Responses of Primary Productivity and Phytoplankton Community to the Atmospheric Nutrient Deposition in the East China Sea
Abstract
:1. Introduction
2. Methods
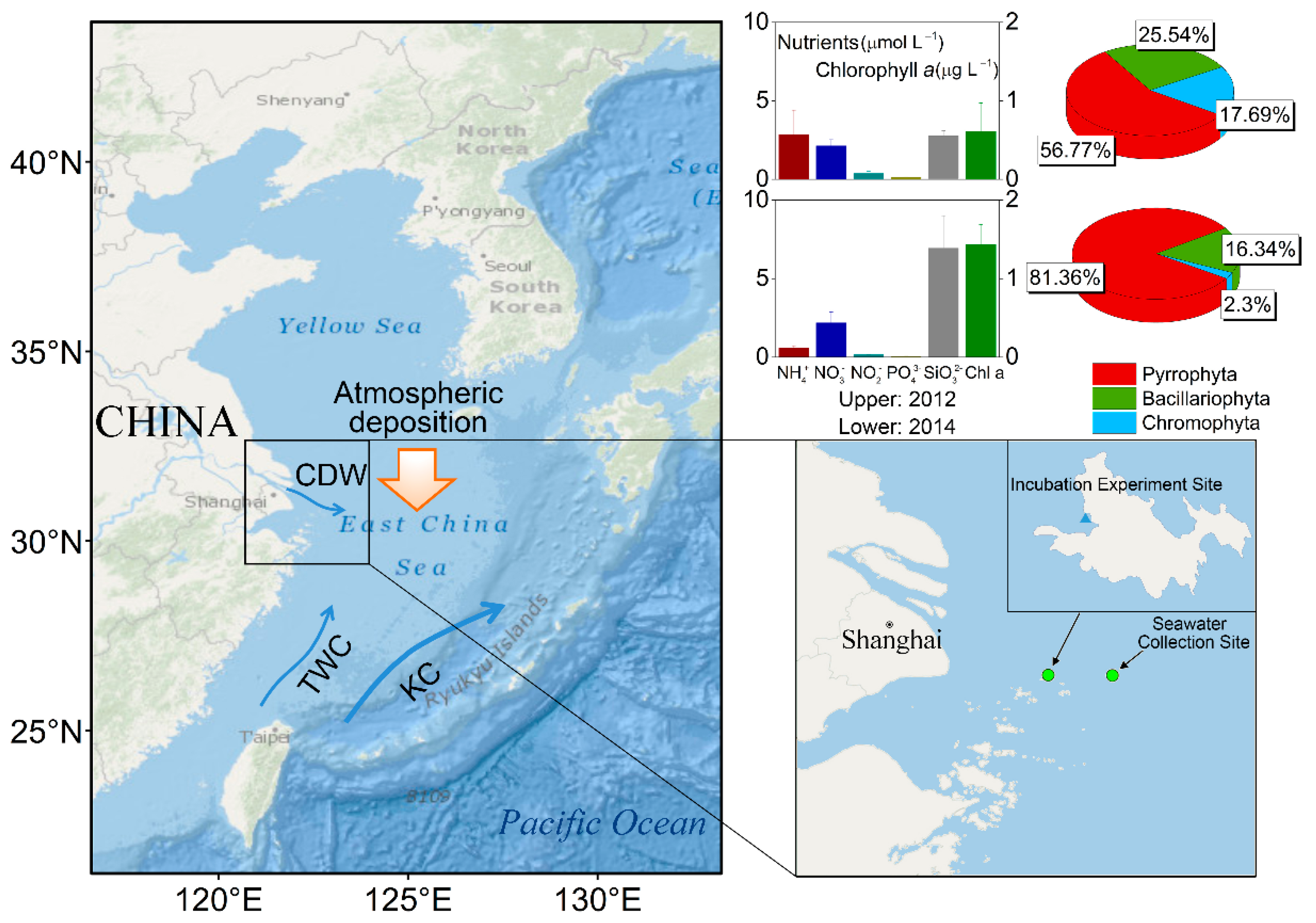
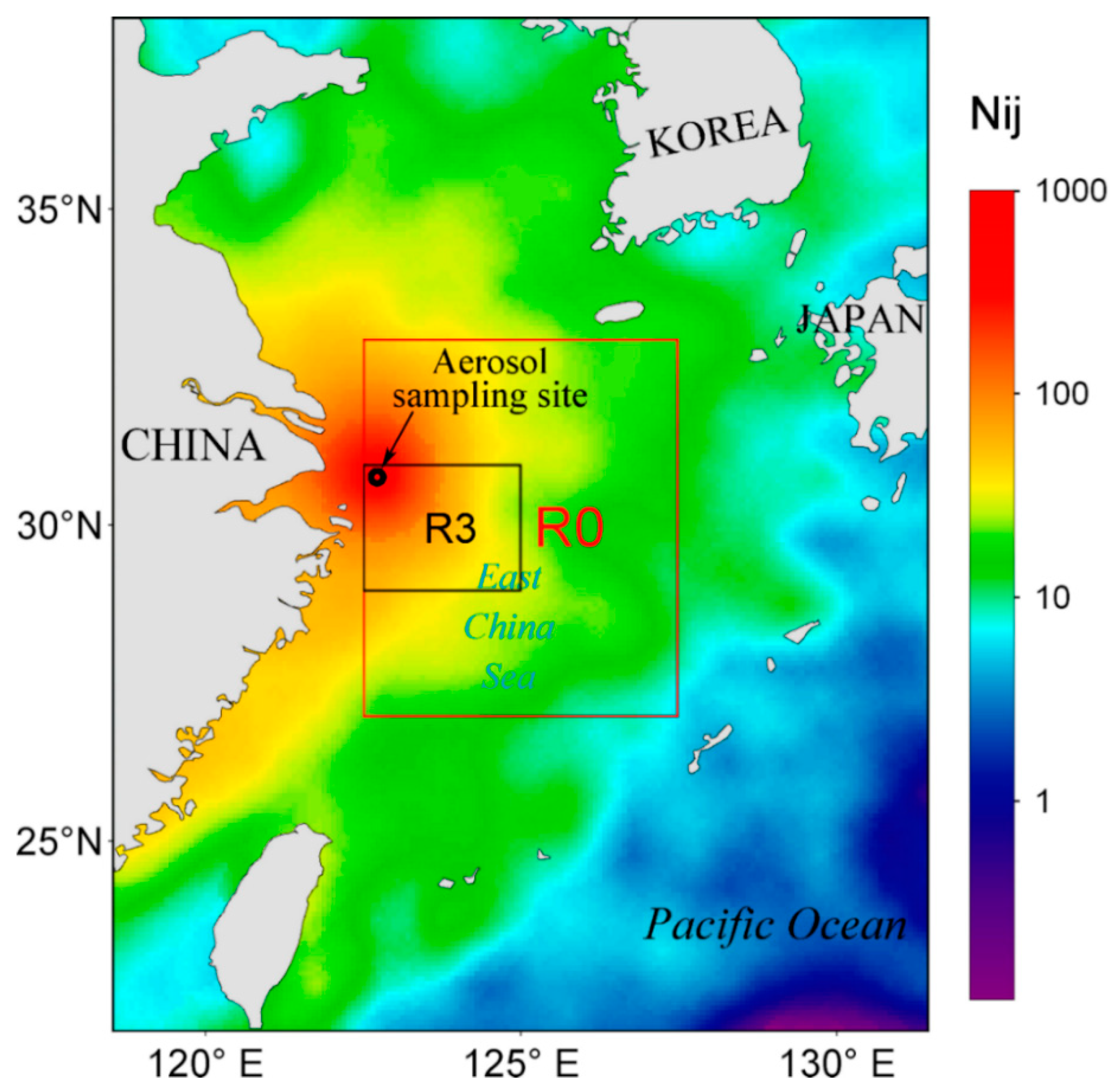
2.1. Aerosol Sample Collection and Dry Flux Estimate
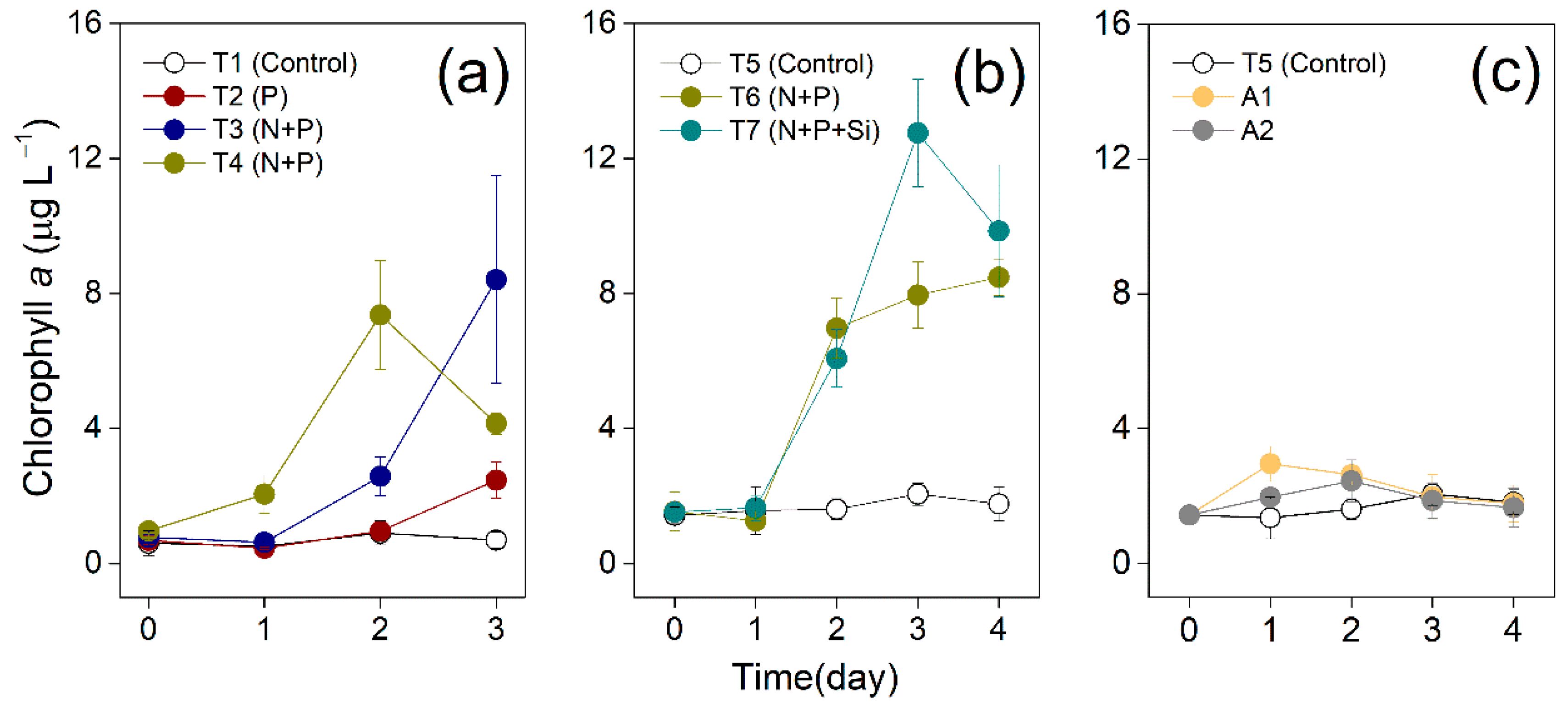
2.2. Bioassay Experiments
2.3. Chl a, Nutrients Measurements, and Phytoplankton Cell Counting
2.4. Statistical Analyses for Correlations between Chl a and Atmospheric Components
3. Results and Discussion
3.1. Changes of Chl a Concentrations
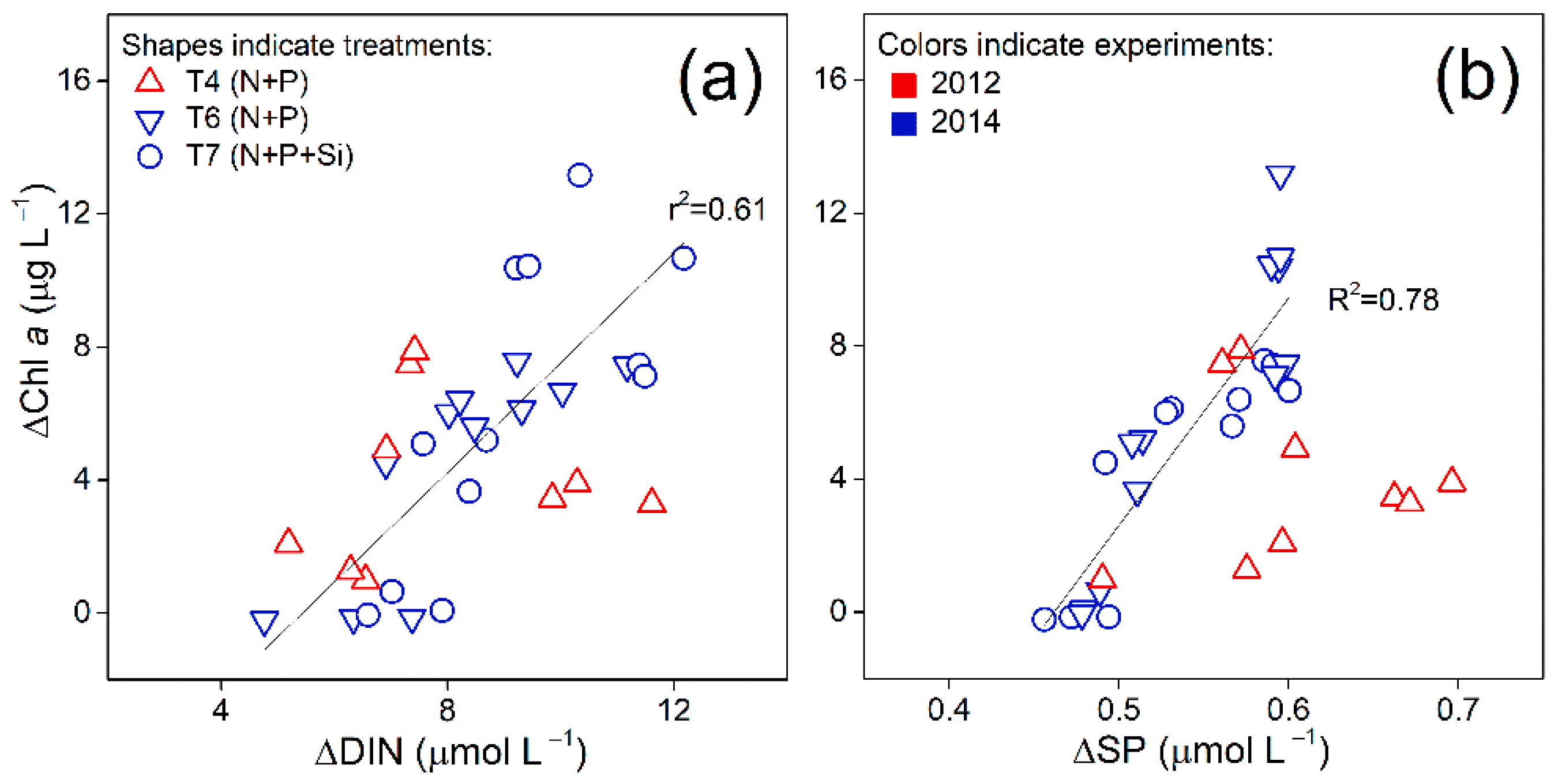
3.2. Correlations between Chl a and Nutrients
3.3. Correlations between Atmospheric N Fluxes and Chl a
3.4. Change of Phytoplankton Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol. Oceanogr. 1988, 33, 823–843. [Google Scholar] [CrossRef]
- Gong, G.C.; Wen, Y.H.; Wang, B.W.; Liu, G.J. Seasonal variation of chlorophyll a concentration, primary production and environmental conditions in the subtropical East China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 1219–1236. [Google Scholar] [CrossRef]
- Chen, C.-T.A.; Andreev, A.; Kim, K.-R.; Yamamoto, M. Roles of Continental Shelves and Marginal Seas in the Biogeochemical Cycles of the North Pacific Ocean. J. Oceanogr. 2004, 60, 17–44. [Google Scholar] [CrossRef]
- Tang, D.; Di, B.; Wei, G.; Ni, I.H.; Oh, I.S.; Wang, S. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia 2006, 568, 245–253. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Zhang, C.; Sun, B.; Wang, X.; Shi, X. Seasonal changes in phytoplankton biomass and dominant species in the Changjiang River Estuary and adjacent seas: General trends based on field survey data 1959–2009. J. Ocean Univ. China 2014, 13, 926–934. [Google Scholar] [CrossRef]
- Li, M.; Xu, K.; Watanabe, M.; Chen, Z. Long-term variations in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem. Estuar. Coast. Shelf Sci. 2007, 71, 3–12. [Google Scholar] [CrossRef]
- Guo, S.; Feng, Y.; Wang, L.; Dai, M.; Liu, Z.; Bai, Y.; Sun, J. Seasonal variation in the phytoplankton community of a continental-shelf sea: The East China Sea. Mar. Ecol. Prog. Ser. 2014, 516, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Chen, Y.; Meng, X.; Wang, F.; Zheng, Z. Phytoplankton community diversity is influenced by environmental factors in the coastal East China Sea. Eur. J. Phycol. 2016, 51, 107–118. [Google Scholar] [CrossRef]
