Effect of Relative Humidity on the Rate of New Particle Formation for Different VOCs
Abstract
:1. Introduction
2. Materials and Methods
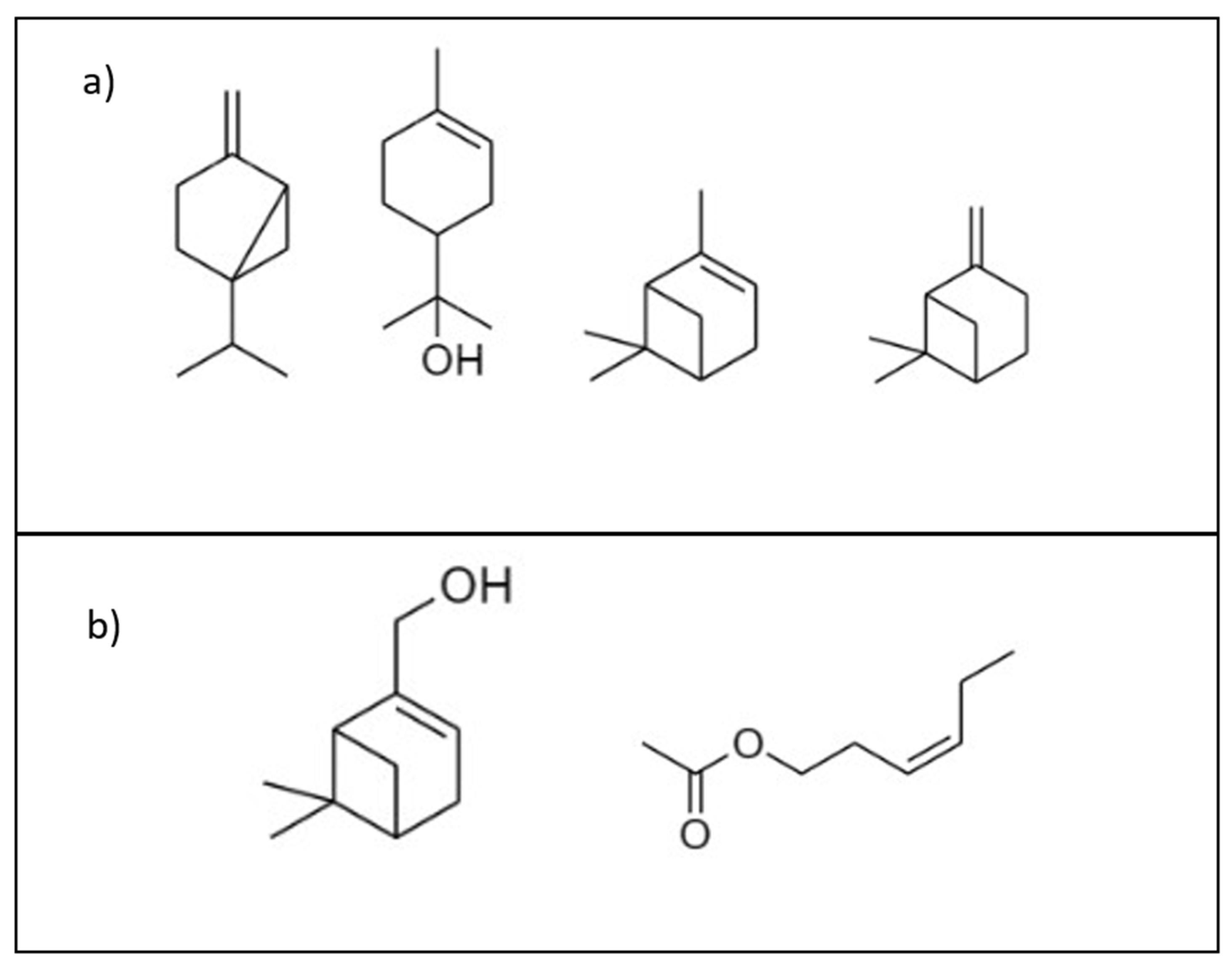
2.1. Reagents
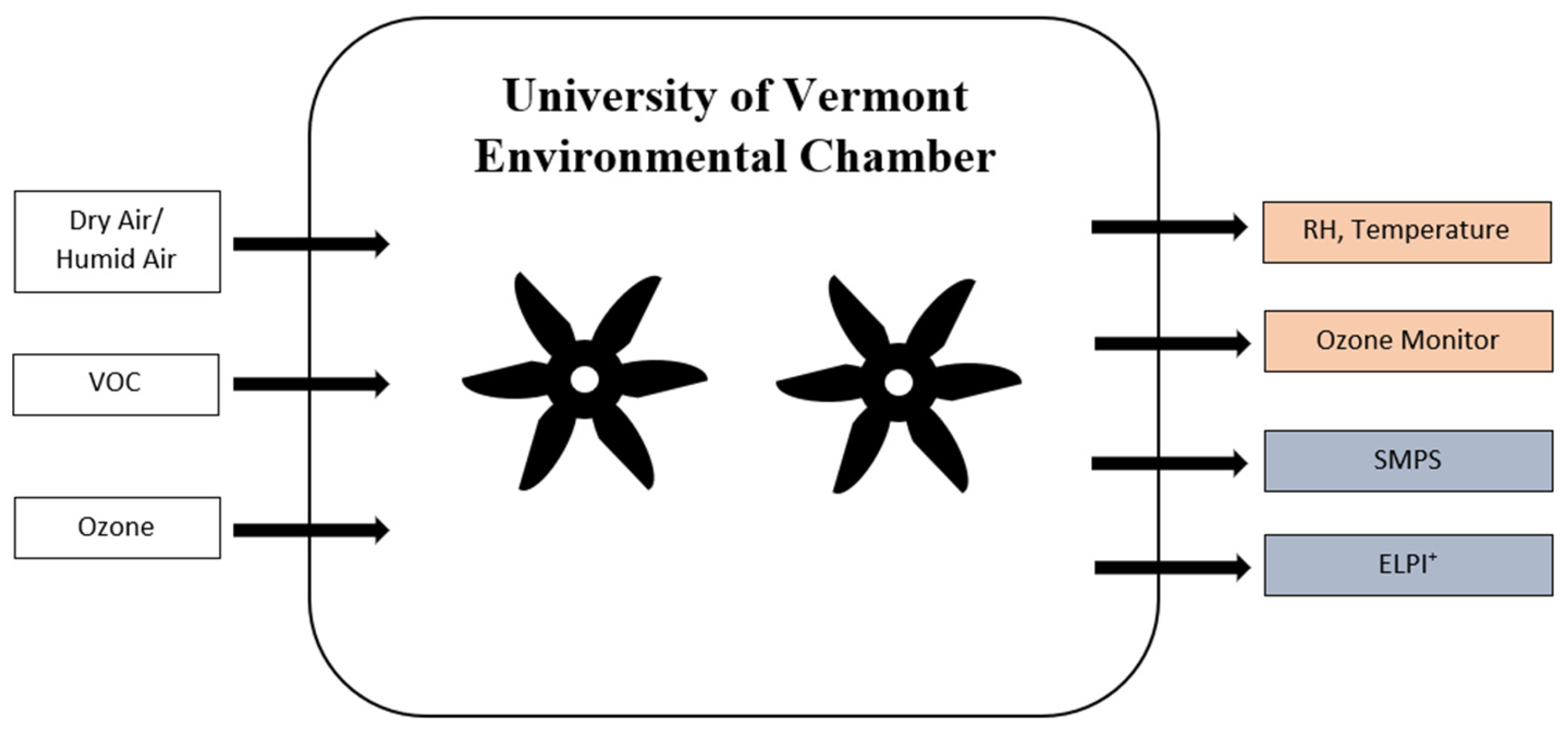
2.2. Instrumentation and Experimental Conditions
2.3. Data Analysis and Terminology
2.4. Methodology
3. Results and Discussion
3.1. Enhancement Factor of NPF (ΔNmax) for Sabinene, α-Terpineol, and Myrtenol as a Function of ξVOC
3.2. Addressing Solubility of VOCs
3.3. Effect of RH on the Rate of NPF for α-Pinene-Derived SOA
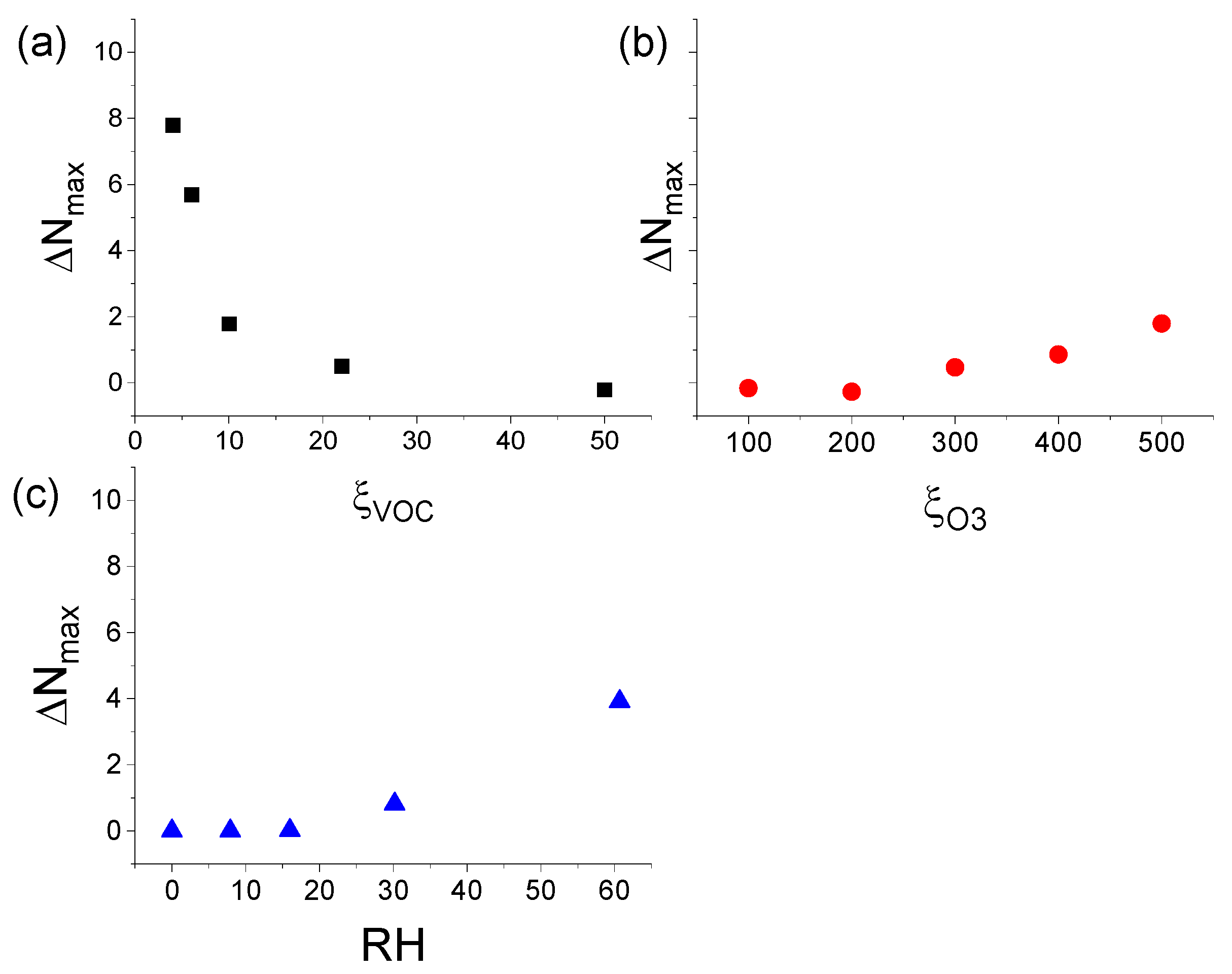
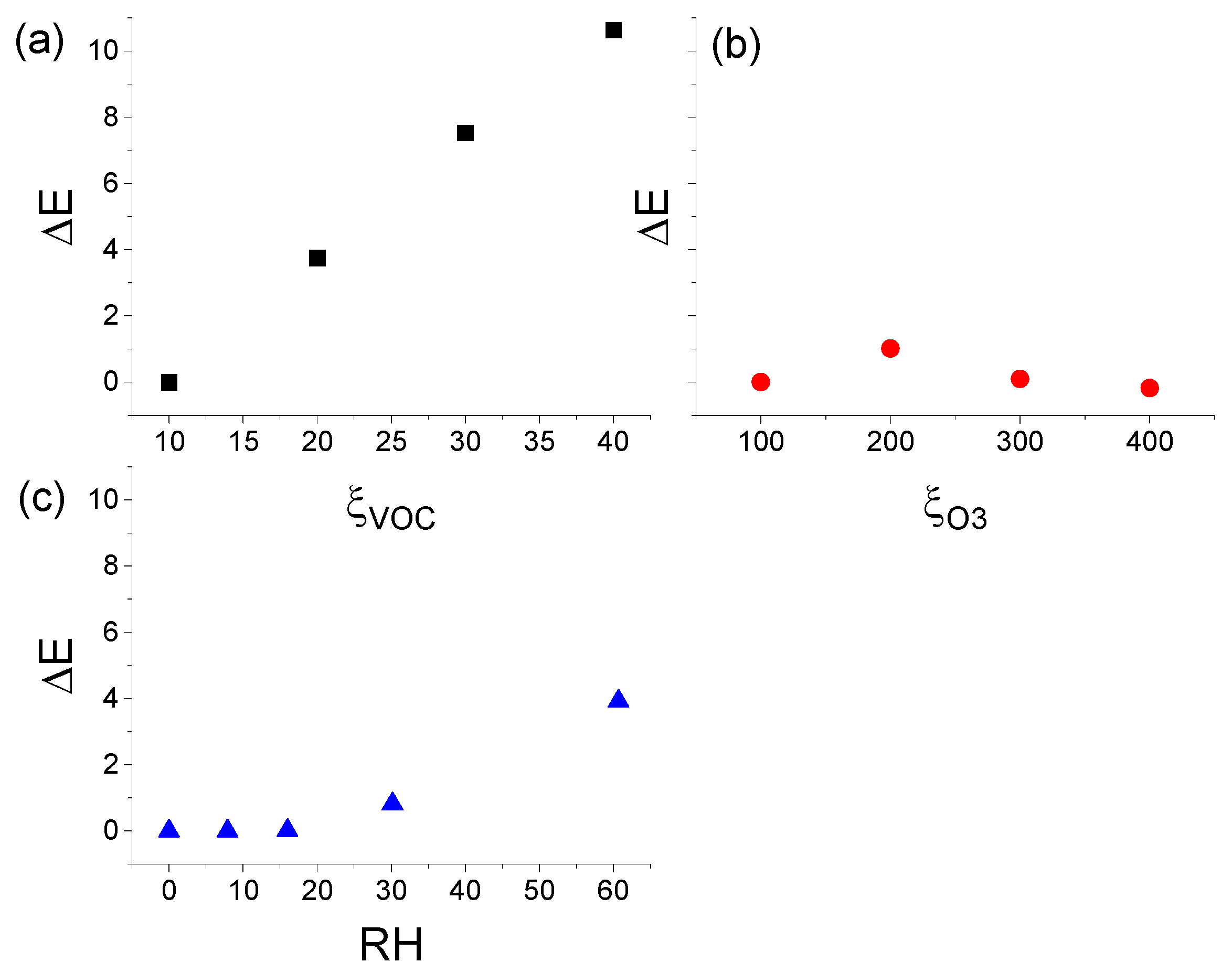
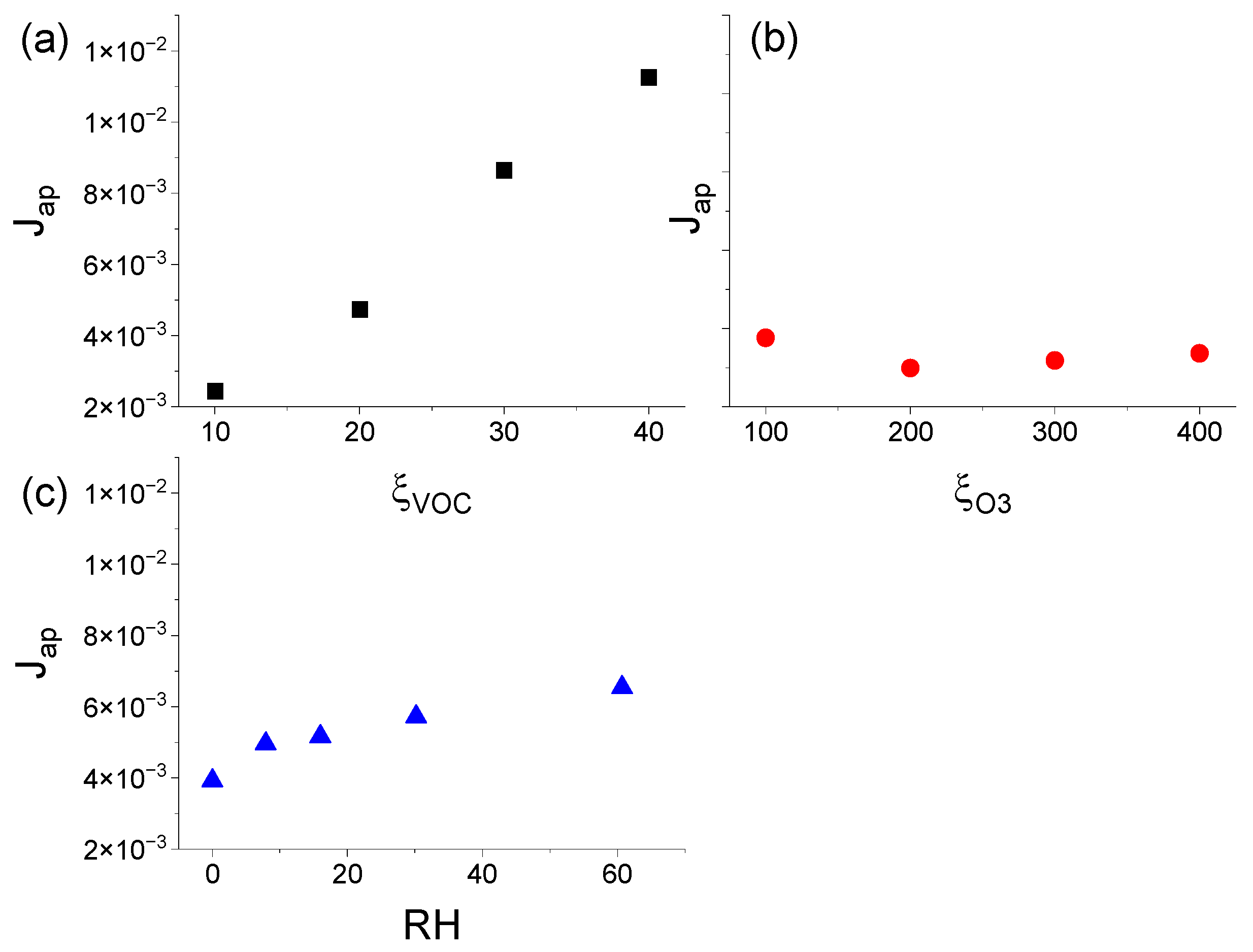
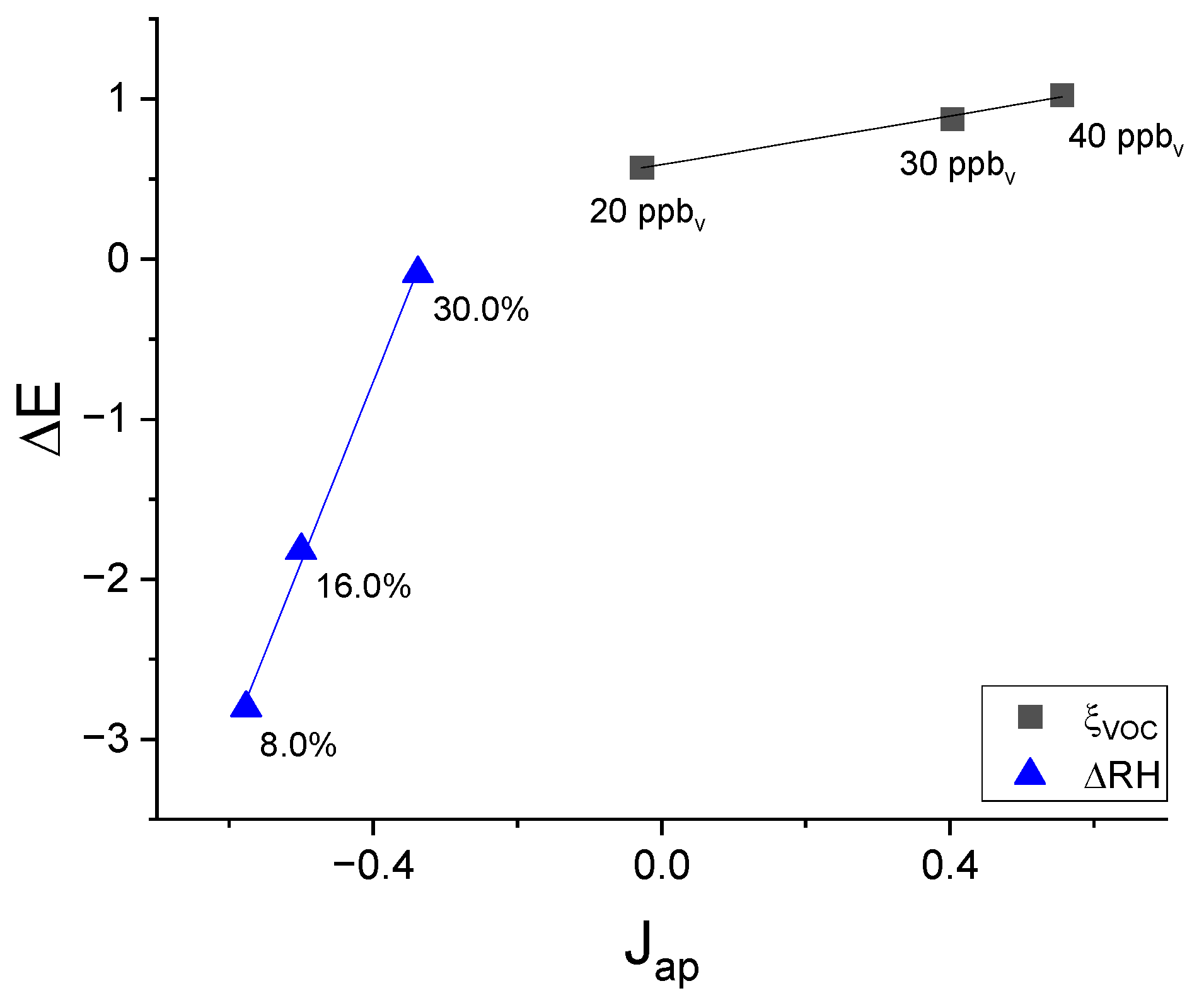
3.3.1. Effect of ξVOC, ξO3, and RH on ΔNmax
3.3.2. Effect of ξVOC, ξO3, and RH on ΔE
3.3.3. Effect of ξVOC, ξO3, and RH on Jap
