Identification and Expression Analysis of the WOX Transcription Factor Family in Foxtail Millet (Setaria italica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Phylogenetic Analysis of WOXs in Foxtail Millet
2.2. Protein Properties and Sequence Analyses of SiWOX Genes
2.3. Sequence Alignment, Gene Structure, and Conserved Motif Analysis of SiWOX Gene Family Members
2.4. Analysis of Promoter Cis-Acting Elements of SiWOX Gene Family Members
2.5. Chromosomal Localization and Collinearity Analysis of SiWOX Gene Family Members
2.6. Analysis of Expression Patterns of SiWOX Gene Family Members
2.7. Response of SiWOX to Plant Hormones
3. Results
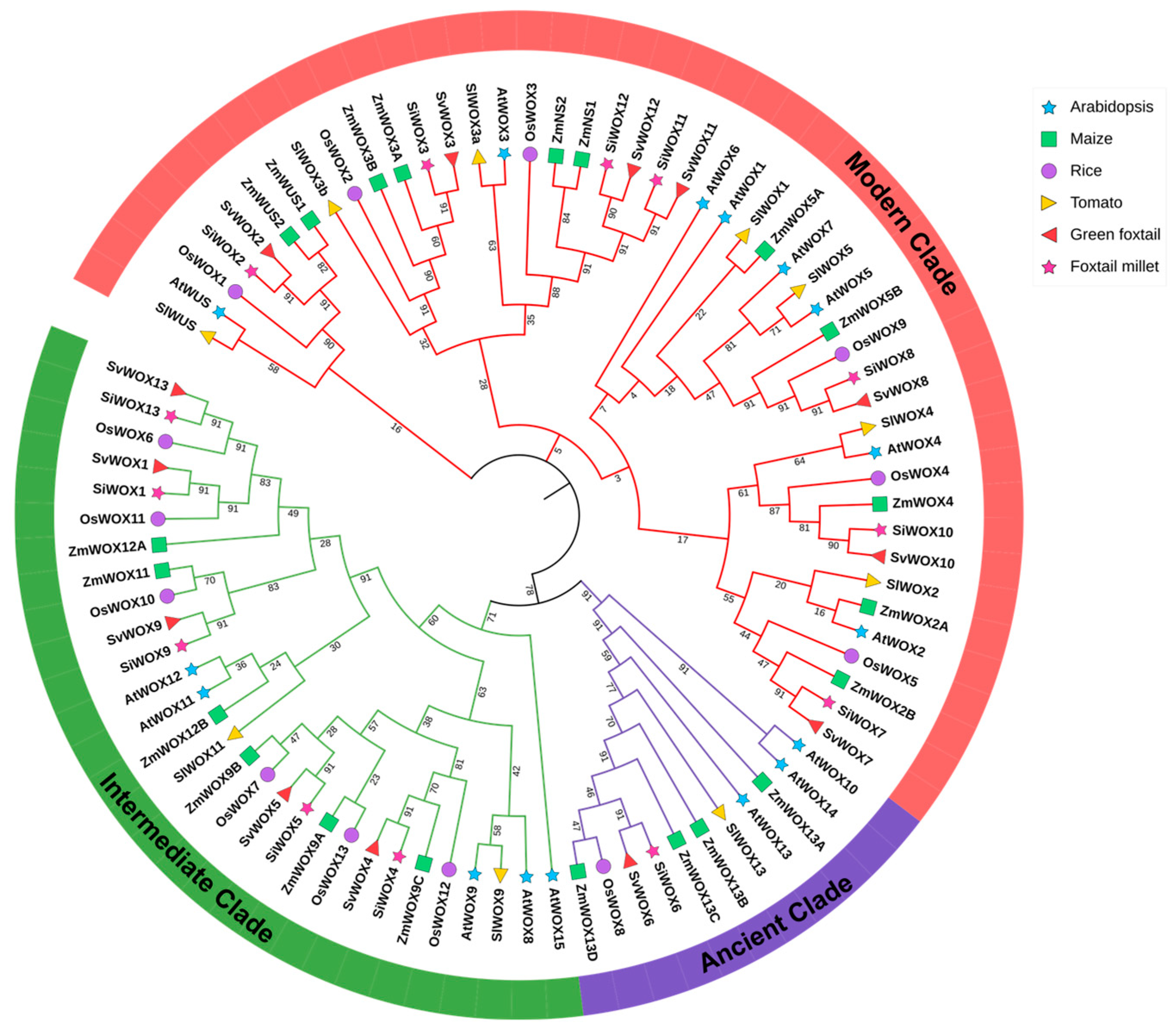
3.1. Identification and Phylogenetic Analysis of SiWOX Gene Family
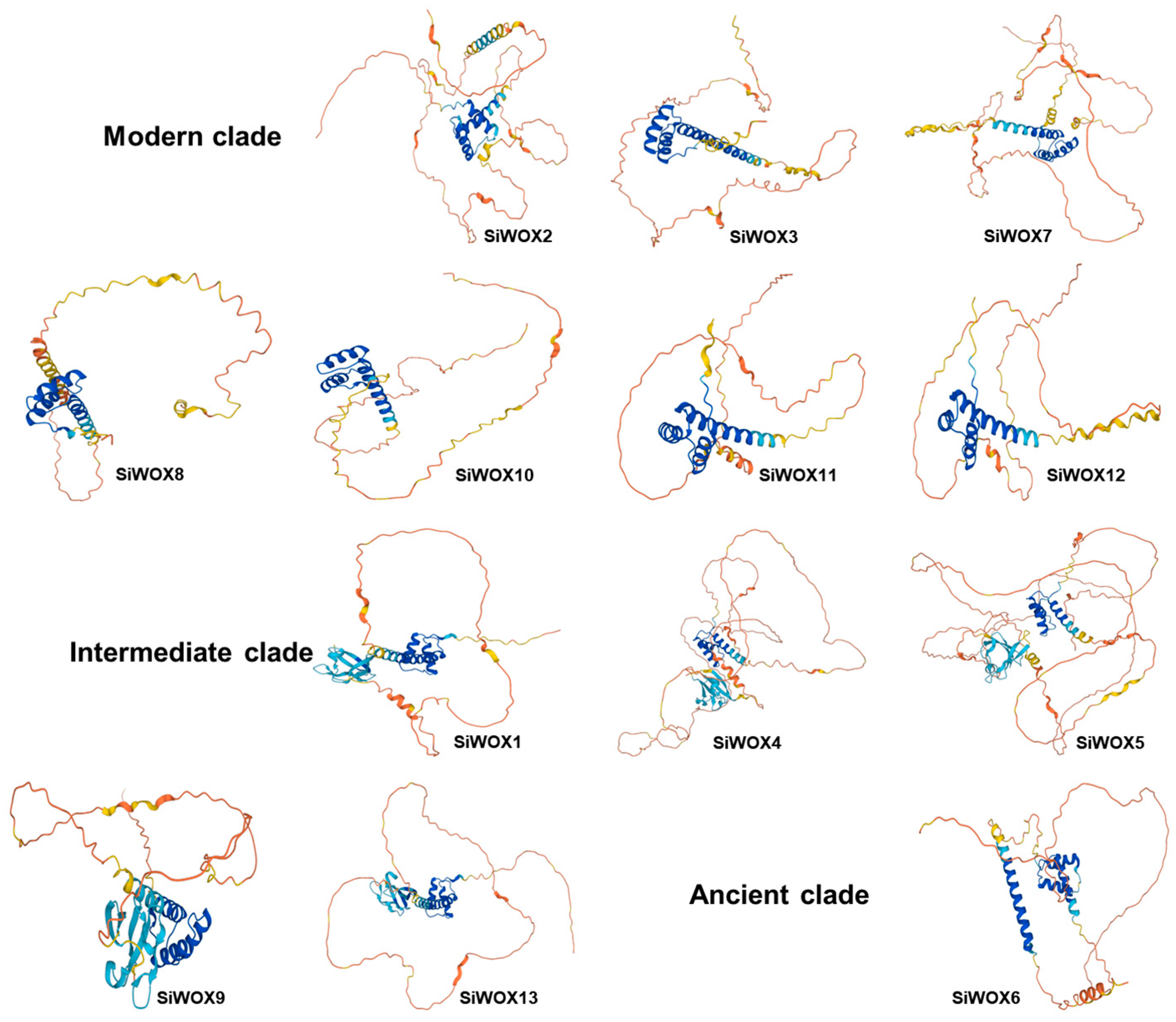
3.2. Structure Characterization of the WOX Proteins of Foxtail Millet
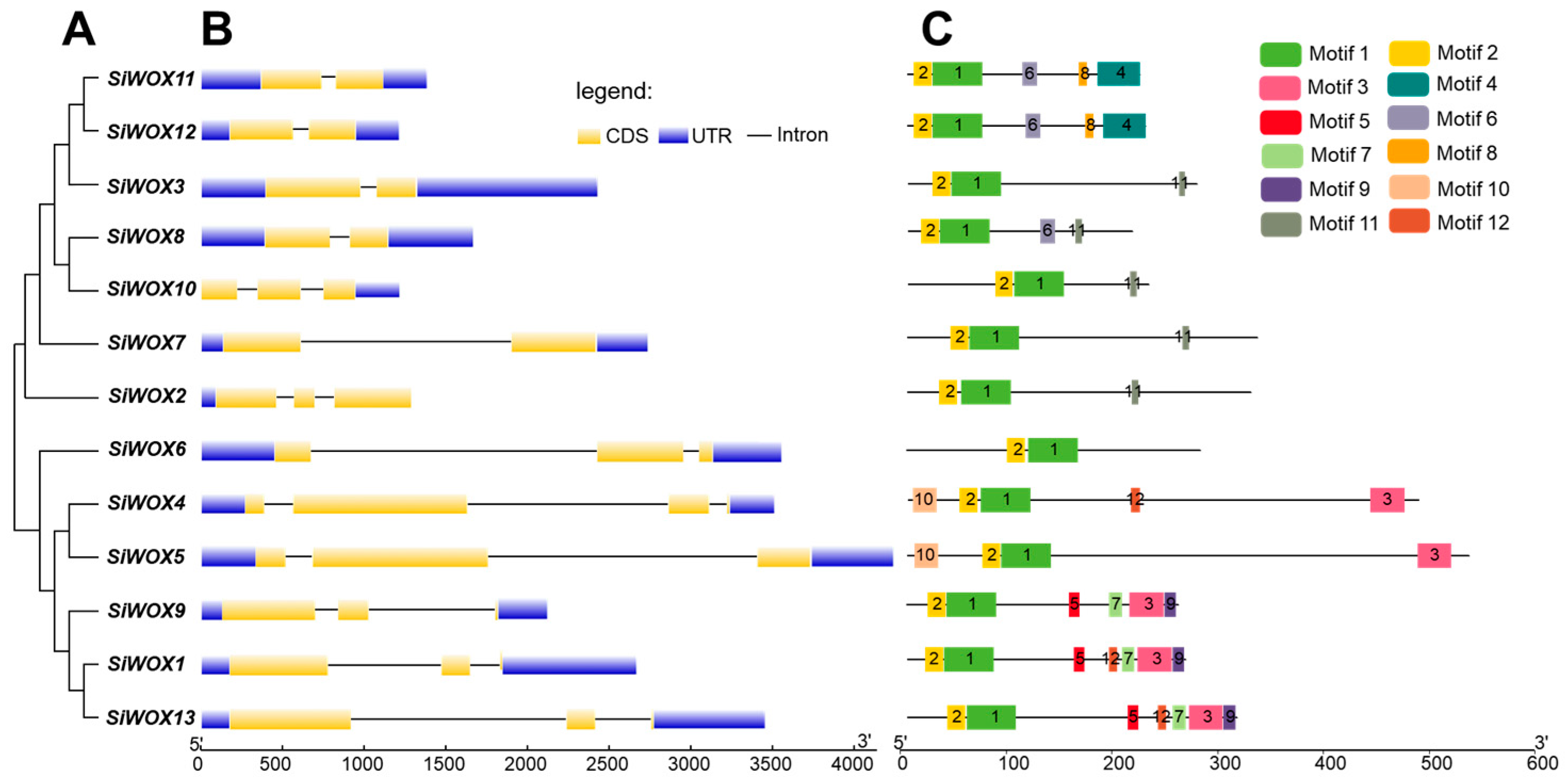
3.3. Sequence Alignment, Gene Structure, and Conserved Motif Analysis of SiWOX Gene Family Members
3.4. Promoter Cis-Acting Element Analysis of SiWOX Gene Family Members
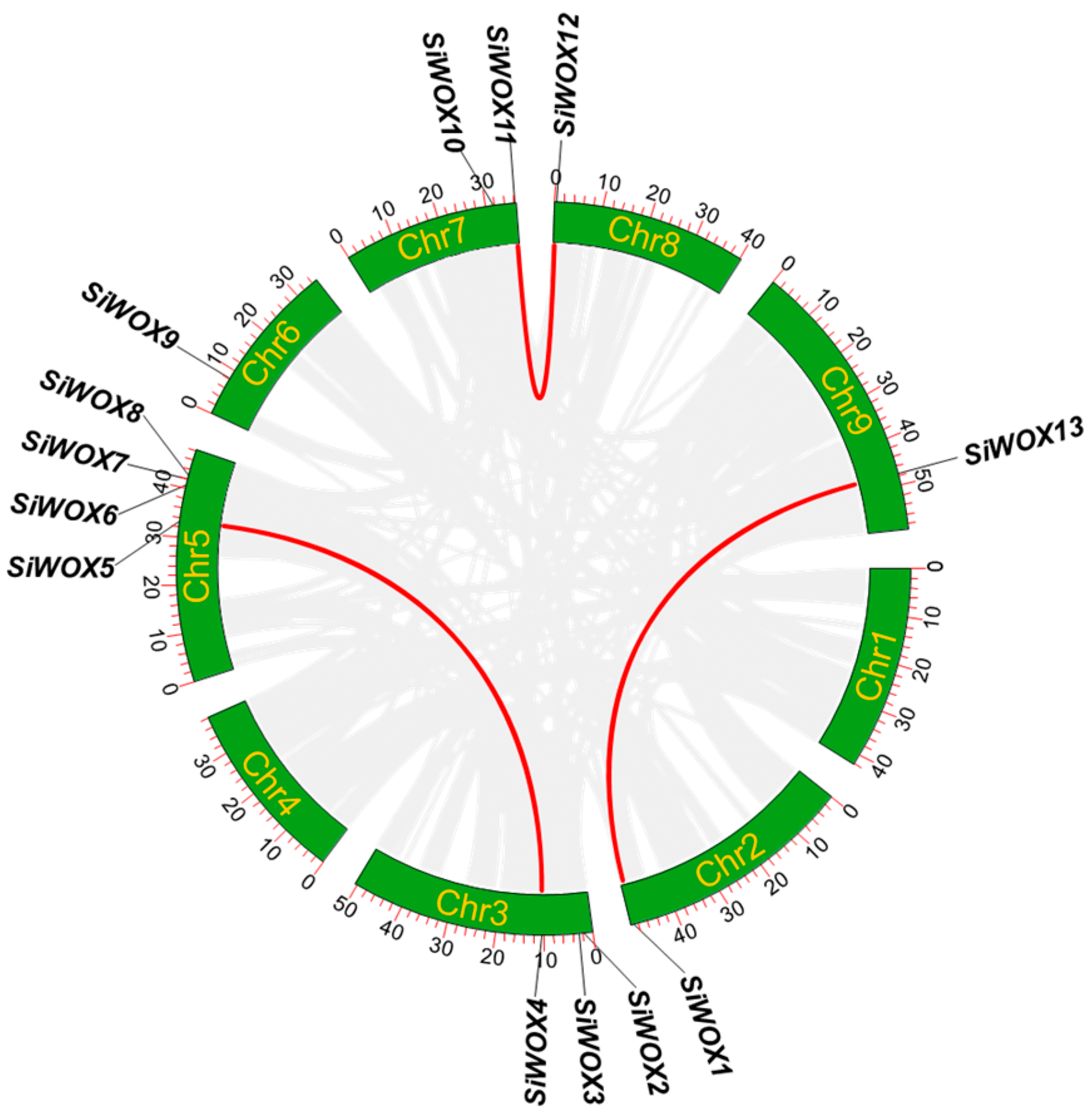
3.5. Chromosomal Localization and Collinearity Analysis of SiWOX Gene Family Members
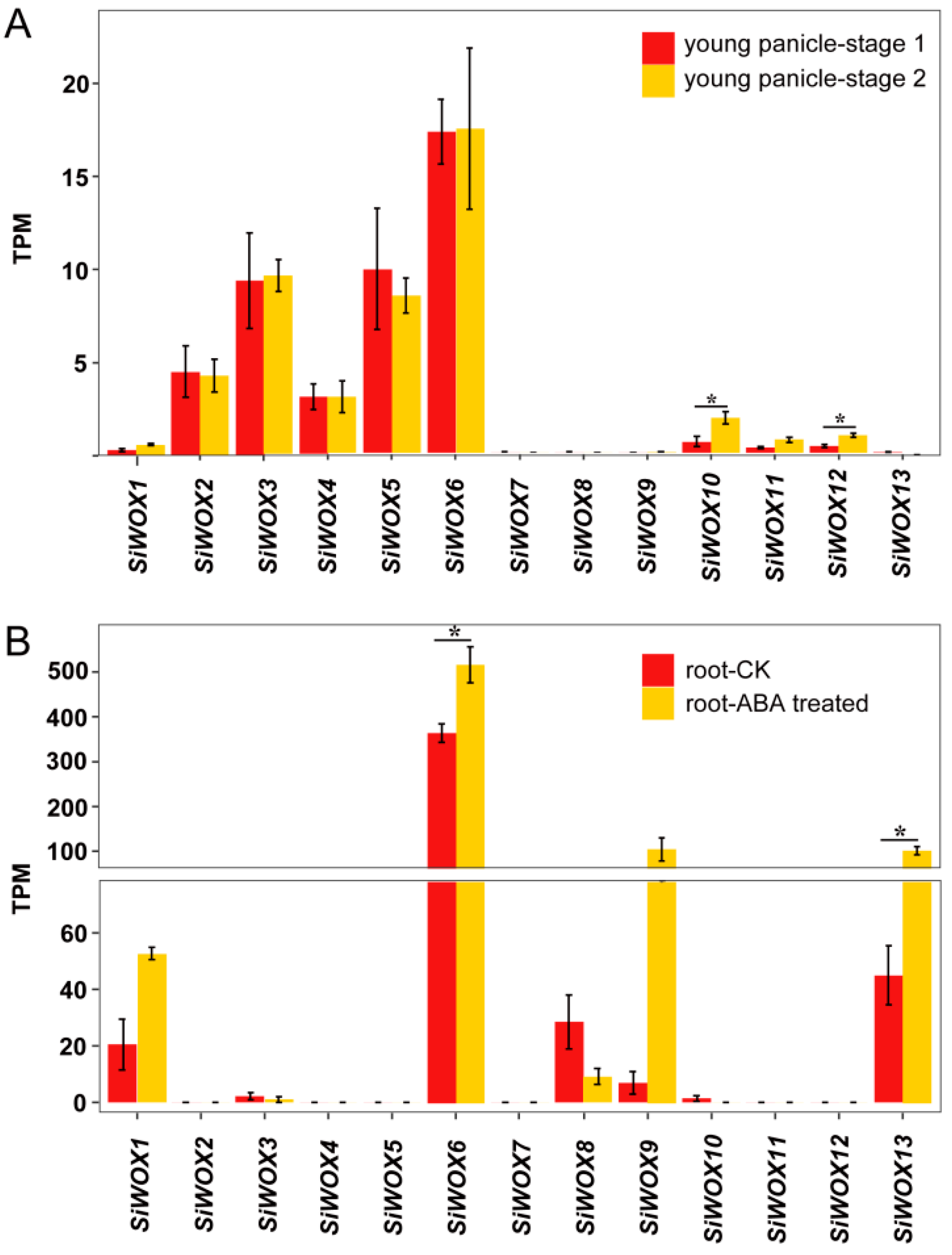
3.6. Analysis of the Expression Patterns of SiWOX Genes
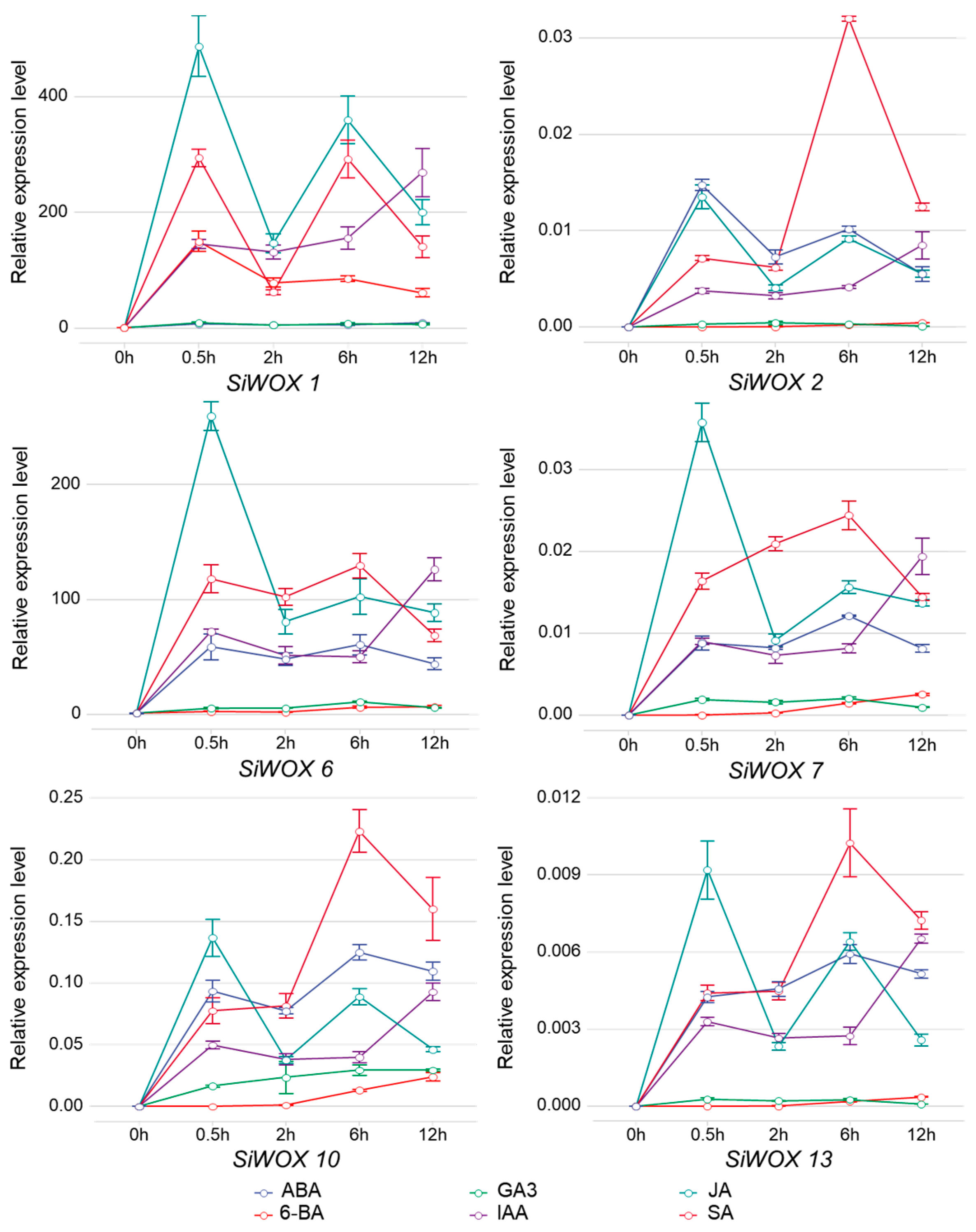
3.7. SiWOX Gene Response to Plant Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Liu, X.; Engstrom, E.M.; Nimchuk, Z.L.; Pruneda-Paz, J.L.; Tarr, P.T.; Sun, Y.; Zhang, Y.; Oh, E.M.; Jacobsen, S.E. Meyerowitz, Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 2015, 517, 377–380. [Google Scholar] [CrossRef]
- Li, X.; Hamyat, M.; Liu, C.; Ahmad, S.; Gao, X.; Guo, C.; Li, R.; Wang, Y.; Abdullah, M.; Gao, X. Identification and Characterization of the WOX Family Genes in Five Solanaceae Species Reveal Their Conserved Roles in Peptide Signaling. Genes 2018, 9, 260. [Google Scholar] [CrossRef]
- Van der Graaff, E.; Laux, T.; Rensing, S.A. Rensing, The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Affolter, M.; Bürglin, T. Homeodomain proteins. Annu. Rev. Biochem. 1994, 63, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Richardt, S.; Lang, D.; Reski, R.; Frank, W.; Rensing, S.A. Rensing, PlanTAPDB, a Phylogeny-Based Resource of Plant Transcription-Associated Proteins. Plant Physiol. 2007, 143, 1452–1466. [Google Scholar] [CrossRef]
