Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Processing
2.2. Data Collection and Processing
3. Results
3.1. Correlation between Air Pollution Variables and COVID-19 Infections, Deaths, and Mortality Rates
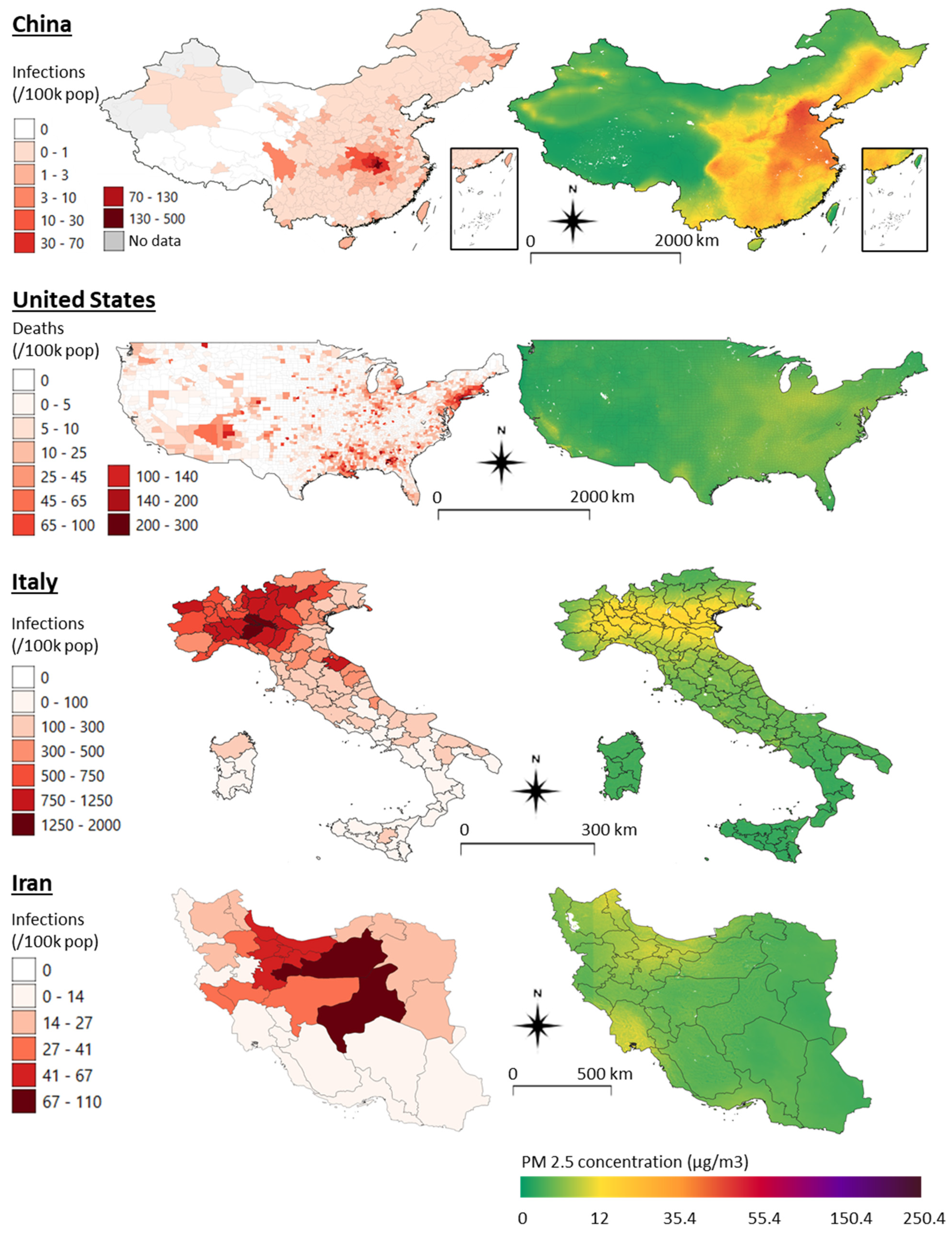
3.2. COVID-19 Distribution, Clusters, and Air Quality Maps
3.3. Previous Literature Account
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Unit | Count | Mean | std | min | 25% | 50% | 75% | Max | Range | iqr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| China | PM25_sat | ug/m3 | 347 | 30.31 | 15.80 | 2.08 | 19.11 | 29.09 | 40.01 | 70.98 | 68.90 | 20.89 |
| NO2_sat | ppb | 347 | 1.96 | 1.96 | 0.06 | 0.54 | 1.26 | 3.06 | 13.75 | 13.69 | 2.52 | |
| PM25_gr | AQI | 308 | 110.78 | 27.79 | 38.20 | 92.63 | 111.55 | 128.51 | 186.96 | 148.76 | 35.88 | |
| PM10_gr | AQI | 308 | 63.61 | 22.98 | 19.96 | 46.26 | 60.90 | 75.67 | 170.27 | 150.31 | 29.40 | |
| CO_gr | AQI | 308 | 9.71 | 3.97 | 2.39 | 7.11 | 8.74 | 11.85 | 27.01 | 24.63 | 4.74 | |
| NO2_gr | AQI | 308 | 13.72 | 5.40 | 3.17 | 9.63 | 13.40 | 17.41 | 28.57 | 25.40 | 7.78 | |
| O3_gr | AQI | 308 | 25.83 | 5.87 | 14.22 | 21.70 | 25.17 | 28.98 | 50.54 | 36.31 | 7.28 | |
| SO2_gr | AQI | 308 | 13.24 | 7.96 | 1.14 | 7.88 | 10.97 | 16.46 | 40.58 | 39.44 | 8.58 | |
| US | PM25_sat | ug/m3 | 3104 | 9.37 | 2.68 | 2.32 | 7.26 | 9.68 | 11.54 | 15.57 | 13.24 | 4.28 |
| NO2_sat | ppb | 3103 | 1.59 | 1.25 | 0.17 | 0.73 | 1.20 | 2.13 | 14.97 | 14.80 | 1.40 | |
| PM25_gr | ug/m3 | 429 | 7.22 | 2.10 | 0.00 | 5.88 | 7.37 | 8.66 | 15.73 | 15.73 | 2.78 | |
| PM10_gr | ug/m3 | 203 | 16.09 | 6.36 | 4.60 | 12.41 | 15.43 | 18.62 | 40.64 | 36.04 | 6.21 | |
| CO_gr | ppm | 158 | 0.25 | 0.10 | 0.04 | 0.19 | 0.25 | 0.30 | 0.82 | 0.78 | 0.12 | |
| NO2_gr | ppb | 248 | 14.63 | 7.93 | 1.08 | 7.88 | 14.50 | 20.65 | 36.73 | 35.65 | 12.76 | |
| O3_gr | ppm | 751 | 0.05 | 0.00 | 0.03 | 0.04 | 0.05 | 0.05 | 0.06 | 0.03 | 0.00 | |
| SO2_gr | ppb | 316 | 2.61 | 5.56 | -0.38 | 0.57 | 1.30 | 2.51 | 75.47 | 75.85 | 1.94 | |
| Italy (provinces) | PM25_sat | ug/m3 | 107 | 12.82 | 6.08 | 4.50 | 7.68 | 11.62 | 18.10 | 25.37 | 20.86 | 10.42 |
| NO2_sat | ppb | 107 | 2.67 | 2.42 | 0.39 | 0.99 | 1.61 | 3.95 | 11.56 | 11.16 | 2.96 | |
| PM25_gr | ug/m3 | 90 | 16.37 | 4.89 | 6.00 | 13.00 | 15.37 | 19.46 | 29.00 | 23.00 | 6.46 | |
| PM10_gr | ug/m3 | 101 | 23.67 | 5.61 | 13.67 | 19.75 | 22.50 | 26.75 | 41.00 | 27.33 | 7.00 | |
| Italy (regions) | PM25_sat | ug/m3 | 21 | 11.29 | 4.73 | 4.90 | 7.73 | 10.48 | 13.84 | 20.59 | 15.69 | 6.11 |
| NO2_sat | ppb | 21 | 1.93 | 1.68 | 0.48 | 0.83 | 1.35 | 2.25 | 7.08 | 6.59 | 1.42 | |
| PM25_gr | ug/m3 | 19 | 15.19 | 3.50 | 9.50 | 13.00 | 14.67 | 16.05 | 22.96 | 13.46 | 3.05 | |
| PM10_gr | ug/m3 | 21 | 22.15 | 4.40 | 15.60 | 20.40 | 21.29 | 22.92 | 34.67 | 19.07 | 2.52 | |
| Iran | PM25_sat | ug/m3 | 31 | 10.97 | 3.07 | 5.89 | 8.74 | 10.47 | 13.74 | 16.43 | 10.54 | 5.00 |
| NO2_sat | ppb | 31 | 0.47 | 0.47 | 0.10 | 0.22 | 0.31 | 0.42 | 2.24 | 2.14 | 0.21 | |
| France | PM25_sat | ug/m3 | 96 | 10.23 | 2.42 | 6.22 | 8.13 | 9.90 | 11.79 | 16.49 | 10.27 | 3.66 |
| NO2_sat | ppb | 96 | 2.37 | 1.75 | 0.55 | 1.20 | 1.80 | 3.02 | 8.79 | 8.24 | 1.82 | |
| Spain | PM25_sat | ug/m3 | 43 | 7.05 | 1.13 | 2.36 | 6.49 | 6.93 | 7.54 | 10.10 | 7.74 | 1.05 |
| NO2_sat | ppb | 43 | 1.02 | 0.45 | 0.10 | 0.69 | 0.91 | 1.27 | 2.69 | 2.58 | 0.58 | |
| Germany | PM25_sat | ug/m3 | 401 | 13.95 | 1.53 | 8.91 | 13.10 | 13.86 | 14.93 | 18.47 | 9.56 | 1.83 |
| NO2_sat | ppb | 401 | 4.77 | 2.04 | 1.48 | 3.48 | 4.19 | 5.24 | 11.86 | 10.38 | 1.76 | |
| UK | PM25_sat | ug/m3 | 364 | 10.51 | 2.64 | 2.16 | 9.06 | 11.29 | 12.07 | 15.37 | 13.21 | 3.01 |
| NO2_sat | ppb | 362 | 5.47 | 2.24 | 0.48 | 4.12 | 5.26 | 6.62 | 10.23 | 9.76 | 2.50 |
Appendix B
| China | US | Italy (Provinces) | Italy (Regions) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | ||||
| PM25_sat | 345 | 0.58 | <0.001 | 3102 | 0.38 | <0.001 | 105 | 0.27 | <0.001 | 19 | 0.30 | 0.065 | |||
| NO2_sat | 345 | 0.63 | <0.001 | 3101 | 0.54 | <0.001 | 105 | 0.36 | <0.001 | 19 | 0.42 | 0.007 | |||
| PM25_gr | 306 | 0.34 | <0.001 | 427 | 0.33 | <0.001 | 88 | 0.31 | <0.001 | 17 | 0.45 | 0.008 | |||
| PM10_gr | 306 | 0.21 | <0.001 | 201 | 0.27 | <0.001 | 99 | 0.38 | <0.001 | 19 | 0.69 | <0.001 | |||
| CO_gr | 306 | 0.09 | 0.022 | 156 | 0.40 | <0.001 | |||||||||
| NO2_gr | 306 | 0.37 | <0.001 | 246 | 0.52 | <0.001 | |||||||||
| O3_gr | 306 | 0.13 | <0.001 | 749 | 0.01 | 0.824 | |||||||||
| SO2_gr | 306 | 0.15 | <0.001 | 314 | −0.09 | 0.016 | |||||||||
