Sensitivity of Chlorophyll Variability to Specific Growth Rate of Phytoplankton Equation over the Yangtze River Estuary in a Physical–Biogeochemical Model
Abstract
:1. Introduction
2. Data and Methodology
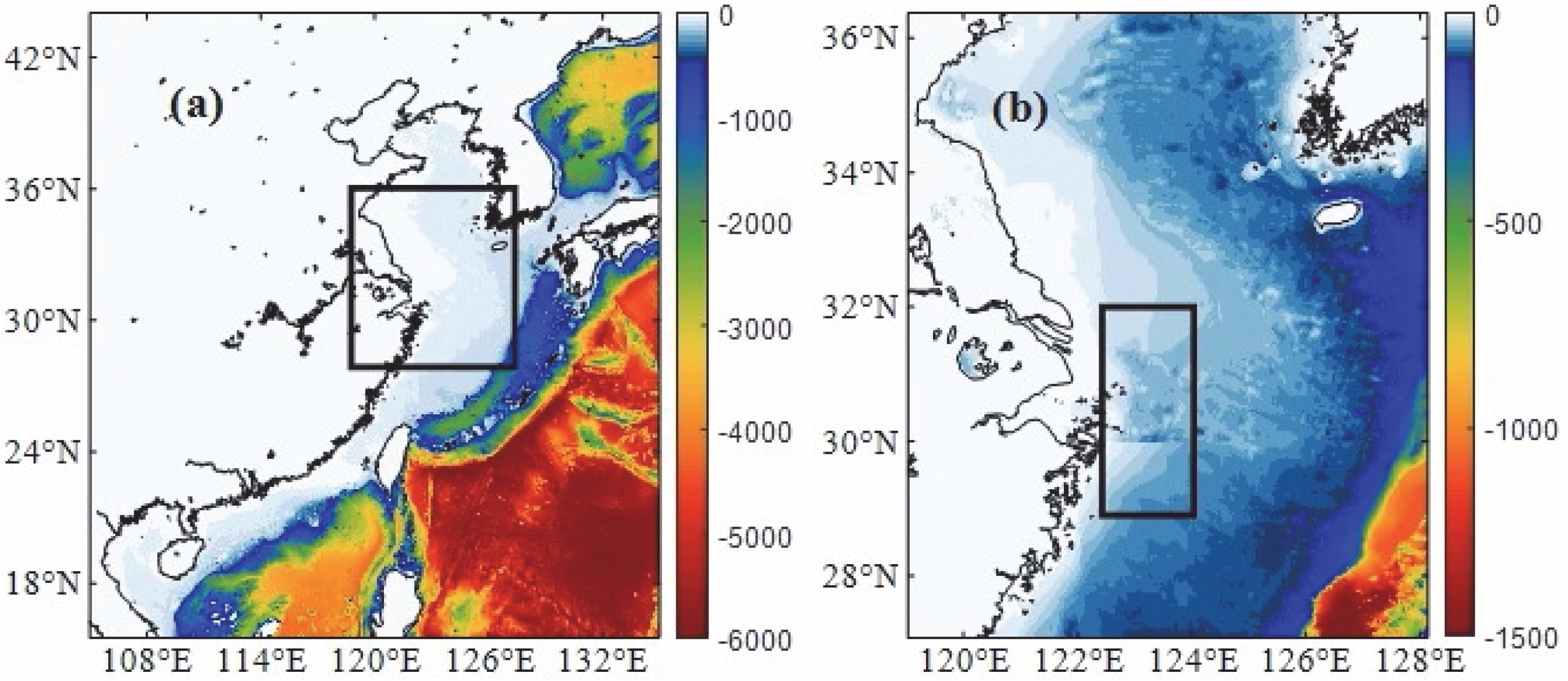
2.1. Model and Data
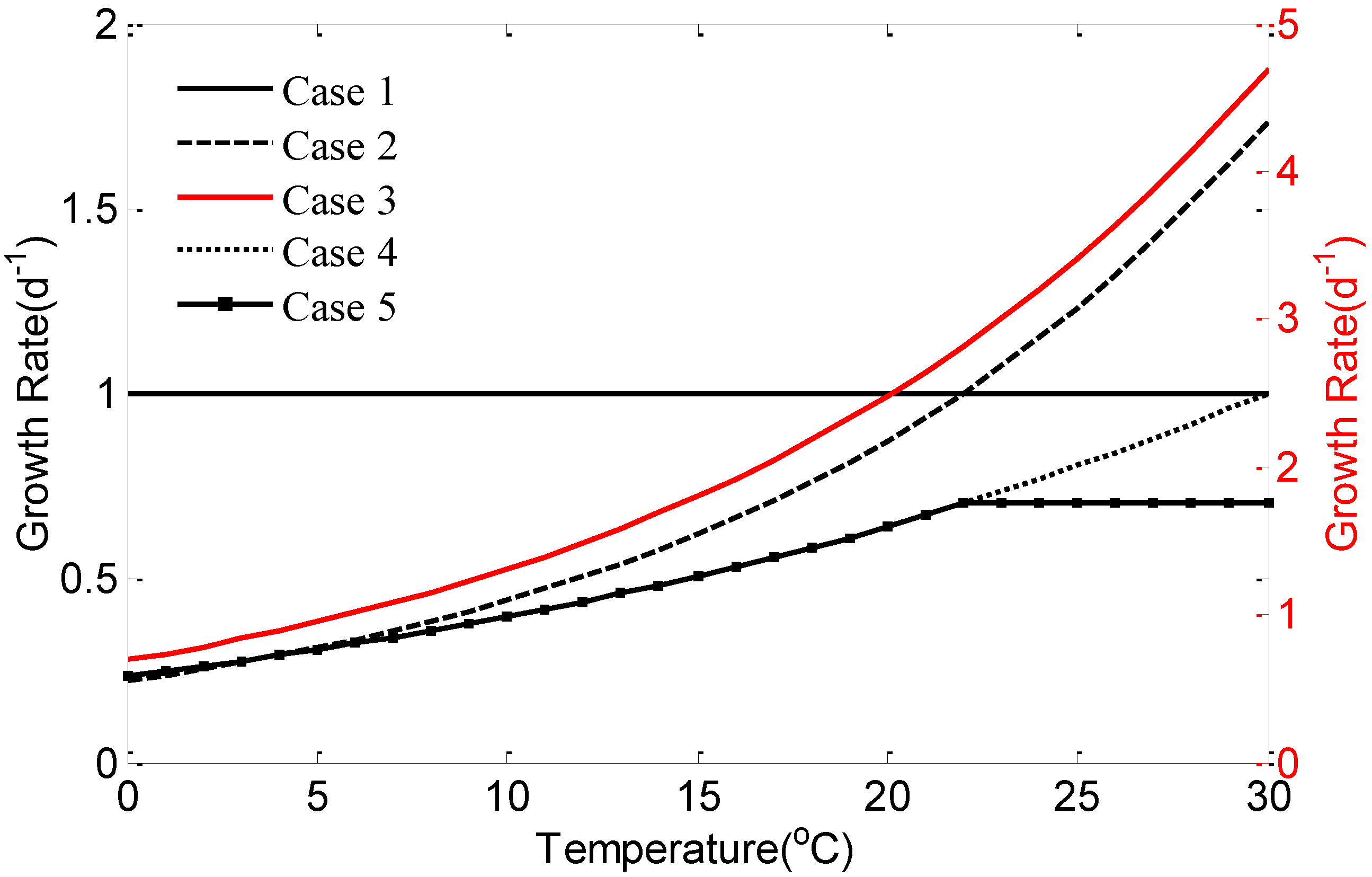
2.2. Experiment Design
3. Results
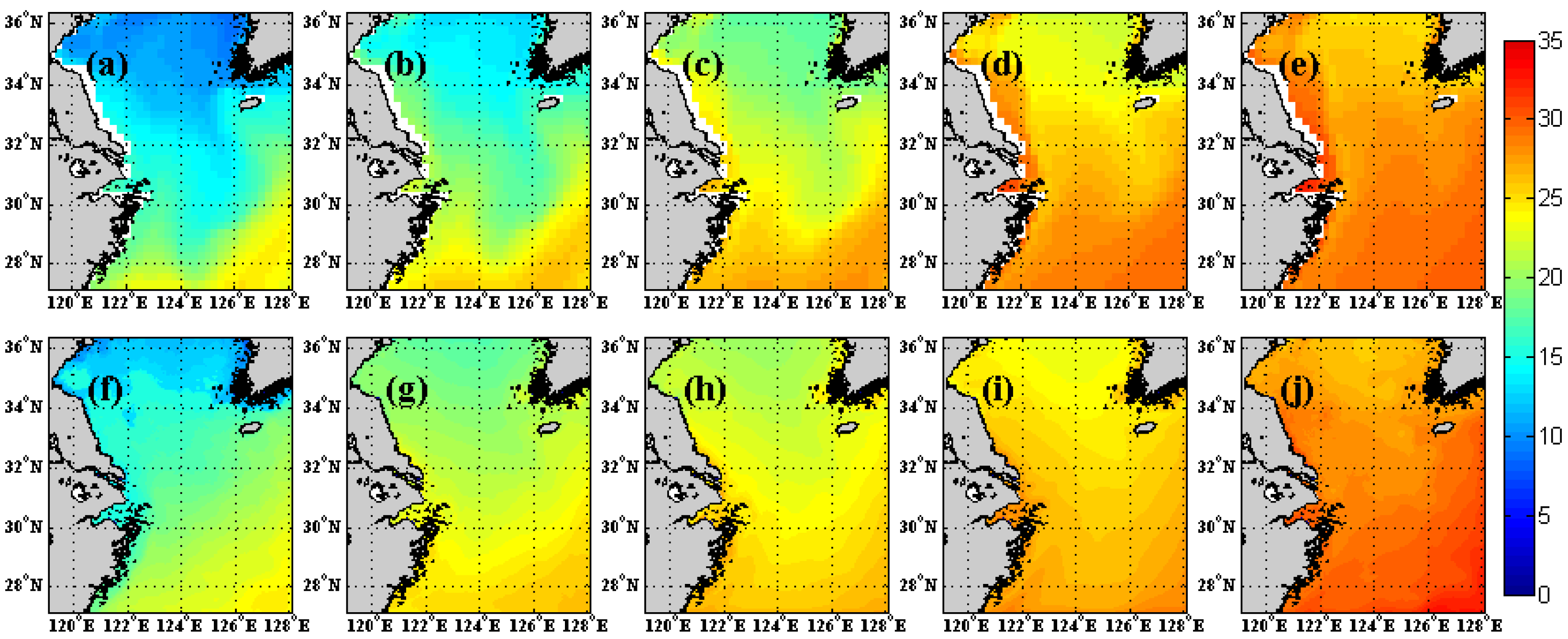
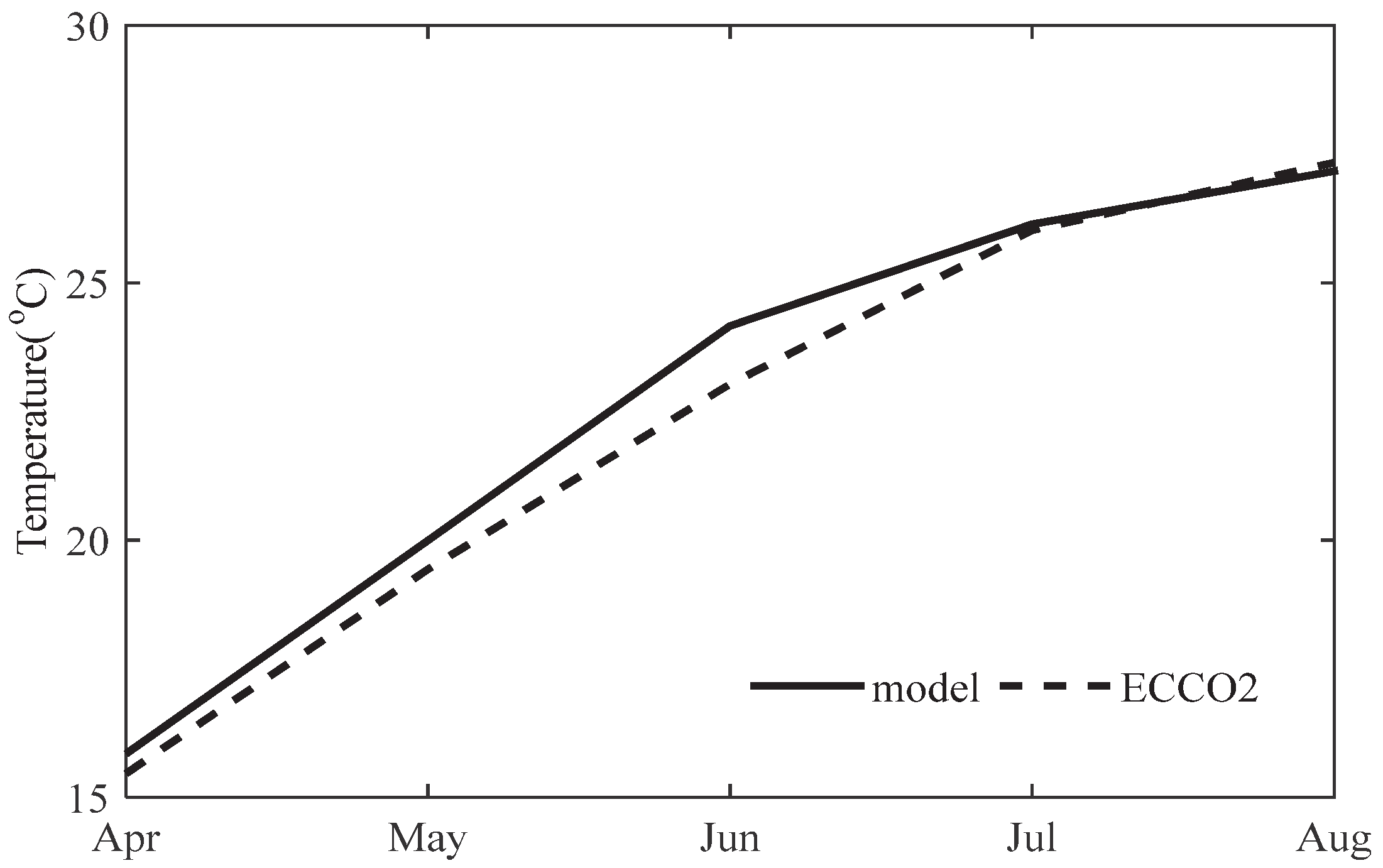
3.1. Comparison of SST with ECCO2 SST Product
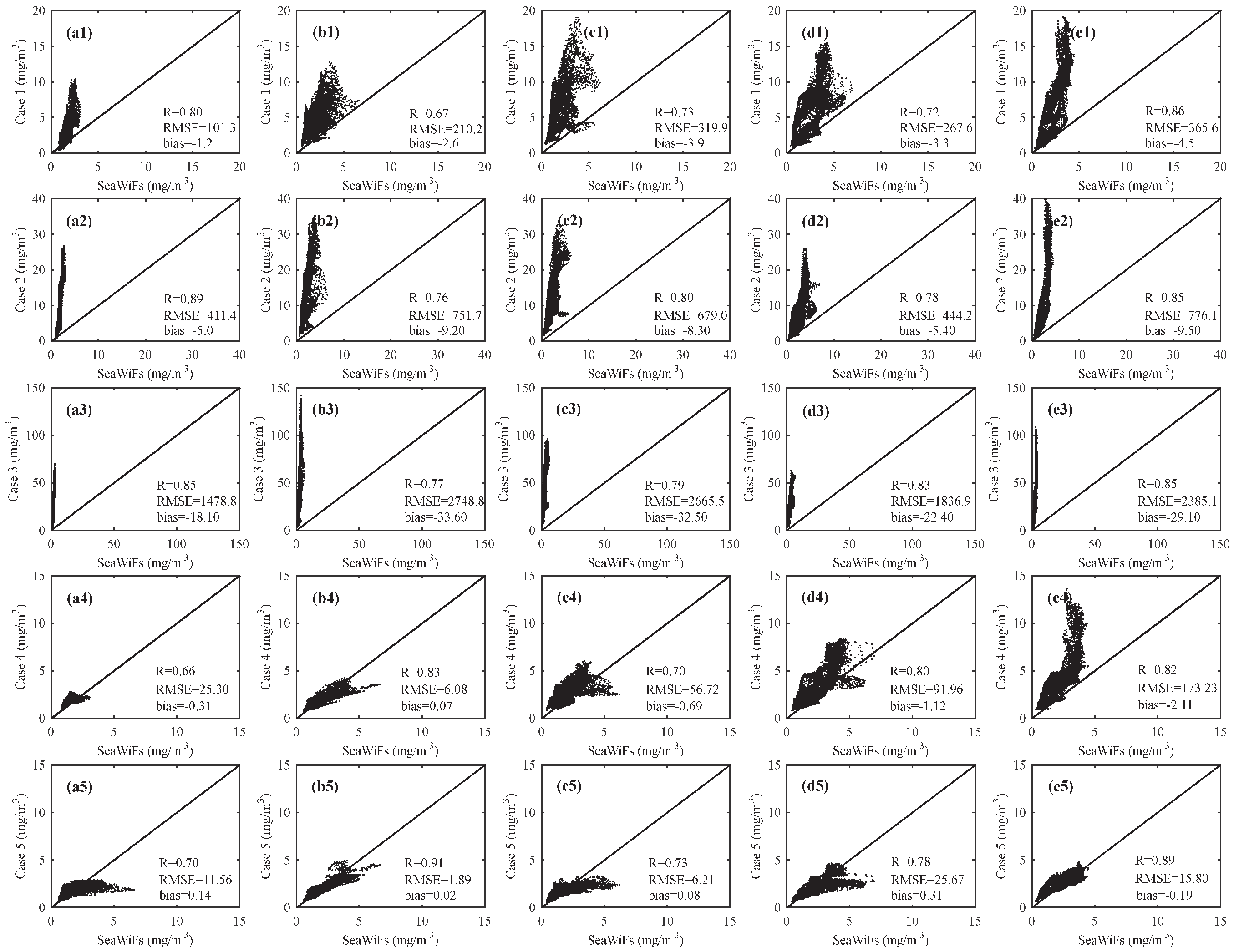
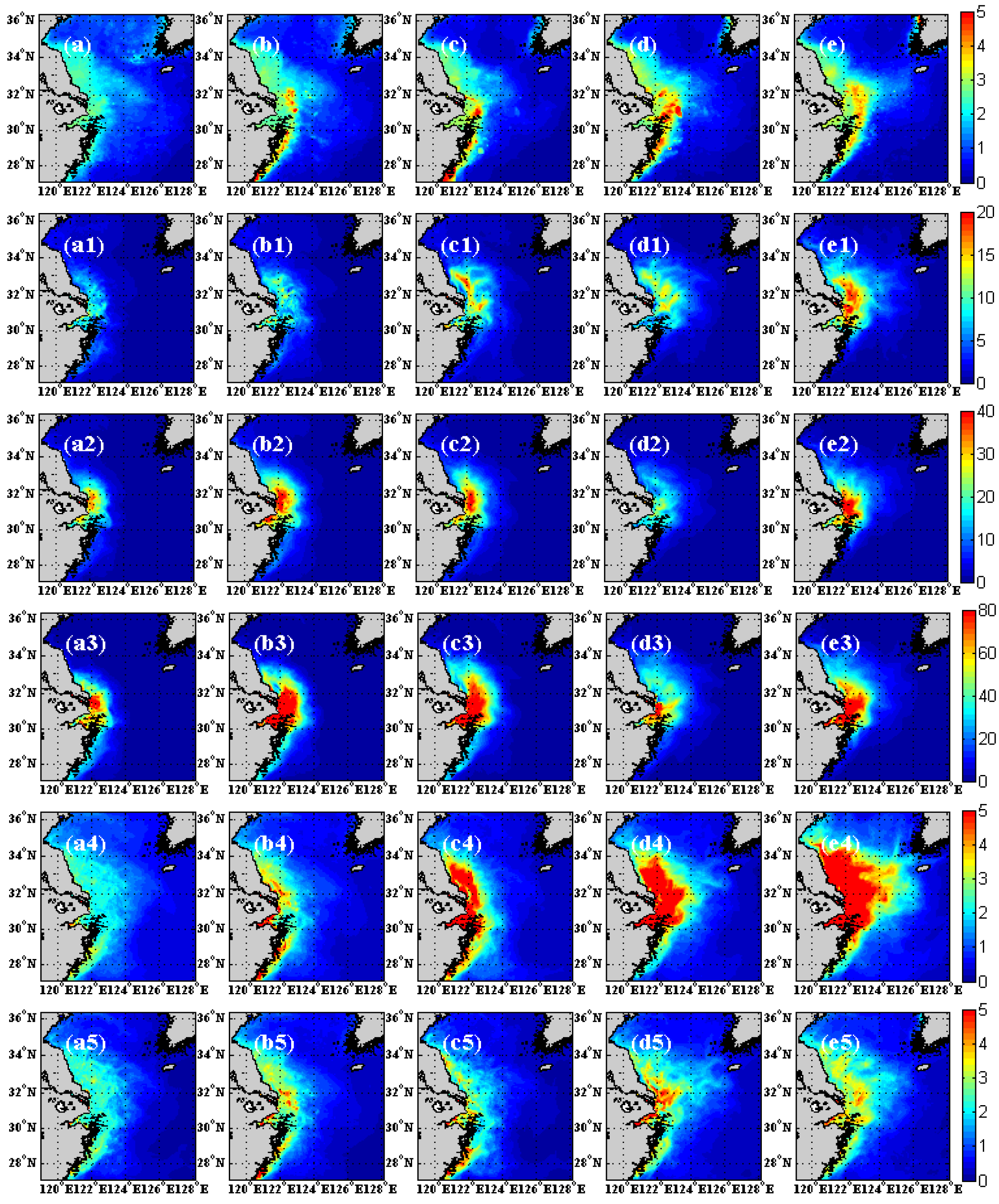
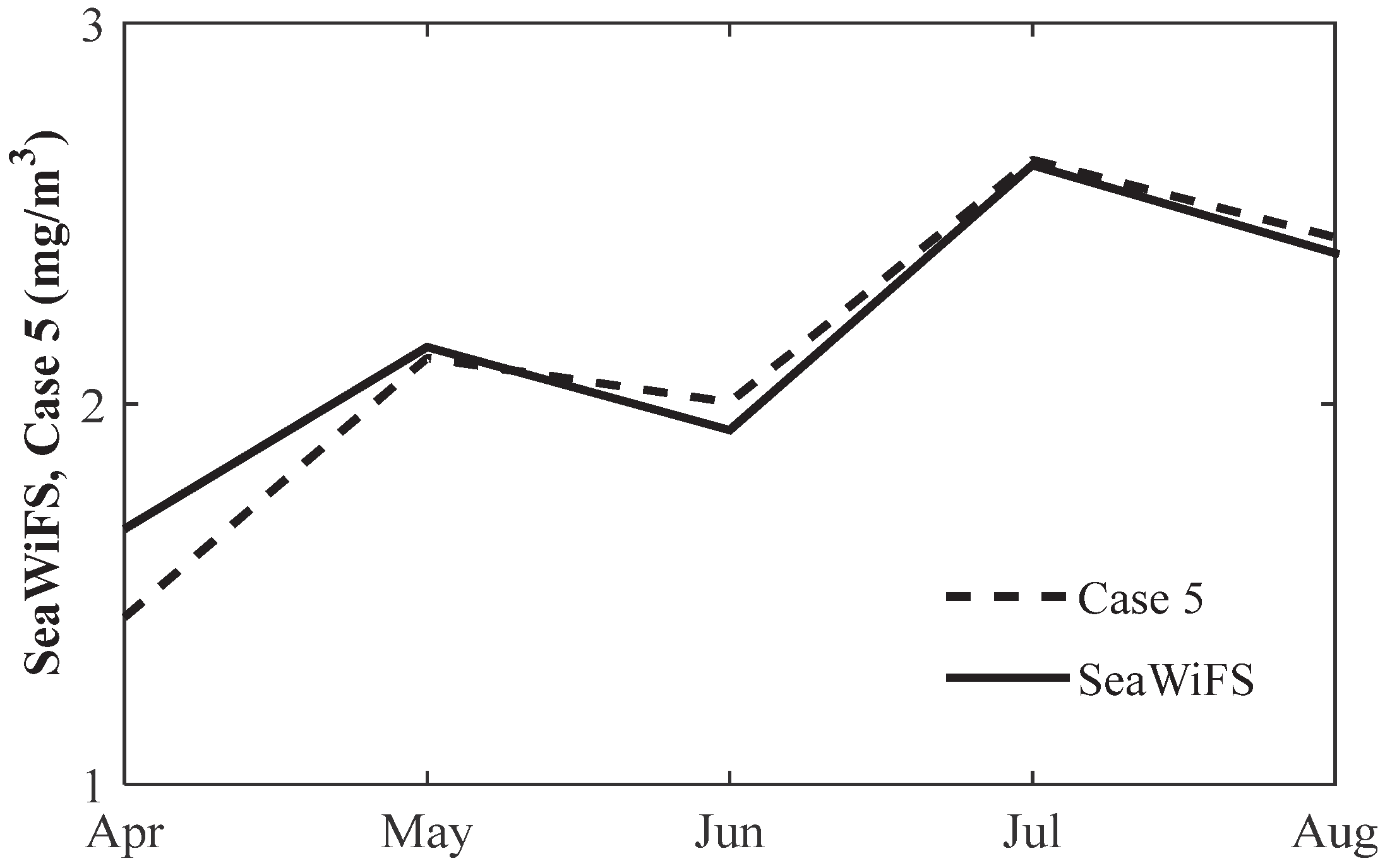
3.2. Comparison of Chlorophyll with Observation
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Agawin, N.; Duarte, C.M.; Agusti, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 2000, 45, 591–600. [Google Scholar] [CrossRef]
- Zinser, E.R.; Johnson, Z.I.; Coe, A. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol. Oceanogr. 2007, 52, 2205–2220. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Connor, D.J.; Thomann, R.V. A dynamic model of the phytoplankton population in the Sacramento-San Joaquin Delta. Adv. Chem. 1971, 106, 131–180. [Google Scholar]
- Mclaren, I.A. Effects of temperature on growth of zooplankton, and the adaptive value of vertical migration. J. Fish. Res. Board Can. 1963, 20, 685–727. [Google Scholar] [CrossRef]
- Eppley, R.W. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972, 70, 1063–1085. [Google Scholar]
- Goldman, J.G.; Carpenter, E.J. A kinetic approach to the effect of temperature on algal growth. Limnol. Oceanogr. 1974, 19, 756–766. [Google Scholar] [CrossRef]