- Palovaara, J.; Hakman, I. Conifer WOX-related homeodomain transcription factors, developmental consideration and expression dynamic of WOX2 during Picea abies somatic embryogenesis. Plant Mol. Biol. 2008, 66, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Ding, Z.; Wang, Q.; Zhang, D.; Xu, J. Origins and Evolution of WUSCHEL-Related Homeobox Protein Family in Plant Kingdom. Sci. World J. 2014, 2014, 534140. [Google Scholar] [CrossRef] [PubMed]
- Haecker, A.; Groß-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arab. Thaliana Dev. 2004, 131, 657–668. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis WUSCHEL Is a Bifunctional Transcription Factor That Acts as a Repressor in Stem Cell Regulation and as an Activator in Floral Patterning. Plant Cell 2009, 21, 3493–3505. [Google Scholar] [CrossRef]
- Paponov, I.A.; Teale, W.; Lang, D.; Paponov, M.; Reski, R.; Rensing, S.A.; Palme, K. The evolution of nuclear auxin signalling. BMC Evol. Biol. 2009, 9, 126. [Google Scholar] [CrossRef]
- Laux, T.; Mayer, K.F.X.; Berger, J.; Jürgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [CrossRef]
- Mayer, K.F.X.; Schoof, H.; Haecker, A.; Lenhard, M.; Jürgens, G.; Laux, T. Role of WUSCHEL in Regulating Stem Cell Fate in the Arabidopsis Shoot Meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gross-Hardt, R.; Lenhard, M.; Laux, T. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 2002, 16, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, F.; Sarkar, A.K.; Tucker, E.J.; Laux, T. WUSCHEL regulates cell differentiation during anther development. Dev. Biol. 2007, 302, 154–159. [Google Scholar] [CrossRef]
- Nardmann, J.; Werr, W. The Shoot Stem Cell Niche in Angiosperms: Expression Patterns of WUS Orthologues in Rice and Maize Imply Major Modifications in the Course of Mono- and Dicot Evolution. Mol. Biol. Evol. 2006, 23, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Xia, Y.; Li, Z.; Jing, D.; Du, J.; Niu, N.; Ma, S.; Wang, J.; Song, Y.; et al. Identification of the WUSCHEL-Related Homeobox (WOX) Gene Family, and Interaction and Functional Analysis of TaWOX9 and TaWUS in Wheat. Int. J. Mol. Sci. 2020, 21, 1581. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E. Cell signalling at the shoot meristem. Nat. Rev. Mol. Cell Biol. 2001, 2, 276–284. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jürgens, G.; Laux, T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Thomas, L.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 2020, 117, 22561–22571. [Google Scholar] [CrossRef]
