Feng, Y., Wan, Y., Su, C., Cui, Y., Xu, W., Hou, H., & Zhu, X. (2023). Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses. Genes, 14(7), 1344. https://doi.org/10.3390/genes14071344