| Iran | France | Spain | Germany | UK | |||||||||||
| df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | |
| PM25_sat | 29 | 0.56 | <0.001 | 94 | 0.34 | <0.001 | 41 | 0.44 | <0.001 | 399 | 0.14 | <0.001 | 362 | 0.44 | <0.001 |
| NO2_sat | 29 | 0.56 | <0.001 | 94 | 0.44 | <0.001 | 41 | 0.25 | 0.018 | 399 | 0.40 | <0.001 | 360 | 0.39 | <0.001 |
References
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Perlman, S. Another decade, another coronavirus. N. Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.M.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pneumonia of Unknown Cause—China. Available online: https://www.who.int/csr/don/05-january−2020-pneumonia-of-unkown-cause-china/en/ (accessed on 21 April 2020).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattiuzzi, C.; Lippi, G. Which lessons shall we learn from the 2019 novel coronavirus outbreak? Ann. Transl. Med. 2020, 8, 48. [Google Scholar] [CrossRef]
- McCloskey, B.; Heymann, D.L. SARS to novel coronavirus—Old lessons and new lessons. Epidemiol. Infect. 2020, 148, e22. [Google Scholar] [CrossRef] [Green Version]
- Nay, O. Can a virus undermine human rights? Lancet Public Health 2020, 5, e238–e239. [Google Scholar] [CrossRef]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air pollution and mortality in the Medicare population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef]
- Krewski, D. Evaluating the effects of ambient air pollution on life expectancy. N. Engl. J. Med. 2009, 360, 413–415. [Google Scholar] [CrossRef] [Green Version]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Tager, I. Chronic Exposure and Susceptibility to Oxidant Air Pollutants. In Lung Biology In Health Disease; Foster, W.M., Costa, D.L., Eds.; Routledge: Milton Park, UK, 2005; Volume 204, p. 259. [Google Scholar]
- Ebenstein, A.; Fan, M.; Greenstone, M.; He, G.; Zhou, M. New evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River Policy. Proc. Natl. Acad. Sci. USA 2017, 114, 10384. [Google Scholar] [CrossRef] [Green Version]
- Brauer, M.; Amann, M.; Burnett, R.T.; Cohen, A.; Dentener, F.; Ezzati, M.; Henderson, S.B.; Krzyzanowski, M.; Martin, R.V.; Dingenen, R.V.; et al. Exposure Assessment for Estimation of the Global Burden of Disease Attributable to Outdoor Air Pollution. Environ. Sci. Technol. 2012, 46, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.R.; Viana, V.P.; Müller, A.M.; Livi, F.P.; Dalcin, P.D.T.R. Respiratory viral infections and effects of meteorological parameters and air pollution in adults with respiratory symptoms admitted to the emergency room. Influenza Other Respir. Viruses 2014, 8, 42–52. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.-F.; Froines, J.; Zhao, J.; Wang, H.; Yu, S.-Z.; Detels, R. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ. Health 2003, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolla, M.; Sorgenti, M.; Gentile, C.; Bishara, M.M. Air Pollution and Lung Diseases. In Clinical Handbook of Air Pollution-Related Diseases; Springer: New York, NY, USA, 2018; pp. 327–339. [Google Scholar]
- Bayram, H.; Sapsford, R.J.; Abdelaziz, M.M.; Khair, O.A. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 2001, 107, 287–294. [Google Scholar] [CrossRef]
- Johannson, K.A.; Vittinghoff, E.; Lee, K.; Balmes, J.R.; Ji, W.; Kaplan, G.G.; Kim, D.S.; Collard, H.R. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur. Respir. J. 2014, 43, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Ko, F.W.; Tam, W.; Wong, T.W.; Chan, D.P.; Tung, A.H.; Lai, C.K.; Hui, D.S. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007, 62, 780–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thillai, M.; Moller, D.R.; Meyer, K.C. Clinical Handbook of Interstitial Lung Disease; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, Y.; Ding, Z.; Xiang, Q.; Wang, W.; Huang, L.; Mao, F. Short-term effects of ambient PM1 and PM2.5 air pollution on hospital admission for respiratory diseases: Case-crossover evidence from Shenzhen, China. Int. J. Hyg. Environ. Health 2020, 224, 113418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.G.; Xia, Y.; Shang, K.Z.; Cheng, Y.F.; Xu, L.; Ning, G.C.; Zhao, W.J.; LI, N.R. Association between ambient air pollution and hospital emergency admissions for respiratory and cardiovascular diseases in Beijing: A time series study. J. Biomed. Environ. Sci. 2015, 28, 352–363. [Google Scholar]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.; Yang, C.X.; Timens, W.; Bossé, Y.; Sin, D.D. SARS-CoV−2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir. Med. 2020, 8, e50–e51. [Google Scholar] [CrossRef]