- Daum, G.; Medzihradszky, A.; Suzaki, T.; Lohmann, J.U. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14619–14624. [Google Scholar] [CrossRef]
- Lopes, F.L.; Galvan-Ampudia, C.; Landrein, B. WUSCHEL in the shoot apical meristem: Old player, new tricks. J. Exp. Bot. 2021, 72, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Leibfried, A.; To, J.P.; Busch, W.; Stehling, S.; Kehle, A.; Demar, M.; Kieber, J.J.; Lohmann, J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005, 438, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Berckmans, B.; Kirschner, G.; Gerlitz, N.; Stadler, R.; Simon, R. CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiol. 2019, 182, 1776–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tucker, E.; Hermann, M.; Laux, T. A Molecular Framework for the Embryonic Initiation of Shoot Meristem Stem Cells. Dev. Cell 2017, 40, 264–277.e4. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Attuluri, V.P.S.; Robert, H.S. Transcriptional control of Arabidopsis seed development. Planta 2022, 255, 90. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Okada, K. A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev. 2001, 15, 3355–3364. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Deveaux, Y.; Toffano-Nioche, C.; Claisse, G.; Thareau, V.; Morin, H.; Laufs, P.; Moreau, H.; Kreis, M.; Lecharny, A. Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 2008, 8, 291. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Ito, T.; Tanaka, H.; Favero, D.S.; Kawamura, A.; Sakamoto, S.; Wakazaki, M.; Tameshige, T.; Fujii, H.; et al. Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection. Plant Physiol. 2022, 188, 425–441. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Fujimoto, H.; et al. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Shao, G.; Xiong, J.; Jiao, Y.; Wang, J.; Liu, G.; Meng, X.; Liang, Y.; Xiong, G.; Wang, Y.; et al. MONOCULM 3, an Ortholog of WUSCHEL in Rice, Is Required for Tiller Bud Formation. J. Genet. Genom. 2015, 42, 71–78. [Google Scholar] [CrossRef]
- Takuya, S.; Makoto, S.; Motoyuki, A.; Masahiro, M.; Yasuo, N.; Hiro-Yuki, H. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 2004, 131, 5649–5657. [Google Scholar]