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forster, P.; Storelvmo, T.; Armour, K.; Collins, W.; Dufresne, J.-L.; Frame, D.; Lunt, D.J.; Mauritsen, T.; Palmer, M.D.; Watanabe, M.; et al. Climate Change 2021—The Physical Science Basis: Working Group i, the Earth’s Energy Budget, Climate Feedbacks and Climate Sensitivity; IPCC: Geneva, Switzerland, 2023; pp. 923–1054. [Google Scholar]
- Zhang, C.Q.; Guo, Y.D.; Shen, H.R.; Luo, H.; Pullinen, I.; Schmitt, S.H.; Wang, M.J.; Fuchs, H.; Kiendler-Scharr, A.; Wahner, A.; et al. Contrasting Influence of Nitrogen Oxides on the Cloud Condensation Nuclei Activity of Monoterpene-Derived Secondary Organic Aerosol in Daytime and Nighttime Oxidation. Geophys. Res. Lett. 2023, 50, e2022GL102110. [Google Scholar] [CrossRef]
- Jo, D.S.; Nault, B.A.; Tilmes, S.; Gettelman, A.; Mccluskey, C.S.; Hodzic, A.; Henze, D.K.; Nawaz, M.O.; Fung, K.M.; Jimenez, J.L. Global Health and Climate Effects of Organic Aerosols from Different Sources. Environ. Sci. Technol. 