- Harrod, K.S.; Jaramillo, R.J.; Rosenberger, C.L.; Wang, S.Z.; Berger, J.A.; McDonald, J.D.; Reed, M.D. Increased susceptibility to RSV infection by exposure to inhaled diesel engine emissions. Am. J. Respir. Cell Mol. Biol. 2003, 28, 451–463. [Google Scholar] [CrossRef]
- Lambert, A.L.; Mangum, J.B.; DeLorme, M.P.; Everitt, J.I. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol. Sci. 2003, 72, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Burge, H.A. Biological Airborne Pollutants. In Lung Biology In Health Disease, Foster, W.M., Costa, D.L., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; Volume 204, p. 329. [Google Scholar]
- Wong, G.; Ko, F.; Lau, T.; Li, S.; Hui, D.; Pang, S.; Leung, R.; Fok, T.; Lai, C. Temporal relationship between air pollution and hospital admissions for asthmatic children in Hong Kong. Clin. Exp. Allergy 2001, 31, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Dong, L.; Qi, J.; Shao, C.; Zhong, X.; Gao, D.; Cao, W.; Gao, J.; Bai, R.; Long, G.; Chu, C. Concentration and size distribution of total airborne microbes in hazy and foggy weather. Sci. Total Environ. 2016, 541, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zou, Z.; Zheng, Y.; Li, J.; Shen, F.; Wu, C.-Y.; Wu, Y.; Hu, M.; Yao, M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016, 550, 751–759. [Google Scholar] [CrossRef]
- Li, Y.; Fu, H.; Wang, W.; Liu, J.; Meng, Q.; Wang, W. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmos. Environ. 2015, 122, 439–447. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Ye, Q.; Fu, J.-F.; Mao, J.-H.; Shang, S.-Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. Res. 2016, 23, 20178–20185. [Google Scholar] [CrossRef]
- Chen, P.-S.; Tsai, F.T.; Lin, C.K.; Yang, C.-Y.; Chan, C.-C.; Young, C.-Y.; Lee, C.-H. Ambient influenza and avian influenza virus during dust storm days and background days. Environ. Health Perspect. 2010, 118, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhang, W.; Li, S.; Williams, G.; Liu, C.; Morgan, G.G.; Jaakkola, J.J.; Guo, Y. Is short-term exposure to ambient fine particles associated with measles incidence in China? A multi-city study. Environ. Res. 2017, 156, 306–311. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, X.; Tao, Y.; Mi, S.; Huang, J.; Zhang, Q. The effects of air pollution and meteorological factors on measles cases in Lanzhou, China. Environ. Sci. Pollut. Res. 2020, 27, 13524–13533. [Google Scholar] [CrossRef]
- Contini, D.; Costabile, F. Does Air Pollution Influence COVID−19 Outbreaks? Atmosphere 2020, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Pallavicini, A.; Ruscio, M.; Piscitelli, P.; Colao, A.; Miani, A. Searching for SARS-COV−2 on Particulate Matter: A Possible Early Indicator of COVID−19 Epidemic Recurrence. Int. J. Environ. Res. Public Health 2020, 17, 2986. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID−19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, M.U.G.; Yang, C.-H.; Gutierrez, B.; Wu, C.-H.; Klein, B.; Pigott, D.M.; du Plessis, L.; Faria, N.R.; Li, R.; Hanage, W.P.; et al. The effect of human mobility and control measures on the COVID−19 epidemic in China. Science 2020, 368, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID−19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef]
- Guan, W.-J.; Zheng, X.-Y.; Chung, K.F.; Zhong, N.-S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Pansini, R.; Fornacca, D. COVID−19 Higher Mortality in Chinese Regions With Chronic Exposure to Lower Air Quality. Front. Public Health 2021, 8, 597753. [Google Scholar] [CrossRef] [PubMed]
- Cereda, D.; Tirani, M.; Rovida, F.; Demicheli, V.; Ajelli, M.; Poletti, P.; Trentini, F.; Guzzetta, G.; Marziano, V.; Barone, A.; et al. The early phase of the COVID−19 outbreak in Lombardy, Italy. arXiv E Prints 2020, 2003, 09320. [Google Scholar]
- Apolone, G.; Montomoli, E.; Manenti, A.; Boeri, M.; Sabia, F.; Hyseni, I.; Mazzini, L.; Martinuzzi, D.; Cantone, L.; Milanese, G.; et al. Unexpected detection of SARS-CoV−2 antibodies in the prepandemic period in Italy. Tumori J. 2020. [Google Scholar] [CrossRef]
- Sajadi, M.M.; Habibzadeh, P.; Vintzileos, A.; Shokouhi, S.; Miralles-Wilhelm, F.; Amoroso, A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID−19). JAMA Netw Open 2020, 3, e2011834. [Google Scholar] [CrossRef]
- Lipsitch, M. Seasonality of SARS-CoV−2: Will COVID−19 Go Away on Its Own in Warmer Weather? Available online: https://ccdd.hsph.harvard.edu/will-covid−19-go-away-on-its-own-in-warmer-weather/ (accessed on 23 March 2020).