- Brush, M.; Brawley, J.W.; Nixon, S.W. Modeling phytoplankton production: Problems with the Eppley curve and an empirical alternative. Mar. Ecol. Prog. Ser. 2002, 238, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Bissinger, J.E.; Montagnes, D.J.S.; Sharples, J. Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnol. Oceanogr. 2008, 53, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Moisan, J.R.; Moisan, T.A.; Abbott, M.R. Modelling the effect of temperature on the maximum growth rates of phytoplankton populations. Ecol. Modell. 2002, 153, 197–215. [Google Scholar] [CrossRef]
- Deng, J.; Huang, L.W.; Wu, G.X.; Yu, R.C. 3D fine resolution modeling of China yellow east sea’s circulation with complete forcing in winter. J. Wuhan Univ. Technol. 2003, 2, 161–165. [Google Scholar]
- Liu, X.Q.; Yin, B.S.; Hou, Y.J. The dynamic of circulation and temperature-salinity structure in the Changjiang mouth and its adjacent marine area, II Major characteristics of the circulation. Oceanol. Limnol. Sin. 2008, 4, 312–320. [Google Scholar]
- Chen, C.S.; Zhu, J.R.; Beardsley, R.C. Physical-biological sources for dense algal blooms near the Changjiang River. Geophys. Res. Lett. 2003, 30, 1515. [Google Scholar] [CrossRef] [Green Version]
- Shchepetkin, A.F.; McWilliams, J.C. The Regional Ocean Modeling System (ROMS): A split-explicit, free-surface, topography following coordinates ocean model. Ocean. Model. 2005, 9, 347–404. [Google Scholar] [CrossRef]
- Lvanov, L.M.; Collins, C.A.; Marchesiello, P. One model validation for meso/submesoscale currents: Metrics and application to ROMS off Central California. Ocean. Model. 2009, 28, 209–225. [Google Scholar]
- Chai, F.; Lindley, S.T.; Barber, R.T. Origin and maintenance of a high NO3 condition in the equatorial Pacific. Deep Sea Res. Part II 1996, 43, 1031–1064. [Google Scholar] [CrossRef]