- Yool, A.; Tyrrell, T. Role of diatoms in regulating the ocean’s silicon cycle. Glob. Biogeochem. Cycles 2003, 17, 1103. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E. Stoichiometric nutrient balance and origin of coastal eutrophication. Mar. Pollut. Bull. 1995, 30, 41–46. [Google Scholar] [CrossRef]
- Egge, J.K. Are diatoms poor competitors at low phosphate concentrations? J. Mar. Syst. 1998, 16, 191–198. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J. Phycol. 2000, 36, 903–913. [Google Scholar] [CrossRef]
- Glibert, P.M.; Magnien, R.; Lomas, M.W.; Alexander, J.; Tan, C.; Haramoto, E.; Trice, M.; Kana, T.M. Harmful algal blooms in the Chesapeake and Coastal Bays of Maryland, USA: Comparison of 1997, 1998, and 1999 events. Estuaries 2001, 24, 875–883. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Ruo, R.; Paid, S.C.; Liu, C.T.; Wong, G.T.F. Exchange of water masses between the East China Sea and the Kuroshio off northeastern Taiwan. Cont. Shelf Res. 1995, 15, 19–39. [Google Scholar] [CrossRef]
- Zhang, J. Nutrient elements in large Chinese estuaries. Cont. Shelf Res. 1996, 16, 1023–1045. [Google Scholar] [CrossRef]
- Chen, Y.; Mills, S.; Street, J.; Golan, D.; Post, A.; Jacobson, M.; Paytan, A. Estimates of atmospheric dry deposition and associated input of nutrients to Gulf of Aqaba seawater. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Duce, R.A.; LaRoche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.G.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S.; et al. Impacts of Atmospheric Anthropogenic Nitrogen on the Open Ocean. Science 2008, 320, 893–897. [Google Scholar] [CrossRef] [Green Version]
- Mackey, K.R.M.; van Dijken, G.L.; Mazloom, S.; Erhardt, A.M.; Ryan, J.; Arrigo, K.R.; Paytan, A. Influence of atmospheric nutrients on primary productivity in a coastal upwelling region. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef] [Green Version]
- Duce, R.A. The Impact of Atmospheric Nitrogen, Phosphorus, and Iron Species on Marine Biological Productivity. In The Role of Air-Sea Exchange in Geochemical Cycling; Buat-Ménard, P., Ed.; Springer: Dordrecht, The Netherlands, 1986; pp. 497–529. [Google Scholar] [CrossRef]
- Paerl, H.W. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnol. Oceanogr. 1997, 42, 1154–1165. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Whitall, D.R. Anthropogenically-derived atmospheric nitrogen deposition, marine eutrophication and harmful algal bloom expansion: Is there a link? Ambio 1999, 28, 307–311. [Google Scholar]