- Ficetola, G.F.; Rubolini, D. Containment measures limit environmental effects on COVID−19 early outbreak dynamics. Sci. Total Environ. 2021, 761, 144432. [Google Scholar] [CrossRef]
- Luo, W.; Majumder, M.S.; Liu, D.; Poirier, C.; Mandl, K.D.; Lipsitch, M.; Santillana, M. The role of absolute humidity on transmission rates of the COVID−19 outbreak. medRxiv 2020. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Auzenbergs, M.; Jafari, Y.; Liu, Y.; Flasche, S.; Lowe, R. Effective transmission across the globe: The role of climate in COVID−19 mitigation strategies. Lancet Planet. Health 2020, 4, e172. [Google Scholar] [CrossRef]
- Srivastava, A. COVID−19 and air pollution and meteorology-an intricate relationship: A review. Chemosphere 2021, 263, 128297. [Google Scholar] [CrossRef]
- Stier, A.J.; Berman, M.G.; Bettencourt, L.M.A. COVID−19 attack rate increases with city size. arXiv 2020, 2003, 10376. [Google Scholar]
- Jung, S.-M.; Akhmetzhanov, A.R.; Hayashi, K.; Linton, N.M.; Yang, Y.; Yuan, B.; Kobayashi, T.; Kinoshita, R.; Nishiura, H. Real-time estimation of the risk of death from novel coronavirus (COVID−19) infection: Inference using exported cases. J. Clin. Med. 2020, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Findlater, A.; Bogoch, I.I. Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Hoehl, S.; Rabenau, H.; Berger, A.; Kortenbusch, M.; Cinatl, J.; Bojkova, D.; Behrens, P.; Böddinghaus, B.; Götsch, U.; Naujoks, F.; et al. Evidence of SARS-CoV−2 Infection in Returning Travelers from Wuhan, China. N. Engl. J. Med. 2020, 382, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.-T.; Marimuthu, K.; Chia, P.-Y.; Koh, V.; Chiew, C.J.; De Wang, L.; Young, B.E.; Chan, M.; Vasoo, S.; Ling, L.-M.; et al. SARS-CoV−2 Infection among Travelers Returning from Wuhan, China. N. Engl. J. Med. 2020, 382, 1476–1478. [Google Scholar] [CrossRef] [Green Version]
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.; Fu, H.; et al. Estimates of the severity of COVID−19 disease. medRxiv 2020, 20, 669–677. [Google Scholar] [CrossRef]
- Wells, C.R.; Sah, P.; Moghadas, S.M.; Pandey, A.; Shoukat, A.; Wang, Y.; Wang, Z.; Meyers, L.A.; Singer, B.H.; Galvani, A.P. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc. Natl. Acad. Sci. 2020, 117, 7504. [Google Scholar] [CrossRef] [Green Version]
- Read, J.M.; Bridgen, J.R.E.; Cummings, D.A.T.; Ho, A.; Jewell, C.P. Novel coronavirus 2019-nCoV: Early estimation of epidemiological parameters and epidemic predictions. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Menkir, T.F.; Chin, T.; Hay, J.A.; Surface, E.D.; De Salazar, P.M.; Buckee, C.O.; Watts, A.; Khan, K.; Sherbo, R.; Yan, A.W.C.; et al. Estimating internationally imported cases during the early COVID−19 pandemic. Nat. Commun. 2021, 12, 311. [Google Scholar] [CrossRef]
- Soriano-Paños, D.; Arias-Castro, J.H.; Reyna-Lara, A.; Martínez, H.J.; Meloni, S.; Gómez-Gardeñes, J. Vector-borne epidemics driven by human mobility. Phys. Rev. Res. 2020, 2, 013312. [Google Scholar] [CrossRef] [Green Version]
- Arenas, A.; Cota, W.; Gómez-Gardeñes, J.; Gómez, S.; Granell, C.; Matamalas, J.T.; Soriano-Paños, D.; Steinegger, B. Modeling the Spatiotemporal Epidemic Spreading of COVID−19 and the Impact of Mobility and Social Distancing Interventions. Phys. Rev. X 2020, 10, 041055. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Watts, A.; Thomas-Bachli, A.; Huber, C.; Kraemer, M.U.G.; Khan, K. Potential for global spread of a novel coronavirus from China. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef]
- De Salazar, P.M.; Niehus, R.; Taylor, A.; Buckee, C.O.; Lipsitch, M. Using predicted imports of 2019-nCoV cases to determine locations that may not be identifying all imported cases. medRxiv 2020. [Google Scholar] [CrossRef]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the effect of individual variation on disease emergence. Nature 2005, 438, 355–359. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, Y.; Wang, M.; Zeng, Q.; Wang, X.; Wang, M.; Zheng, Z.; Li, X.; Zhang, Y.; Wang, T.; et al. Identification of a super-spreading chain of transmission associated with COVID−19. medRxiv 2020. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID−19 for cancer patients. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- Olds, J.L.; Kabbani, N. Is nicotine exposure linked to cardiopulmonary vulnerability to COVID−19 in the general population? FEBS J. 2020, 287, 3651–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Smoking and COVID−19. Available online: https://www.who.int/publications/i/item/smoking-and-covid−19 (accessed on 4 June 2020).