- Chen, Z.; Cortes, L.; Gallavotti, A. Genetic dissection of cis-regulatory control of ZmWUSCHEL1 expression by type-B RESPONSE REGULATORS. Plant Physiol. 2023, 194, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Hu, Y.; Zhao, Y.; Liu, H.; Zhou, D.X. A WUSCHEL-LIKE HOMEOBOX gene represses a YABBY gene expression required for rice leaf development. Plant Physiol. 2007, 144, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y.; Lu, Z.; Yang, L.; Gao, R.; Lu, J.; Li, J.; Xiong, G. Glabrous Rice 1, encoding a homeodomain protein, regulates trichome development in rice. Rice 2012, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, K.; Wang, Y.; Peng, Y.; Hu, F.; Wen, L.; Han, B.; Qian, Q.; Teng, S. A WUSCHEL-like homeobox gene, OsWOX3B responses to NUDA/GL-1 locus in rice. Rice 2012, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, M.J.; Freeling, M. The narrow sheath leaf domain deletion: A genetic tool used to reveal developmental homologies among modified maize organs. Plant J. 1998, 13, 547–561. [Google Scholar]
- Chen, Y. Study on Transcription Factor SlWOX13 Regulating Tomato Growth and Development through Gibberellin Pathway. Master’s Thesis, Chongqing University, Chongqing, China, 2022. [Google Scholar]
- Jiang, G.; Li, Z.; Ding, X.; Zhou, Y.; Lai, H.; Jiang, Y.; Duan, X. WUSCHEL-related homeobox transcription factor SlWOX13 regulates tomato fruit ripening. Plant Physiol. 2023, 194, 2322–2337. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Quan, Z.; Cheng, S.; Xu, X.; Pan, S.; Xie, M.; Zeng, P.; Yue, Z.; Wang, W.; et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549–554. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Li, X.; Shen, H.; Gao, J.; Hou, S.; Zhang, B.; Mayes, S.; Bennett, M.; Ma, J.; et al. A mini foxtail millet with an Arabidopsis-like life cycle as a C(4) model system. Nat. Plants 2020, 6, 1167–1178. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, R.; Wang, Y.; Zhi, H.; Tang, S.; Jia, G.; Diao, X. Genome-Wide Characterization and Haplotypic Variation Analysis of the YUC Gene Family in Foxtail Millet (Setaria italica). Int. J. Mol. Sci. 2023, 24, 15637. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Chen, S.; Zhang, H.; Li, L. Genome-Wide Identification and Characterization of CLAVATA3/EMBRYO SURROUNDING REGION (CLE) Gene Family in Foxtail Millet (Setaria italica, L.). Genes 2023, 14, 2046. [Google Scholar] [CrossRef]