- Chai, F.; Dugdale, R.C.; Peng, T.H. One-dimensional ecosystem model of the equatorial Pacific upwelling system. Part I: Model development and silicon and nitrogen cycle. Deep Sea Res. Part II 2002, 49, 2713–2745. [Google Scholar] [CrossRef]
- Xiu, P.; Chai, F. Modeled biogeochemical responses to mesoscale eddies in the South China. J. Geophys. Res. Oceans 2011, 116, C10. [Google Scholar] [CrossRef] [Green Version]
- Xiu, P.; Chai, F. Spatial and temporal variability in phytoplankton carbon, chlorophyll, and nitrogen in the North Pacific. J. Geophys. Res. Oceans 2012, 117, C11023. [Google Scholar] [CrossRef]
- Xiu, P.; Chai, F. Connections between physical, optical and biogeochemical processes in the Pacific Ocean. Prog. Oceanogr. 2014, 122, 30–53. [Google Scholar] [CrossRef]
- Garcia, H.E.; Locarnini, R.A.; Boyer, T.P. World Ocean Atlas 2005, Nutrients (Phosphate, Nitrate, Silicate); Government Printing Office: Washington, DC, USA, 2006; pp. 2–14. [Google Scholar]
- Kalnay, E.; Kanamitsu, M.; Kistler, R. The NCEP/NCAR 40-year reanalysis project. Bull. Am. Meteorol. Soc. 1996, 77, 437–471. [Google Scholar] [CrossRef]
- Kondo, J. Air-sea bulk transfer coefficients in diabatic conditions. Bound. Layer Meteorol. 1975, 9, 91–112. [Google Scholar] [CrossRef]
- Large, W.G.; Pond, S. Sensible and latent heat flux measurements over the ocean. J. Phys. Oceanogr. 1982, 12, 464–482. [Google Scholar] [CrossRef]
- Key, R.M.; Kozyr, A.; Sabine, C.L. A global ocean carbon climatology: Results from GLODAP. Global Biogeochem. Cycles 2004, 18, GB4031. [Google Scholar] [CrossRef]
- Marshall, J.C.; Adcroft, A.; Hill, C. A finite-volume, incompressible Navier Stokes model for studies of the ocean on parallel computers. J. Geophys. Res. Oceans 1997, 102, 5753–5766. [Google Scholar] [CrossRef]