- Comunian, S.; Dongo, D.; Milani, C.; Palestini, P. Air Pollution and Covid−19: The Role of Particulate Matter in the Spread and Increase of Covid−19’s Morbidity and Mortality. Int. J. Environ. Res. Public Health 2020, 17, 4487. [Google Scholar] [CrossRef] [PubMed]
- Copat, C.; Cristaldi, A.; Fiore, M.; Grasso, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. The role of air pollution (PM and NO2) in COVID−19 spread and lethality: A systematic review. Environ. Res. 2020, 191, 110129. [Google Scholar] [CrossRef]
- Lanchipa-Ale, T.; Moreno-Salazar, K.; Luque-Zúñiga, B. COVID−19 perspective on air pollution. Rev. Soc. Científica Parag. 2020, 25, 155–182. [Google Scholar] [CrossRef]
- Fattorini, D.; Regoli, F. Role of the chronic air pollution levels in the Covid−19 outbreak risk in Italy. Environ. Pollut. 2020, 264, 114732. [Google Scholar] [CrossRef]
- Wu, X.; Braun, D.; Schwartz, J.; Kioumourtzoglou, M.A.; Dominici, F. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Sci. Adv. 2020. [Google Scholar] [CrossRef]
- Travaglio, M.; Popovic, R.; Yu, Y.; Leal, N.; Martins, L.M. Links between air pollution and COVID−19 in England. medRxiv 2020. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV−2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guascito, M.R.; Pietrogrande, M.C.; Decesari, S.; Contini, D. Oxidative Potential of Atmospheric Aerosols. Atmosphere 2021, 12, 531. [Google Scholar] [CrossRef]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2018; Available online: http://www.who.int/phe/publications/air-pollution-global-assessment/en/ (accessed on 10 April 2020).
- Kyle, A.D.; Woodruff, T.J.; Buffler, P.A.; Davis, D.L. Use of an Index to Reflect the Aggregate Burden of Long-Term Exposure to Criteria Air Pollutants in the United States. Environ. Health Perspect. 2002, 110, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pansini, R.; Fornacca, D. Initial evidence of higher morbidity and mortality due to SARS-CoV−2 in regions with lower air quality. medRxiv 2020. [Google Scholar] [CrossRef]
- Pansini, R.; Fornacca, D. Higher virulence of COVID−19 in the air-polluted regions of eight severely affected countries. medRxiv 2020. [Google Scholar] [CrossRef]
- Cohen, J.; Kupferschmidt, K. Countries test tactics in ‘war’ against COVID−19. Science 2020, 367, 1287. [Google Scholar] [CrossRef] [Green Version]
- Xinhua. Full Text: Q&A on the Revisions to the Numbers of Confirmed Cases and Fatalities of COVID−19 in Wuhan. Available online: http://www.xinhuanet.com/english/2020−04/17/c_138984721.htm (accessed on 17 April 2020).
- Legido-Quigley, H.; Mateos-García, J.T.; Campos, V.R.; Gea-Sánchez, M.; Muntaner, C.; McKee, M. The resilience of the Spanish health system against the COVID−19 pandemic. Lancet Public Health 2020, 5, e251–e252. [Google Scholar] [CrossRef] [Green Version]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Hsu, N.C.; Kahn, R.A.; Levy, R.C.; Lyapustin, A.; Sayer, A.M.; Winker, D.M. Global Annual PM2.5 Grids from MODIS, MISR and SeaWiFS Aerosol Optical Depth (AOD) with GWR, 1998−2016. Available online: https://doi.org/10.7927/H4ZK5DQS (accessed on 10 April 2020).
- van Donkelaar, A.; Martin, R.V.; Brauer, M.; Hsu, N.C.; Kahn, R.A.; Levy, R.C.; Lyapustin, A.; Sayer, A.M.; Winker, D.M. Global Estimates of Fine Particulate Matter Using a Combined Geophysical-Statistical Method with Information from Satellites. Environ. Sci. Technol. 2016, 50, 3762. [Google Scholar] [CrossRef]
- Geddes, J.A.; Martin, R.V.; Boys, B.L.; van Donkelaar, A. Global 3-Year Running Mean Ground-Level Nitrogen Dioxide (NO2) Grids from GOME, SCIAMACHY and GOME−2; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2017. [Google Scholar]
- Geddes, J.A.; Martin, R.V.; Boys, B.L.; van Donkelaar, A. Long-term Trends Worldwide in Ambient NO2 Concentrations Inferred from Satellite Observations for Exposure Assessment. Environ. Health Perspect. 2016, 124, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Su, F.; Pei, T.; Zhang, A.; Du, Y.; Luo, B.; Cao, Z.; Wang, J.; Yuan, W.; Zhu, Y.; et al. COVID−19: Challenges to GIS with Big Data. Geogr. Sustain. 2020, 1, 77–87. [Google Scholar] [CrossRef]
- Donkelaar, A.v.; Martin, R.V.; Brauer, M.; Boys, B.L. Use of Satellite Observations for Long-Term Exposure Assessment of Global Concentrations of Fine Particulate Matter. Environ. Health Perspect. 2014, 123, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Wartenberg, D. Multivariate Spatial Correlation: A Method for Exploratory Geographical Analysis. Geographical Analysis 2010, 17, 263–283. [Google Scholar] [CrossRef]
- Anselin, L.; Syabri, I.; Smirnov, O. Visualizing multivariate spatial correlation with dynamically linked windows. In Proceedings of the CSISS Workshop on New Tools for Spatial Data Analysis, Santa Barbara, CA, USA, 20–23 March 2002. [Google Scholar]
- Ogen, Y. Response to the commentary by Alexandra A. Chudnovsky on ‘Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID−19) fatality’. Sci. Total Environ. 2020, 139239. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID−19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020, 6, eabd4049. [Google Scholar] [CrossRef]
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID−19 mortality in the United States. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Pluchino, A.; Biondo, A.E.; Giuffrida, N.; Inturri, G.; Latora, V.; Le Moli, R.; Rapisarda, A.; Russo, G.; Zappalà, C. A novel methodology for epidemic risk assessment of COVID−19 outbreak. Sci. Rep. 2021, 11, 5304. [Google Scholar] [CrossRef]
- Andree, B.P.J. Incidence of COVID−19 and Connections with Air Pollution Exposure: Evidence from the Netherlands. Available online: http://documents.worldbank.org/curated/en/462481587756439003/Incidence-of-COVID−19-and-Connections-with-Air-Pollution-Exposure-Evidence-from-the-Netherlands (accessed on 5 May 2020).
- Cole, M.A.; Ozgen, C.; Strobl, E. Air Pollution Exposure and Covid−19 in Dutch Municipalities. Environ. Resour. Econ. 2020, 76, 581–610. [Google Scholar] [CrossRef]
- Nawahda, A. The Rises of Coronavirus Disease (COVID−19) Death Rate in Japan with High PM2.5. Available online: https://www.researchgate.net/publication/341333433_The_Rises_of_Coronavirus_Disease_COVID−19_death_rate_in_Japan_with_high_PM25 (accessed on 20 June 2020).