- Chen, H.; Ge, W. Identification, Molecular Characteristics, and Evolution of GRF Gene Family in Foxtail Millet (Setaria italica, L.). Front. Genet. 2022, 12, 727674. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, J.; Liu, J.; Yin, J.; Zhang, D. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 2010, 52, 1016–1026. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Ma, D.; Liu, C. Identification and Evolutionary Analysis of Cotton (Gossypium hirsutum) WOX Family Genes and Their Potential Function in Somatic Embryogenesis. Int. J. Mol. Sci. 2023, 24, 11077. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Cao, Y.; Wei, B.; Gu, L. A Comprehensive Identification and Expression Analysis of the WUSCHEL Homeobox-Containing Protein Family Reveals Their Special Role in Development and Abiotic Stress Response in Zea mays, L. Int. J. Mol. Sci. 2023, 25, 441. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; Wang, Z.; Li, S. Molecular characterization and transcriptional analysis of VrWOX genes in mungbean [Vigna radiate (L.) Wilczek]. Sheng Wu Gong. Cheng Xue Bao 2023, 39, 566–585. [Google Scholar]
- Riccucci, E.; Vanni, C.; Vangelisti, A.; Fambrini, M.; Giordani, T.; Cavallini, A.; Mascagni, F.; Pugliesi, C. Genome-Wide Analysis of WOX Multigene Family in Sunflower (Helianthus annuus, L.). Int. J. Mol. Sci. 2023, 24, 3352. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, L.; Liu, J.; Sun, Y.; Li, B.; Wang, L.; Ren, Z.; Chen, C. Pan-genome Analysis of WOX Gene Family and Function Exploration of CsWOX9 in Cucumber. Int. J. Mol. Sci. 2023, 24, 17568. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; He, Y.; Liu, B.; Xu, Y.; Zhao, G. Genome-Wide Identification and Characterization Analysis of WUSCHEL-Related Homeobox Family in Melon (Cucumis melo, L.). Int. J. Mol. Sci. 2023, 24, 12326. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Gao, T.; Wang, C.; Wang, X.; Chen, C.; Tian, M.; Yang, W. In silico genome wide identification and expression analysis of the WUSCHEL-related homeobox gene family in Medicago sativa. Genom. Inf. 2022, 20, e19. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, C.; He, Q.; Zhang, J.; Liang, H.; Lu, Z.; Xie, K.; Tang, S.; Zhou, Y.; Liu, B.; et al. A complete reference genome assembly for foxtail millet and Setaria-db, a comprehensive database for Setaria. Mol. Plant 2024, 17, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, H.W.; Li, C.H.; Pao, W.K. Cytological and Genetical Studies of the Interspecific Cross of the Cultivated Foxtail Millet, Setaria Italica (L.) Beauv., and the Green Foxtail Millet, S. Viridis, L.1. Agron. J. 1945, 37, 32–54. [Google Scholar] [CrossRef]
- Soyk, S.; Lemmon, Z.H.; Sedlazeck, F.J.; Jiménez-Gómez, J.M.; Alonge, M.; Hutton, S.F.; Van Eck, J.; Schatz, M.C.; Lippman, Z.B. Duplication of a domestication locus neutralized a cryptic variant that caused a breeding barrier in tomato. Nat. Plants 2019, 5, 471–479. [Google Scholar] [CrossRef]
- Alonge, M.; Wang, X.; Benoit, M.; Soyk, S.; Pereira, L.; Zhang, L.; Suresh, H.; Ramakrishnan, S.; Maumus, F.; Ciren, D.; et al. Major Impacts of Widespread Structural Variation on Gene Expression and Crop Improvement in Tomato. Cell 2020, 182, 145–161.e23. [Google Scholar] [CrossRef]
- Lye, Z.N.; Purugganan, M.D. Copy Number Variation in Domestication. Trends Plant Sci. 2019, 24, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, X.; Paterson, A.H. Paterson, Different patterns of gene structure divergence following gene duplication in Arabidopsis. BMC Genom. 2013, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- Doust, A.N.; Devos, K.M.; Gadberry, M.D.; Gale, M.D.; Kellogg, E.A. The genetic basis for inflorescence variation between foxtail and green millet (poaceae). Genetics 2005, 169, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, B.; Wang, X.; Li, H.; Han, Y. Foxtail millet: Nutritional and eating quality, and prospects for genetic improvement. Front. Agric. Sci. Eng. 2015, 2, 124–133. [Google Scholar] [CrossRef]
- Hou, D.; Chen, J.; Ren, X.; Wang, C.; Diao, X.; Hu, X.; Zhang, Y.; Shen, Q. A whole foxtail millet diet reduces blood pressure in subjects with mild hypertension. J. Cereal Sci. 2018, 84, 13–19. [Google Scholar] [CrossRef]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients 2018, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Cabedo, M.; Xie, Y.; Wenkel, S. Wenkel, Homeodomain leucine-zipper proteins and their role in synchronizing growth and development with the environment. J. Integr. Plant Biol. 2014, 56, 518–526. [Google Scholar] [CrossRef]