- Nakamura, T.; Matsumoto, K.; Uematsu, M. Chemical characteristics of aerosols transported from Asia to the East China Sea: An evaluation of anthropogenic combined nitrogen deposition in autumn. Atmos. Environ. 2005, 39, 1749–1758. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Q.; Ma, W.; Chen, L. Atmospheric deposition of inorganic nitrogen to the eastern China seas and its implications to marine biogeochemistry. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Fu, J.; Wang, B.; Chen, Y.; Ma, Q. The influence of continental air masses on the aerosols and nutrients deposition over the western North Pacific. Atmos. Environ. 2018, 172, 1–11. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Guo, L.; Wang, F. Estimate of dry deposition fluxes of nutrients over the East China Sea: The implication of aerosol ammonium to non-sea-salt sulfate ratio to nutrient deposition of coastal oceans. Atmos. Environ. 2013, 69, 131–138. [Google Scholar] [CrossRef]
- Yadav, K.; Sarma, V.V.S.S.; Rao, D.B.; Kumar, M.D. Influence of atmospheric dry deposition of inorganic nutrients on phytoplankton biomass in the coastal Bay of Bengal. Mar. Chem. 2016, 187, 25–34. [Google Scholar] [CrossRef]
- Wan, X.; Wu, Z.; Chang, Z.; Zhang, X. Reanalysis of atmospheric flux of nutrients to the South Yellow Sea and the East China Sea. J. Mar. Environ. Sci. 2002, 21, 14–18. [Google Scholar]
- Duce, R.A.; Tindale, N.W. Atmospheric transport of iron and its deposition in the ocean. Limnol. Oceanogr. 1991, 36, 1715–1726. [Google Scholar] [CrossRef]
- Guo, C.; Yu, J.; Ho, T.Y.; Wang, L.; Song, S.; Kong, L.; Liu, H. Dynamics of phytoplankton community structure in the South China Sea in response to the East Asian aerosol input. Biogeosciences 2012, 9, 1519–1536. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-W.; Lee, K.; Najjar, R.G.; Jeong, H.-D.; Jeong, H.J. Increasing N Abundance in the Northwestern Pacific Ocean Due to Atmospheric Nitrogen Deposition. Science 2011, 334, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-N.; Lee, K.; Gruber, N.; Karl, D.M.; Bullister, J.L.; Yang, S.; Kim, T.-W. Increasing anthropogenic nitrogen in the North Pacific Ocean. Science 2014, 346, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.T.F.; Gong, G.C.; Liu, K.K.; Pai, S.C. ‘Excess Nitrate’ in the East China Sea. Estuar. Coast. Shelf Sci. 1998, 46, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.-d.; Wang, X.-l.; Zhan, R. Nutrient conditions in the Yellow Sea and the East China Sea. Estuar. Coast. Shelf Sci. 2003, 58, 127–136. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Kavanaugh, M.T.; Wang, F.; Chen, Y.; Liu, F.; Glover, D.M.; Chien, C.-T.; Paytan, A. Atmospheric and Fluvial Nutrients Fuel Algal Blooms in the East China Sea. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.-T.; Mackey, K.R.M.; Dutkiewicz, S.; Mahowald, N.M.; Prospero, J.M.; Paytan, A. Effects of African dust deposition on phytoplankton in the western tropical Atlantic Ocean off Barbados. Glob. Biogeochem. Cycles 2016, 30, 716–734. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Fogel, M.L. Isotopic characterization of atmospheric nitrogen inputs as sources of enhanced primary production in coastal Atlantic Ocean waters. Mar. Biol. 1994, 119, 635–645. [Google Scholar] [CrossRef]
- Paytan, A.; Mackey, K.R.M.; Chen, Y.; Lima, I.D.; Doney, S.C.; Mahowald, N.; Labiosa, R.; Postf, A.F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Chen, Y.; Wang, B.; Ma, Q.; Wang, F. Responses of phytoplankton community to the input of different aerosols in the East China Sea. Geophys. Res. Lett. 2016, 43, 7081–7088. [Google Scholar] [CrossRef]
- Bishop, J.K.; Davis, R.E.; Sherman, J.T. Robotic observations of dust storm enhancement of carbon biomass in the North Pacific. Science 2002, 298, 817–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Gao, H.; Zhang, J.; Tan, S.; Ren, J.; Liu, C.; Liu, Y.; Yao, X. Examination of causative link between a springbloom and dry/wet deposition of Asian dust in the Yellow Sea, China. J. Geophys. Res. 2012, 117, D17304. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Prasanna Kumar, S. Dust-induced episodic phytoplankton blooms in the Arabian Sea during winter monsoon. J. Geophys. Res. Oceans 2014, 119, 7123–7138. [Google Scholar] [CrossRef]
- Tan, S.-C.; Wang, H. The transport and deposition of dust and its impact on phytoplankton growth in the Yellow Sea. Atmos. Environ. 2014, 99, 491–499. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Meng, X.; Fu, J.; Wang, B. The contribution of anthropogenic sources to the aerosols over East China Sea. Atmos. Environ. 2016, 127, 22–33. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Wang, F.; Meng, X.; Xu, Z.; Zhuang, G. Effects of Asian dust on the atmospheric input of trace elements to the East China Sea. Mar. Chem. 2014, 163, 19–27. [Google Scholar] [CrossRef]
- Park, S.; Chu, P.C. Synoptic distributions of thermal surface mixed layer and thermocline in the southern yellow and East China Seas. J. Oceanogr. 2007, 63, 1021–1028. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Boss, E.S.; Westberry, T.K.; Graff, J.R.; Halsey, K.H.; Milligan, A.J.; Siegel, D.A.; Brown, M.B. Revaluating ocean warming impacts on global phytoplankton. Nat. Clim. Chang. 2016, 6, 323–330. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Zhou, S.; Li, H.; Wang, F. Solubilities and deposition fluxes of atmospheric Fe and Cu over the Northwest Pacific and its marginal seas. Atmos. Environ. 2020, 239, 117763. [Google Scholar] [CrossRef]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT Atmospheric Transport and Dispersion Modeling System. Bull. Am. Meteorol. Soc. 2016, 96, 2059–2077. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, X.Y.; Draxler, R.R. TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ. Model. Softw. 2009, 24, 938–939. [Google Scholar] [CrossRef]
- Ashbaugh, L.L.; Malm, W.C.; Sadeh, W.Z. A Residence Time Probability Analysis of Sulfur Concentrations at Grand Canyon National Park. Atmos. Environ. (1967) 1985, 19, 1263–1270. [Google Scholar] [CrossRef]
- Lee Chen, Y.-L.; Chen, H.-Y. Nitrate-based new production and its relationship to primary production and chemical hydrography in spring and fall in the East China Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 1249–1264. [Google Scholar] [CrossRef]
- Gong, G.-C.; Chang, J.; Chiang, K.-P.; Hsiung, T.-M.; Hung, C.-C.; Duan, S.-W.; Codispoti, L.A. Reduction of primary production and changing of nutrient ratio in the East China Sea: Effect of the Three Gorges Dam? Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Fisher, T.; Peele, E.; Ammerman, J.H., Jr. Nutrient Limitation of Phytoplankton in Chesapeake Bay. Mar. Ecol. Prog. Ser. 1992, 82, 51–63. [Google Scholar] [CrossRef]
- Justić, D.; Rabalais, N.N.; Turner, R.E.; Dortch, Q. Changes in nutrient structure of river-dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuar. Coast. Shelf Sci. 1995, 40, 339–356. [Google Scholar] [CrossRef]
- Zhou, W.; Huo, W.; Yuan, X.; Yin, K. Distribution features of chlorophyll a and primary productivity in high frequency area of red tide in East China Sea during spring. Ying Yong Sheng Tai Xue Bao (J. Appl. Ecol.) 2003, 14, 1055–1059. [Google Scholar]
- Li, H.; Chen, Y.; Zhou, S.; Wang, F.; Yang, T.; Zhu, Y.; Ma, Q. Change of dominant phytoplankton groups in the eutrophic coastal sea due to atmospheric deposition. Sci. Total Environ. 2021, 753, 141961. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Cloning and functional characterization of ammonium transporters from the marine diatom Cylindrotheca fusiformis (Bacillariophceae). J. Phycol. 2005, 41, 105–113. [Google Scholar] [CrossRef]
- Sunda, W. Feedback Interactions between Trace Metal Nutrients and Phytoplankton in the Ocean. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowen, R.J.; Tett, P.; Jones, K.J. Predicting marine eutrophication: The yield of chlorophyll from nitrogen in Scottish coastal waters. Mar. Ecol. Prog. Ser. 1992, 85, 153–161. [Google Scholar] [CrossRef]