- Mele, M.; Magazzino, C. Pollution, economic growth, and COVID−19 deaths in India: A machine learning evidence. Environ. Sci. Pollut. Res. 2021, 28, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Evans, G.J.; To, T.M.; Brook, J.R.; Burnett, R.T. An ecological analysis of long-term exposure to PM2.5 and incidence of COVID−19 in Canadian health regions. Environ. Res. 2020, 191, 110052. [Google Scholar] [CrossRef]
- Qiao, L.; Lau, S.-F.; Mudie, L. Estimates show Wuhan death toll far higher than official figure. Available online: https://www.rfa.org/english/news/china/wuhan-deaths−03272020182846.html (accessed on 30 March 2020).
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID−19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. Ambient nitrogen dioxide pollution and spread ability of COVID−19 in Chinese cities. Ecotoxicol. Environ. Saf. 2020. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Fu, S.; Xu, X.; Li, L.; Ma, Y.; Zhou, J.; Yao, J.; Liu, X.; Zhang, X.; et al. An effect assessment of Airborne par-ticulate matter pollution on COVID−19: A multi-city Study in China. medRxiv 2020. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, J.; Wang, W.; Liu, Z.; Kan, H.; Qiu, Y.; Meng, X.; Wang, W. Association of particulate matter pollution and case fatality rate of COVID−19 in 49 Chinese cities. Sci. Total Environ. 2020, 741, 140396. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J. COVID−19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources. Environ. Pollut. 2020, 265, 115126. [Google Scholar] [CrossRef]
- EEA. European Environment Agency—Air quality in Europe—2018. Available online: http://www.eea.europa.eu/publications/air-quality-in-europe−2018/ (accessed on 10 April 2020).
- Conticini, E.; Frediani, B.; Caro, D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV−2 lethality in Northern Italy? Environ. Pollut. 2020. [Google Scholar] [CrossRef] [PubMed]
- Piccolomini, E.L.; Zama, F. Monitoring Italian COVID−19 spread by a forced SEIRD model. PLoS ONE 2020, 15, e0237417. [Google Scholar] [CrossRef] [PubMed]
- Milan, S.; Treré, E. A widening data divide: COVID−19 and the Global South. Available online: https://data-activism.net/2020/04/bigdatasur-a-widening-data-divide-covid−19-and-the-global-south/ (accessed on 31 April 2020).
- Singh, P.; Dey, S.; Purohit, B.; Dixit, K.; Chakraborty, S. Robust association between short-term ambient PM2.5 exposure and COVID prevalence in India. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Kumar, S. Effect of meteorological parameters on spread of COVID−19 in India and air quality during lockdown. Sci. Total Environ. 2020, 745, 141021. [Google Scholar] [CrossRef]
- Velásquez, R.M.A.; Lara, J.V.M. Gaussian approach for probability and correlation between the number of COVID−19 cases and the air pollution in Lima. Urban Clim. 2020, 33, 100664. [Google Scholar] [CrossRef] [PubMed]
- Bolaño-Ortiz, T.R.; Camargo-Caicedo, Y.; Puliafito, S.E.; Ruggeri, M.F.; Bolaño-Diaz, S.; Pascual-Flores, R.; Saturno, J.; Ibarra-Espinosa, S.; Mayol-Bracero, O.L.; Torres-Delgado, E.; et al. Spread of SARS-CoV−2 through Latin America and the Caribbean region: A look from its economic conditions, climate and air pollution indicators. Environ. Res. 2020, 191, 109938. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.F.; Jalaludin, J.; Latif, M.T. Demystifying a Possible Relationship between COVID−19, Air Quality and Meteorological Factors: Evidence from Kuala Lumpur, Malaysia. Aerosol Air Qual. Res. 2020, 20, 1520–1529. [Google Scholar] [CrossRef]
- Notari, A.; Torrieri, G. COVID−19 transmission risk factors. arXiv Preprint 2020, arXiv:2005.03651. [Google Scholar]
- Forster, P.; Forster, L.; Renfrew, C.; Forster, M. Phylogenetic network analysis of SARS-CoV−2 genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 9241–9243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovanetti, M.; Angeletti, S.; Benvenuto, D.; Ciccozzi, M. A doubt of multiple introduction of SARS-CoV-2 in Italy: A preliminary overview. J. Med Virol. 2020, 92, 1634–1636. [Google Scholar] [CrossRef]
- Luo, C.; Yao, L.; Zhang, L.; Yao, M.; Chen, X.; Wang, Q.; Shen, H. Possible Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV−2) in a Public Bath Center in Huai’an, Jiangsu Province, China. JAMA Netw. Open 2020, 3, e204583. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Lloyd-Smith, J.O.; De Wit, E.; Munster, V.J.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; et al. Aerosol and Surface Stability of SARS-CoV−2 as Compared with SARS-CoV−1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Licen, S.; Perrone, M.G.; Piazzalunga, A.; Borelli, M.; Palmisani, J.; Di Gilio, A.; et al. Potential role of particulate matter in the spreading of COVID−19 in Northern Italy: First observational study based on initial epidemic diffusion. BMJ Open 2020, 10, e039338. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV−2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Setti, L.; Passarini, F.; De Gennaro, G.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Di Gilio, A.; Torboli, V.; Fontana, F.; et al. SARS-Cov−2RNA found on particulate matter of Bergamo in Northern Italy: First evidence. Environ. Res. 2020, 188, 109754. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M. An index to quantify environmental risk of exposure to future epidemics of the COVID−19 and similar viral agents: Theory and practice. Environ. Res. 2020, 191, 110155. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Goldberg, M.S. Methodological Considerations for Epidemiological Studies of Air Pollution and the SARS and COVID−19 Coronavirus Outbreaks. Environ. Heal. Perspect. 2020, 128, 095001. [Google Scholar] [CrossRef]