- Wunsch, C.; Heimbach, P. Practical global ocean state estimation. Phys. D Nonlinear Phenom. 2007, 230, 197–208. [Google Scholar] [CrossRef]
- Wunsch, C.; Heimbach, P.; Ponte, R.M. The global general circulation of the ocean estimated by the ECCO-Consortium. Oceanography 2009, 22, 88–103. [Google Scholar] [CrossRef]
- Zhou, F.; Chai, F.; Huang, D.J. Investigation of hypoxia off the Changjiang Estuary using a coupled model of ROMS-CoSiNE. Prog. Oceanogr. 2017, 159, 237–254. [Google Scholar] [CrossRef]
- Zhang, J. Nutrient elements in large Chinese estuaries. Cont. Shelf Res. 1996, 16, 1023–1045. [Google Scholar] [CrossRef]
- Liu, X.C.; Shen, H.T.; Huang, Q.H. Concentration variation and flux estimation of dissolved inorganic nutrient from the Changjiang River into its estuary. Oceanol. Limnol. Sin. 2002, 33, 332–340. [Google Scholar]
- Shen, Z.L.; Liu, Q. Nutrients in the Changjiang River. Environ. Monit. Assess 2008, 153, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Yu, Z.M.; Shen, Z.L. Nutrient characteristics in the Yangtze River Estuary and the adjacent East China Sea before and after impoundment of the Three Gorges Dam. Sci. Total Environ. 2009, 407, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.Z.; Liu, R.Y. Construction of Three Gorge Dam Project and the Ecology and Environment in the Estuary Science Press; Beijing Publication: Beijing, China, 1994; p. 343. [Google Scholar]
- Domingues, R.B.; Barbosa, A.; Galvão, H. Nutrients, light and phytoplankton succession in a temperate estuary (the Guadiana, south-western Iberia). Estuar. Coast. Shelf Sci. 2005, 64, 249–260. [Google Scholar] [CrossRef]