- Karl, D.M. Microbially Mediated Transformations of Phosphorus in the Sea: New Views of an Old Cycle. Annu. Rev. Mar. Sci. 2014, 6, 279–337. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, H.; Yao, X.; Shi, Z.; Shi, J.; Yu, Y.; Meng, L.; Guo, X. Phytoplankton growth response to Asian dust addition in the northwest Pacific Ocean versus the Yellow Sea. Biogeosciences 2018, 15, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Chu, Q.; Zhang, C.; He, J.; Gao, H. Impact of Dust and Haze Addition on Phytoplankton Biomass and Community Structure in the South China Sea. China Environ. Sci. 2018, 38, 3512–3523. [Google Scholar]
- Wang, F.J.; Chen, Y.; Guo, Z.G.; Gao, H.W.; Mackey, K.R.; Yao, X.H.; Zhuang, G.S.; Paytan, A. Combined effects of iron and copper from atmospheric dry deposition on ocean productivity. Geophys. Res. Lett. 2017, 44, 2546–2555. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global Iron Connections Between Desert Dust, Ocean Biogeochemistry, and Climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Altieri, K.E.; Hastings, M.G.; Peters, A.J.; Oleynik, S.; Sigman, D.M. Isotopic evidence for a marine ammonium source in rainwater at Bermuda. Glob. Biogeochem. Cycles 2014, 28, 1066–1080. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nature 2013, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Capone, D.G.; Knapp, A.N. A marine nitrogen cycle fix? Nature 2007, 445, 159–160. [Google Scholar] [CrossRef]
- Wang, J.; Kan, J.; Qian, G.; Chen, J.; Xia, Z.; Zhang, X.; Liu, H.; Sun, J. Denitrification and anammox: Understanding nitrogen loss from Yangtze Estuary to the east China sea (ECS). Environ. Pollut. 2019, 252, 1659–1670. [Google Scholar] [CrossRef]
- Agawin, N.S.; Duarte, C.; Agustí, S. Response of Mediterranean Synechococcus growth and loss rates to experimental nutrient inputs. Mar. Ecol. Prog. Ser. 2000, 206, 97–106. [Google Scholar] [CrossRef]
- Lippemeier, S.; Frampton, D.M.F.; Blackburn, S.I.; Geier, S.C.; Negri, A.P. Influence of phosphorus limitation on toxicity and photosynthesis of Alexandrium minutum (Dinophyceae) monitored by in-line detection of variable chlorophyll fluorescence. J. Phycol. 2003, 39, 320–331. [Google Scholar] [CrossRef]
- Mulderij, G.; Van Donk, E.; Roelofs, J.G.M. Differential sensitivity of green algae to allelopathic substances from Chara. Hydrobiologia 2003, 491, 261–271. [Google Scholar] [CrossRef]