2023, 57, 13793–13807. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Carlson, B.E.; Yung, Y.L.; Lv, D.R.; Hansen, J.; Penner, J.E.; Liao, H.; Ramaswamy, V.; Kahn, R.A.; Zhang, P.; et al. Scattering and absorbing aerosols in the climate system. Nat. Rev. Earth Environ. 2022, 3, 363–379. [Google Scholar] [CrossRef]
- Shrivastava, M.; Cappa, C.D.; Fan, J.; Goldstein, A.H.; Guenther, A.B.; Jimenez, J.L.; Kuang, C.; Laskin, A.; Martin, S.T.; Ng, N.L.; et al. Recent advances in understanding secondary organic aerosol: Implications for global climate forcing. Rev. Geophys. 2017, 55, 509–559. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipila, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Shrivastava, M.; Easter, R.C.; Liu, X.H.; Zelenyuk, A.; Singh, B.; Zhang, K.; Ma, P.L.; Chand, D.; Ghan, S.; Jimenez, J.L.; et al. Global transformation and fate of SOA: Implications of low-volatility SOA and gas-phase fragmentation reactions. J. Geophys. Res. Atmos. 2015, 120, 4169–4195. [Google Scholar] [CrossRef]
- Olenius, T.; Bergström, R.; Kubecka, J.; Myllys, N.; Elm, J. Reducing chemical complexity in representation of new-particle formation: Evaluation of simplification approaches. Environ. Sci. Atmos. 2023, 3, 552–567. [Google Scholar] [CrossRef]
- Bender, F.A.M.; Frey, L.; McCoy, D.T.; Grosvenor, D.P.; Mohrmann, J.K. Assessment of aerosol-cloud-radiation correlations in satellite observations, climate models and reanalysis. Clim. Dyn. 2019, 52, 4371–4392. [Google Scholar] [CrossRef]
- Kulmala, M.; Petaja, T.; Ehn, M.; Thornton, J.; Sipila, M.; Worsnop, D.R.; Kerminen, V.M. Chemistry of Atmospheric Nucleation: On the Recent Advances on Precursor Characterization and Atmospheric Cluster Composition in Connection with Atmospheric New Particle Formation. Annu. Rev. Phys. Chem. 2014, 65, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, D.; Cai, R.L.; Blichner, S.M.; Kontkanen, J.; Zhou, P.T.; Makkonen, R.; Kerminen, V.M.; Kulmala, M.; Riipinen, I.; Kangasluoma, J. Atmospheric nanoparticle growth. Rev. Mod. Phys. 2023, 95, 045002. [Google Scholar] [CrossRef]
- Kirkby, J.; Amorim, A.; Baltensperger, U.; Carslaw, K.S.; Christoudias, T.; Curtius, J.; Donahue, N.M.; El Haddad, I.; Flagan, R.C.; Gordon, H.; et al. Atmospheric new particle formation from the CERN CLOUD experiment. Nat. Geosci. 2023, 16, 948–957. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and Growth of Nanoparticles in the Atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef]
- Riccobono, F.; Schobesberger, S.; Scott, C.E.; Dommen, J.; Ortega, I.K.; Rondo, L.; Almeida, J.; Amorim, A.; Bianchi, F.; Breitenlechner, M.; et al. Oxidation Products of Biogenic Emissions Contribute to Nucleation of Atmospheric Particles. Science 2014, 344, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Kupc, A.; Williamson, C.J.; Hodshire, A.L.; Kazil, J.; Ray, E.; Bui, T.P.; Dollner, M.; Froyd, K.D.; McKain, K.; Rollins, A.; et al. The potential role of organics in new particle formation and initial growth in the remote tropical upper troposphere. Atmos. Chem. Phys. 2020, 20, 15037–15060. [Google Scholar] [CrossRef]
- Elm, J.; Ayoubi, D.; Engsvang, M.; Jensen, A.B.; Knattrup, Y.; Kubecka, J.; Bready, C.J.; Fowler, V.R.; Harold, S.E.; Longsworth, O.M.; et al. Quantum chemical modeling of organic enhanced atmospheric nucleation: A critical review. Wires Comput. Mol. Sci. 2023, 13, 1662. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, Y.; Li, Z.Y.; Liu, L.; Zhang, X.H.; Zheng, J.; Kerminen, V.M.; Kulmala, M.; Jiang, J.K.; Cai, R.L.; et al. The dependence of new particle formation rates on the interaction between cluster growth, evaporation, and condensation sink. Environ. Sci. Atmos. 2023, 3, 168–181. [Google Scholar] [CrossRef]