- Costanzo, E.; Trehin, C.; Vandenbussche, M. The role of WOX genes in flower development. Ann. Bot. 2014, 114, 1545–1553. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, M.M.; Ran, F.; Guo, P.C.; Ke, Y.Z.; Wu, Y.W.; Wen, J.; Li, P.F.; Li, J.N.; Du, H. Global Analysis of WOX Transcription Factor Gene Family in Brassica napus Reveals Their Stress- and Hormone-Responsive Patterns. Int. J. Mol. Sci. 2018, 19, 3470. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Liu, C.; Li, W.; Zhang, Z.L.; Gao, X.M.; Zhou, H.; Guo, Y.F. Genome-wide identification, phylogenetic analysis and expression profiling of the WOX family genes in Solanum lycopersicum. Hereditas 2016, 38, 444–460. [Google Scholar] [PubMed]
- Chen, Z.; Li, W.; Gaines, C.; Buck, A.; Galli, M.; Gallavotti, A. Structural variation at the maize WUSCHEL1 locus alters stem cell organization in inflorescences. Nat. Commun. 2021, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, P.; Swentowsky, K.W.; Jackson, D. Cultivating potential: Harnessing plant stem cells for agricultural crop improvement. Mol. Plant 2024, 17, 50–74. [Google Scholar] [CrossRef] [PubMed]
- Doust, A.N.; Kellogg, E.A. Inflorescence diversification in the panicoid “bristle grass” clade (Paniceae, Poaceae): Evidence from molecular phylogenies and developmental morphology. Am. J. Bot. 2002, 89, 1203–1222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhou, D.X.; Zhao, Y. WUSCHEL-related homeobox gene WOX11 increases rice drought resistance by controlling root hair formation and root system development. Plant Signal. Behav. 2016, 11, e1130198. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.T.; Tameshige, T.; Takahara, M.; Mitsuda, N.; Okada, K. The functional balance between the WUSCHEL-RELATED HOMEOBOX1 gene and the phytohormone auxin is a key factor for cell proliferation in Arabidopsis seedlings. Plant Biotechnol. 2018, 35, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Minh-Thu, P.T.; Kim, J.S.; Chae, S.; Jun, K.M.; Lee, G.S.; Kim, D.E.; Cheong, J.J.; Song, S.I.; Nahm, B.H.; Kim, Y.K. A WUSCHEL Homeobox Transcription Factor, OsWOX13, Enhances Drought Tolerance and Triggers Early Flowering in Rice. Mol. Cells 2018, 41, 781–798. [Google Scholar] [PubMed]
- Rodriguez-Leal, D.; Xu, C.; Kwon, C.T.; Soyars, C.; Demesa-Arevalo, E.; Man, J.; Liu, L.; Lemmon, Z.H.; Jones, D.S.; Van Eck, J.; et al. Lippman, Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat. Genet. 2019, 51, 786–792. [Google Scholar] [CrossRef]