- Riegman, R.; De Boer, M.; de Senerpont Domis, L. Growth of harmful marine algae in multispecies cultures. J. Plankton Res. 1996, 18, 1851–1866. [Google Scholar] [CrossRef]
- Dugdale, R.C.; Wilkerson, F.P. Silicate regulation of new production in the equatorial Pacific upwelling. Nature 1998, 391, 270–273. [Google Scholar] [CrossRef]
- Martin-Jezequel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Egge, J.K.; Aksnes, D. Silicate as Regulating Nutrient in Phytoplankton Competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Cochlan, W.; Price, N.; Harrison, P. Effects of irradiance on nitrogen uptake by phytoplankton: Comparison of frontal and stratified communities. Mar. Ecol. Prog. Ser. 1991, 69, 103–116. [Google Scholar] [CrossRef]
- Kudela, R.; Cochlan, W. Nitrogen and carbon uptake kinetics and the influence of irradiance for a red tide bloom of Southern California. Aquat. Microb. Ecol. 2000, 21, 31–47. [Google Scholar] [CrossRef]
- Leong, S.C.Y.; Maekawa, M.; Taguchi, S. Carbon and nitrogen acquisition by the toxic dinoflagellate Alexandrium tamarense in response to different nitrogen sources and supply modes. Harmful Algae 2010, 9, 48–58. [Google Scholar] [CrossRef]
- Paasche, E.; Bryceson, I.; Tangen, K. Interspecific variation in dark nitrogen uptake by dinoflagellates. J. Phycol. 2004, 20, 394–401. [Google Scholar] [CrossRef]
- Marr, J.C.; Jackson, A.E.; McLachlan, J.L. Occurrence of Prorocentrum lima, a DSP toxin-producing species from the Atlantic coast of Canada. J. Appl. Phycol. 1992, 4, 17–24. [Google Scholar] [CrossRef]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Li, F.; Tan, L.; Wang, J. Nutrients structure changes impact the competition and succession between diatom and dinoflagellate in the East China Sea. Sci. Total Environ. 2017, 574, 499–508. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, G.; Wan, A.; Zhao, Z.; Wang, S.; Liu, Q. Nutrient-limitation induced diatom-dinoflagellate shift of spring phytoplankton community in an offshore shellfish farming area. Mar. Pollut. Bull. 2019, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liss, P.S.; Hatton, A.D.; Malin, G.; Nightingale, P.D.; Turner, S.M. Marine sulphur emissions. Philos. Trans. R. Soc. Lond. Ser. B 1997, 352, 159–169. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Keller, M.D. Dimethyl Sulfide Production and Marine Phytoplankton: The Importance of Species Composition and Cell Size. Biol. Oceanogr. 1989, 6, 375–382. [Google Scholar] [CrossRef]