- Furnas, M.; Mitchell, A.; Skuza, M. In the other 90%: Phytoplankton responses to enhanced nutrient availability in the Great Barrier Reef Lagoon. Mar. Pollut. Bull. 2005, 51, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W. Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecol. Eng. 2006, 26, 40–54. [Google Scholar] [CrossRef]
- Wang, X.C.; Chao, Y.; Zhang, H.C. Modeling tides and their influence on the circulation in Prince William Sound, Alaska. Cont. Shelf Res. 2013, 63, S126–S137. [Google Scholar] [CrossRef]
- Berdeal, I.B.; Hickey, B.M.; Kawase, M. Influence of wind stress and ambient flow on a high discharge river plume. J. Geophys. Res. Oceans 2002, 107, 3130. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.B.; Wang, J. Interannual variability and sensitivity study of the ocean circulation and thermohaline structure in Prince William Sound, Alaska. Cont. Shelf Res. 2004, 24, 393–411. [Google Scholar] [CrossRef]
- Mooers, C.N.K.; Wu, X.L.; Bang, I. Performance of a nowcast/forcast system for Prince William Sound, Alaska. Cont. Shelf Res. 2007, 29, 42–60. [Google Scholar] [CrossRef]
- Gong, C.G.; Wen, Y.H.; Wang, B.W. Seasonal variation of chlorophyll a concentration, primary production and environmental conditions in the subtropical East China Sea. Deep Sea Res. Part II 2003, 50, 1219–1236. [Google Scholar] [CrossRef]
- Geider, R.J.; MacIntyre, H.L.; Kana, T.M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 1998, 43, 679–694. [Google Scholar] [CrossRef]
- Moore, J.K.; Doney, S.C.; Kleypas, J.A. An intermediate complexity marine ecosystem model for the global domain. Deep Sea Res. 2002, 49, 403–462. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Boss, E.; Chai, F. The value of adding optics to ecosystem models: A case study. Biogeosciences 2007, 4, 817–835. [Google Scholar] [CrossRef] [Green Version]