- Geng, L.; Tan, M.; Deng, Q.; Wang, Y.; Zhang, T.; Hu, X.; Ye, M.; Lian, X.; Zhou, D.X.; Zhao, Y. Transcription factors WOX11 and LBD16 function with histone demethylase JMJ706 to control crown root development in rice. Plant Cell 2024, koad318. [Google Scholar] [CrossRef]


| Gene Name | Gene ID | Number of Amino Acids | Molecular Weight | Isoelectric Point | Instability Index | Aliphatic Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| SiWOX1 | Si2g43190 | 263 | 27.50 | 7.84 | 62.06 | 63.57 | −0.264 | Nucleus |
| SiWOX2 | Si3g03660 | 325 | 33.49 | 8.61 | 60.8 | 62.06 | −0.306 | Nucleus |
| SiWOX3 | Si3g05200 | 273 | 28.59 | 7.66 | 66.92 | 69.3 | −0.137 | Nucleus |
| SiWOX4 | Si3g14560 | 483 | 50.91 | 6.92 | 67.92 | 82.11 | −0.19 | Nucleus |
| SiWOX5 | Si5g27130 | 531 | 54.80 | 7.16 | 57.26 | 70.96 | −0.2 | Nucleus |
| SiWOX6 | Si5g36340 | 278 | 30.91 | 6 | 50.26 | 59.42 | −0.733 | Nucleus |
| SiWOX7 | Si5g37740 | 331 | 35.19 | 8.93 | 78.12 | 62.51 | −0.419 | Nucleus |
| SiWOX8 | Si5g38630 | 212 | 24.07 | 8.36 | 52.12 | 68.07 | −0.703 | Nucleus |
| SiWOX9 | Si6g09430 | 257 | 27.89 | 7.22 | 64.8 | 65.41 | −0.352 | Nucleus |
| SiWOX10 | Si7g26980 | 228 | 25.39 | 9.26 | 66.32 | 74.87 | −0.534 | Nucleus |
| SiWOX11 | Si7g33510 | 220 | 24.01 | 8.83 | 68.04 | 52.95 | −0.673 | Nucleus |
| SiWOX12 | Si8g01120 | 225 | 24.55 | 8.98 | 71.07 | 51.33 | −0.732 | Nucleus |
| SiWOX13 | Si9g41130 | 312 | 32.00 | 6.74 | 69 | 63.27 | −0.249 | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, L.; Li, Y.; Ma, C.; Meng, X.; Han, Y.; Li, H.; Huang, M.; Qin, Y.; Ren, X. Identification and Expression Analysis of the WOX Transcription Factor Family in Foxtail Millet (Setaria italica L.). Genes 2024, 15, 476. https://doi.org/10.3390/genes15040476
Nan L, Li Y, Ma C, Meng X, Han Y, Li H, Huang M, Qin Y, Ren X. Identification and Expression Analysis of the WOX Transcription Factor Family in Foxtail Millet (Setaria italica L.). Genes. 2024; 15(4):476. https://doi.org/10.3390/genes15040476
Chicago/Turabian StyleNan, Lizhang, Yajun Li, Cui Ma, Xiaowei Meng, Yuanhuai Han, Hongying Li, Mingjing Huang, Yingying Qin, and Xuemei Ren. 2024. "Identification and Expression Analysis of the WOX Transcription Factor Family in Foxtail Millet (Setaria italica L.)" Genes 15, no. 4: 476. https://doi.org/10.3390/genes15040476