- Patz, J.A.; Hahn, M.B. Climate Change and Human Health: A One Health Approach. In One Health: The Human–Animal–Environment Interfaces in Emerging Infectious Diseases; Mackenzie, J.S., Jeggo, M., Daszak, P., Richt, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 366, pp. 141–171. [Google Scholar]
- Poland, G.A. SARS-CoV−2: A time for clear and immediate action. Lancet Infect. Dis. 2020, 20, 531–532. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P. What can COVID−19 teach us about responding to climate change? Lancet Planet. Heal. 2020, 4, e174. [Google Scholar] [CrossRef]
- Okello, A.L.; Vandersmissen, A.; Welburn, S.C. One Health into action: Integrating Global Health Governance with national priorities in a globalized world. In One Health: The Theory and Practice of Integrated Health Approaches; CABI Publishing: Wallingford, UK, 2015; pp. 283–303. [Google Scholar]
- Atlas, R.M.; Maloy, S. One Health: People, Animals, and the Environment; ASM Press: Washington, CD, USA, 2014. [Google Scholar] [CrossRef]
- Editorial. Prevent and predict. Nat. Ecol. Evol. 2020, 4, 283. Available online: https://www.nature.com/articles/s41559-020-1150-5.pdf (accessed on 10 April 2020). [CrossRef] [Green Version]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.D.M.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.E.; Jones, K.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nat. Cell Biol. 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Johnson, C.K.; Hitchens, P.L.; Pandit, P.S.; Rushmore, J.; Evans, T.S.; Young, C.C.W.; Doyle, M.M. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc. R. Soc. B Boil. Sci. 2020, 287, 20192736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabi, G.; Siddique, R.; Ali, A.; Khan, S. Preventing bat-born viral outbreaks in future using ecological interventions. Environ. Res. 2020, 185, 109460. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Owers, K.A.; Bordes, F. Biodiversity and Emerging Zoonoses. In Confronting Emerging Zoonoses; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; pp. 27–41. [Google Scholar]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nat. Cell Biol. 2020, 584, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.; Farrar, J.; Ihekweazu, C.; Kang, G.; Koopmans, M.; Nkengasong, J. A new twenty-first century science for effective epidemic response. Nat. Cell Biol. 2019, 575, 130–136. [Google Scholar] [CrossRef]
- Ahmed, F.; Ahmed, N.; Pissarides, C.; Stiglitz, J. Why inequality could spread COVID−19. Lancet Public Heal. 2020, 5, e240. [Google Scholar] [CrossRef]


| Measuring Unit | Time Period | Format | Source (Access Date Same As Time Period) | |
|---|---|---|---|---|
| COVID-19 | ||||
| China | Infections, Deaths | Until 23 May 2020 | Tabular Prefecture level | DXY—DX Doctor: http://ncov.dxy.cn/ncovh5/view/en_pneumonia Chinese government health commission |
| Italy | Infections, Deaths | Until 22 May 2020 | Tabular Province and region levels | Github repository: https://github.com/pcm-dpc/COVID-19 Dipartimento della Protezione Civile: http://www.protezionecivile.it/ |
| US | Infections, Deaths | Until 21 May 2020 | Tabular County level | The New York Times Github repository: https://github.com/nytimes/covid-19-data |
| Iran | Infections | Until 22 Mar 2020 | Tabular Province level | IRNA–The Islamic Republic News Agency: https://en.irna.ir/photo/83723991/Iran-s-coronavirus-toll-update-March-22-2020 |
| France | Deaths | Until 22 May 2020 | Tabular Department level | Open Data Platform of the French Government: https://www.data.gouv.fr/fr/datasets/chiffres-cles-concernant-lepidemie-de-covid19-en-france/#_ |
| Spain | Infections, Deaths | Until 2 May 2020 | Tabular Province level | Data from Spanish Ministry of Health. Github: https://github.com/Secuoyas-Experience/covid-19-es |
| Germany | Infections, Deaths | Until 25 May 2020 | Tabular District level | Robert Koch Institut: https://www.rki.de/EN/Home/homepage_node.html |
| UK | Infections, Deaths | Until ca. 1 June 2020 (infections) 24 May 2020 (deaths) | Tabular LTLA/NHS level | Several government sources: https://coronavirus.data.gov.uk/, https://phw.nhs.wales/, https://www.ons.gov.uk/, https://www.nrscotland.gov.uk/, https://www.health-ni.gov.uk/ |
| Population | ||||
| China | No. of residents | Estimates 2017 | Tabular Prefecture level | https://www.citypopulation.de/ Data from Province Governments |
| Italy | 2019 | Tabular Province level | Istat—Italian National Institute of Statistics http://dati.istat.it/ | |
| US | Estimates 2018 | Tabular County level | US Census Bureau (on ESRI ArcGIS): https://www.arcgis.com/home/item.html?id=a00d6b6149b34ed3b833e10fb72ef47b | |
| Iran | 2016 | Tabular Province level | Statistical Center of Iran: https://www.amar.org.ir/ | |
| France | Estimates 2020 | Tabular Department level | Insee—French National Institute of Statistics: https://www.insee.fr/ | |
| Spain | 2019 | Tabular Province level | INE—Spanish National Institute of Statistics: https://www.ine.es/en/index.htm | |
| Germany | Estimates 2018 | Tabular District level | Database of the Federal Statistic Office: https://www-genesis.destatis.de/ | |
| UK | Estimates 2018 | Tabular LTLA/NHS level | U.K. Office of National Statistics https://www.ons.gov.uk/ | |
| Air Quality (ground measures) | ||||
| China PM 2.5, PM 10, O3, NO2, SO2, CO | AQI | 2014 | Tabular GPS points | University of Harvard Dataverse: https://dataverse.harvard.edu Data from http://aqicn.org |