- Li, W.K.W. Temperature Adaptation in Phytoplankton: Cellular and Photosynthetic Characteristics. Environ. Sci. Res. 1980, 19, 259–279. [Google Scholar]
- Li, Y.B. The Study of the Seasonal Occurrence Mechanism of HABs in the Changjiang Estuary and Its Adjacent Sea. Ph.D. Dissertation, Ocean University of China, Qingdao, China, 2008; pp. 68–70. [Google Scholar]
- Lin, J. A Modeling Study of the Phytoplankton Dynamics off the Changjiang Estuary. Ph.D. Dissertation, East China Normal University, Shanghai, China, 2011; pp. 38–39. [Google Scholar]
- Ratkowsky, D.A.; Lowry, R.K.; McMeekin, T.A. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J. Bacteriol. Res. 1983, 154, 1222–1226. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.J.; Yan, T.; Zou, J.Z. Preliminary analysis of the characteristics of red tide areas in Changjiang River Estuary and its adjacent sea. Chin. J. Appl. Ecol. 2003, 14, 1031–1038. [Google Scholar]
- Ye, S.F.; Ji, H.H.; Cao, L. Red tides in the Yangtze River Estuary and adjacent sea areas: Causes and mitigation. Mar. Sci. 2002, 28, 26–32. [Google Scholar]
- Chen, L.Y.L.; Chen, H.Y.; Gong, G.C. Phytoplankton production during a summer coastal upwelling in the East China Sea. Cont. Shelf Res. 2004, 24, 1321–1338. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Jin, J.X. Spatial-Temporal variations of chlorophyll in the adjacent sea area of the Yangtze River estuary influenced by Yangtze River Discharge. Int. J. Environ. Res. Public Health 2015, 12, 5420–5438. [Google Scholar] [CrossRef]
| Parameter Description | Value | Data Source |
|---|---|---|