- Harrison, P.J.; Hu, M.H.; Yang, G.P.; Lu, X. Phosphate limitation in estuarine and coastal waters of China. J. Exp. Mar. Biol. Ecol. 1990, 140, 79–87. [Google Scholar] [CrossRef]



| Experimental Year | Treatment | Nutrients Addition (μmol L−1) | Aerosol Addition (mg L−1) | |||
|---|---|---|---|---|---|---|
| NH4+ | NO3− | PO43− | SiO32− | |||
| 2012 | T1 | 0 | 0 | 0 | 0 | - |
| T2 | 0 | 0 | 0.6 | 0 | - | |
| T3 | 1.0 | 9.0 | 0.6 | 0 | - | |
| T4 | 6.0 | 4.0 | 0.6 | 0 | - | |
| 2014 | T5 | 0 | 0 | 0 | 0 | - |
| T6 | 6.0 | 4.0 | 0.6 | 0 | - | |
| T7 | 6.0 | 4.0 | 0.6 | 10 | - | |
| A1 (31 March 2012) | 1.2 * | 2.1 * | 0.007 * (SP) | - | 1.0 | |
| A2 (27 October 2012) | 3.5 * | 1.8 * | 0.003 * (SP) | - | 1.0 | |
| Treatments | T2(P) | T3(N + P) | T4(N + P) | T6(N + P) | T7(N + P+Si) | Aerosol 1 | Aerosol 2 |
|---|---|---|---|---|---|---|---|
| DINadd (μmol L−1) | 0 | 10 | 10 | 10 | 10 | 3.27 | 5.26 |
| ΔDINmax (μmol L−1) | 2.47 | 12.4 | 10.5 | 10.6 | 11.7 | 3.45 | 1.61 |
| ΔDINmax/DINadd | - | 1.24 | 1.05 | 1.06 | 1.17 | 1.06 | 0.31 |
| SPadd (μmol L−1) | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.02 | 0.01 |
| ΔSPmax (μmol L−1) | 0.68 | 0.68 | 0.68 | 0.60 | 0.60 | 0.02 | 0.01 |
| ΔSPmax/SPadd | 1.13 | 1.14 | 1.13 | 1.00 | 0.99 | 0.97 | 0.94 |
| ΔChl amax (μg L−1) | 1.62 | 7.80 | 6.76 | 7.05 | 11.3 | 1.52 | 1.01 |
| ΔChl amaxDINadd (μg/μmol) | 0.16 | 0.78 | 0.68 | 0.71 | 1.13 | 0.47 | 0.19 |
| ΔChl amax/ΔDINmax (μg/μmol) | 0.66 | 0.63 | 0.64 | 0.67 | 0.97 | 0.44 | 0.63 |
| ΔChl amax/SPadd (μg/μmol) | 2.70 | 13.0 | 11.3 | 11.7 | 18.9 | 80.9 | 94.3 |
| ΔDINmax/ΔSPmax | 3.65 | 18.1 | 15.6 | 17.7 | 19.7 | 190 | 159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Chen, Y.; Wang, F.; Li, H. Responses of Primary Productivity and Phytoplankton Community to the Atmospheric Nutrient Deposition in the East China Sea. Atmosphere 2021, 12, 210. https://doi.org/10.3390/atmos12020210
Ma Q, Chen Y, Wang F, Li H. Responses of Primary Productivity and Phytoplankton Community to the Atmospheric Nutrient Deposition in the East China Sea. Atmosphere. 2021; 12(2):210. https://doi.org/10.3390/atmos12020210
Chicago/Turabian StyleMa, Qingwei, Ying Chen, Fanghui Wang, and Haowen Li. 2021. "Responses of Primary Productivity and Phytoplankton Community to the Atmospheric Nutrient Deposition in the East China Sea" Atmosphere 12, no. 2: 210. https://doi.org/10.3390/atmos12020210
APA StyleMa, Q., Chen, Y., Wang, F., & Li, H. (2021). Responses of Primary Productivity and Phytoplankton Community to the Atmospheric Nutrient Deposition in the East China Sea. Atmosphere, 12(2), 210. https://doi.org/10.3390/atmos12020210