| Italy PM 2.5, PM 10 | μg/m3 | Annual 2013-2016 | Tabular Location name | Ambient Air Quality Database, WHO, April 2018 https://www.who.int/airpollution/data/cities/en/ |
| US PM 2.5, PM 10, O3, NO2, SO2, CO | μg/m3 ppm, ppb | 2019 | Tabular GPS points | EPA—United States Environmental Protection Agency https://www.epa.gov/outdoor-air-quality-data |
| Air Quality (satellite) | ||||
| PM 2.5 | μg/m3 | Annual 1998-2016 | Continuous grid (0.01 arc deg.) | Global Annual PM 2.5 Grids from MODIS, MISR and SeaWiFS Aerosol Optical Depth (AOD) with GWR, v1 https://doi.org/10.7927/H4ZK5DQS |
| NO2 | ppb | 3-year running means (1996-2012) | Continuous grid (0.1 arc deg.) | Global 3-Year Running Mean Ground-Level NO2 Grids from GOME, SCIAMACHY and GOME-2, v1 (1996–2012) https://doi.org/10.7927/H4JW8BTT |
| China | US | Italy (Provinces) | Iran | Spain | Germany | UK | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | |
| population | 337 | 0.23 | <0.001 | 3102 | 0.26 | <0.001 | 105 | 0.00 | 0.951 | 29 | −0.15 | 0.250 | 41 | −0.27 | 0.010 | 399 | 0.03 | 0.317 | 362 | 0.18 | <0.001 |
| pop. dens | 337 | 0.32 | <0.001 | 3102 | 0.30 | <0.001 | 105 | 0.12 | 0.078 | 29 | 0.14 | 0.279 | 41 | −0.33 | 0.002 | 399 | 0.10 | 0.002 | 362 | 0.21 | <0.001 |
| PM 2.5 sat | 337 | 0.28 | <0.001 | 3102 | 0.25 | <0.001 | 105 | 0.62 | <0.001 | 29 | 0.24 | 0.061 | 41 | −0.03 | 0.778 | 399 | −0.07 | 0.046 | 362 | −0.03 | 0.386 |
| NO2 sat | 337 | 0.24 | <0.001 | 3101 | 0.22 | <0.001 | 105 | 0.55 | <0.001 | 29 | 0.40 | <0.001 | 41 | 0.08 | 0.470 | 399 | −0.03 | 0.375 | 360 | 0.06 | 0.086 |
| PM 2.5 gr | 302 | 0.15 | <0.001 | 427 | 0.21 | <0.001 | 88 | 0.34 | <0.001 | ||||||||||||
| PM 10 gr | 302 | 0.04 | 0.330 | 201 | 0.14 | 0.004 | 99 | 0.11 | 0.096 | ||||||||||||
| CO gr | 302 | −0.01 | 0.840 | 156 | 0.18 | 0.001 | |||||||||||||||
| NO2 gr | 302 | 0.12 | 0.002 | 246 | 0.41 | <0.001 | |||||||||||||||
| O3 gr | 302 | −0.03 | 0.477 | 749 | 0.03 | 0.238 | |||||||||||||||
| SO2 gr | 302 | −0.01 | 0.843 | 314 | −0.12 | 0.002 | |||||||||||||||
| China | US | Italy (Regions) | France | Spain | Germany | UK | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | |
| population | 337 | 0.17 | <0.001 | 3102 | 0.36 | <0.001 | 19 | 0.01 | 0.976 | 94 | 0.17 | 0.015 | 41 | −0.25 | 0.019 | 399 | 0.03 | 0.409 | 362 | 0.13 | <0.001 |
| pop. dens | 337 | 0.16 | <0.001 | 3102 | 0.36 | <0.001 | 19 | 0.16 | 0.323 | 94 | 0.24 | <0.001 | 41 | −0.40 | <0.001 | 399 | 0.05 | 0.153 | 362 | 0.29 | <0.001 |
| infect 100k | 337 | 0.39 | <0.001 | 3102 | 0.55 | <0.001 | 19 | 0.83 | <0.001 | . | . | . | 41 | 0.81 | <0.001 | 399 | 0.65 | <0.001 | 362 | 0.46 | <0.001 |
| PM 2.5 sat | 337 | 0.18 | <0.001 | 3102 | 0.24 | <0.001 | 19 | 0.60 | <0.001 | 94 | 0.56 | <0.001 | 41 | −0.09 | 0.385 | 399 | −0.04 | 0.241 | 362 | 0.16 | <0.001 |
| NO2 sat | 337 | 0.16 | <0.001 | 3101 | 0.26 | <0.001 | 19 | 0.51 | <0.001 | 94 | 0.57 | <0.001 | 41 | 0.08 | 0.470 | 399 | −0.05 | 0.118 | 360 | 0.23 | <0.001 |
| PM 2.5 gr | 302 | 0.18 | <0.001 | 427 | 0.24 | <0.001 | 17 | 0.22 | 0.183 | ||||||||||||
| PM 10 gr | 302 | 0.12 | 0.006 | 201 | 0.18 | <.001 | 19 | 0.00 | 1.00 | ||||||||||||
| CO gr | 302 | 0.11 | 0.012 | 156 | 0.20 | <.001 | |||||||||||||||
| NO2 gr | 302 | 0.12 | 0.005 | 246 | 0.42 | <.001 | |||||||||||||||
| O3 gr | 302 | −0.02 | 0.585 | 749 | 0.03 | 0.173 | |||||||||||||||
| SO2 gr | 302 | 0.04 | 0.409 | 314 | −0.08 | 0.028 | |||||||||||||||
| China | US | Italy (Regions) | Spain | Germany | UK | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | df (N-2) | Tau | P Value | |
| PM 2.5 sat | 313 | 0.16 | <0.001 | 2904 | 0.17 | <0.001 | 19 | 0.45 | 0.004 | 41 | −0.09 | 0.408 | 399 | 0.00 | 0.987 | 361 | 0.25 | <0.001 |
| NO2 sat | 313 | 0.14 | 0.001 | 2904 | 0.20 | <0.001 | 19 | 0.34 | 0.031 | 41 | 0.13 | 0.205 | 399 | −0.07 | 0.047 | 360 | 0.20 | <0.001 |
| PM 2.5 gr | 285 | 0.18 | <0.001 | 418 | 0.18 | <0.001 | 17 | 0.07 | 0.674 | |||||||||
| PM 10 gr | 285 | 0.13 | 0.005 | 191 | 0.15 | 0.002 | 19 | 0.08 | 0.654 | |||||||||
| CO gr | 285 | 0.12 | 0.007 | 156 | 0.14 | 0.009 | ||||||||||||
| NO2 gr | 285 | 0.12 | 0.007 | 239 | 0.26 | <0.001 | ||||||||||||
| O3 gr | 285 | −0.03 | 0.482 | 738 | 0.02 | 0.435 | ||||||||||||
| SO2 gr | 285 | 0.06 | 0.178 | 309 | 0.00 | 0.925 | ||||||||||||
| Country | Pollutants (G/S) | Correlation | Comment | References |
|---|---|---|---|---|
| US | PM 2.5 (G) | Positive | Additional cofactors studied | [97,98] |
| Italy | PM 2.5 (G), PM 10 (G), NO2 (G), etc | Positive | Additional cofactors studied | [76,99] |
| Spain, Germany, Italy, France | NO2 (S) | Positive | Differences between countries not considered | [96] |
| Netherlands | PM 2.5 (G) | Positive | Additional cofactors studied | [100,101] |
| Japan | PM 2.5 (G) | Positive | [102] | |
| India | PM 2.5 (G), NO2 (G), CO2 (G) | Positive | [103] | |
| Canada | PM 2.5 (G) | Positive | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pansini, R.; Fornacca, D. Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries. Atmosphere 2021, 12, 795. https://doi.org/10.3390/atmos12060795
Pansini R, Fornacca D. Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries. Atmosphere. 2021; 12(6):795. https://doi.org/10.3390/atmos12060795
Chicago/Turabian StylePansini, Riccardo, and Davide Fornacca. 2021. "Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries" Atmosphere 12, no. 6: 795. https://doi.org/10.3390/atmos12060795
APA StylePansini, R., & Fornacca, D. (2021). Early Spread of COVID-19 in the Air-Polluted Regions of Eight Severely Affected Countries. Atmosphere, 12(6), 795. https://doi.org/10.3390/atmos12060795