| Nitrate (NO3)/mmol·m−3 Silicate (Si(OH)4)/mmol·m−3 Phosphate (PO4)/mmol·m−3 Oxygen (O2)/mmol·m−3 | World Ocean Atlas 2005 (WOA05) [21] | |
| Ammonium (NH4)/mmol·m−3 | 0.0001 mmol·m−3 | Xiu and Chai [20] |
| Total alkalinity (Talk)/mmol·m−3 | GLODAP dataset [25] | |
| Total CO2 (TCO2)/mmol·m−3 | 0.0001 mmol·m−3 | Xiu and Chai [20] |
| Semi-labile DOC (SDOC)/mmol·m−3 | surface to bottom: decreases according to a hyperbolic tangent function from 15 mmol·m−3 to 0.01 mmol·m−3. | |
| Labile DOC (LDOC)/mmol·m−3 | 0–500 m: 2.0 mmol·m−3; 500 m to bottom: 0.01 mmol·m−3. | |
| Colored labile dissolved organic carbon (CLDOC)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Labile DON (LDON)/mmol·m−3 | 9.95 | |
| Semi-labile DON (SDON)/mmol·m−3 | 15.38 | |
| Colored semi-labile (CSDOC)/mmol·m−3 | 0.4 | |
| Detritus-nitrogen (DDN)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Detritus-silicate (DDSI)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Detritus-carbon (DDC)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Bacteria nitrogen (BAC)/mmol·m−3 | surface to bottom: decreases according to a hyperbolic tangent function from 0.03 mmol·m−3 to 0.01 mmol·m−3. | |
| Small phytoplankton (S1)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Diatoms (S2)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Coccolithophorids (S3)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Chlorophyll in small phytoplankton (chl1)/mg·m−3 | 0.0001 mg·m−3 | |
| Chlorophyll in large phytoplankton (chl2)/mg·m−3 | 0.0001 mg·m−3 | |
| Coccolithophorids chlorophyll (chl3)/mg·m−3 | 0.0001 mg·m−3 | |
| Carbon in small phytoplankton (C1)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Carbon in large phytoplankton (C2)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Coccolithophorids carbon (C3)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Micro zooplankton (ZZ1)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Meso zooplankton (ZZ2)/mmol·m−3 | 0.0001 mmol·m−3 | |
| zz1-carbon (ZZC1)/mmol·m−3 | 0.0001 mmol·m−3 | |
| zz2-carbon (ZZC2)/mmol·m−3 | 0.0001 mmol·m−3 | |
| Particulate inorganic carbon (DDCA)/mmol·m−3 | 0.0001 mmol·m−3 |
| Cases | Specific Growth Rate of Phytoplankton Equations | Source |
|---|---|---|
| Case 1 | Q10 = ·1.0 | Eppley [6] |
| Case 2 | = ·e(0.069·(T-Topt)) | Zhou et al. [29] |
| Case 3 | = ·0.69·(1.066T) | Eppley [6] |
| Case 4 | = ·e(−4000.0·(1.0/(T+273.15)−1.0/303.15)) | Geider [43]; Moore et al. [44]; Fujii et al. [45] |
| Case 5 | = ·e(−4000.0·(1.0/(T+273.15)−1.0/303.15)) = ·e(−4000.0·(1.0/(Topt+273.15)−1.0/303.15)) (T > 22 °C) | Lin [47]; Li [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Wang, X.; Xiu, P.; Chai, F.; Chen, Z. Sensitivity of Chlorophyll Variability to Specific Growth Rate of Phytoplankton Equation over the Yangtze River Estuary in a Physical–Biogeochemical Model. Atmosphere 2022, 13, 1748. https://doi.org/10.3390/atmos13111748
Wu Q, Wang X, Xiu P, Chai F, Chen Z. Sensitivity of Chlorophyll Variability to Specific Growth Rate of Phytoplankton Equation over the Yangtze River Estuary in a Physical–Biogeochemical Model. Atmosphere. 2022; 13(11):1748. https://doi.org/10.3390/atmos13111748
Chicago/Turabian StyleWu, Qiong, Xiaochun Wang, Peng Xiu, Fei Chai, and Zhongxiao Chen. 2022. "Sensitivity of Chlorophyll Variability to Specific Growth Rate of Phytoplankton Equation over the Yangtze River Estuary in a Physical–Biogeochemical Model" Atmosphere 13, no. 11: 1748. https://doi.org/10.3390/atmos13111748