- Dada, L.; Lehtipalo, K.; Kontkanen, J.; Nieminen, T.; Baalbaki, R.; Ahonen, L.; Duplissy, J.; Yan, C.; Chu, B.W.; Petaja, T.; et al. Formation and growth of sub-3-nm aerosol particles in experimental chambers. Nat. Protoc. 2020, 15, 1013–1040. [Google Scholar] [CrossRef]
- Li, C.X.; Signorell, R. Understanding vapor nucleation on the molecular level: A review. J. Aerosol Sci. 2021, 153, 105676. [Google Scholar] [CrossRef]
- Kerminen, V.M.; Chen, X.M.; Vakkari, V.; Petaja, T.; Kulmala, M.; Bianchi, F. Atmospheric new particle formation and growth: Review of field observations. Environ. Res. Lett. 2018, 13, 103003. [Google Scholar] [CrossRef]
- Deng, Y.G.; Inomata, S.; Sato, K.; Ramasamy, S.; Morino, Y.; Enami, S.; Tanimoto, H. Temperature and acidity dependence of secondary organic aerosol formation from alpha-pinene ozonolysis with a compact chamber system. Atmos. Chem. Phys. 2021, 21, 5983–6003. [Google Scholar] [CrossRef]
- Heinritzi, M.; Dada, L.; Simon, M.; Stolzenburg, D.; Wagner, A.C.; Fischer, L.; Ahonen, L.R.; Amanatidis, S.; Baalbaki, R.; Baccarini, A.; et al. Molecular understanding of the suppression of new-particle formation by isoprene. Atmos. Chem. Phys. 2020, 20, 11809–11821. [Google Scholar] [CrossRef]
- Kristensen, K.; Jensen, L.N.; Quelever, L.L.J.; Christiansen, S.; Rosati, B.; Elm, J.; Teiwes, R.; Pedersen, H.B.; Glasius, M.; Ehn, M.; et al. The Aarhus Chamber Campaign on Highly Oxygenated Organic Molecules and Aerosols (ACCHA): Particle formation, organic acids, and dimer esters from alpha-pinene ozonolysis at different temperatures. Atmos. Chem. Phys. 2020, 20, 12549–12567. [Google Scholar] [CrossRef]
- Jonsson, A.M.; Hallquist, M.; Ljungstrom, E. Impact of humidity on the ozone initiated oxidation of limonene, Delta(3)-carene, and alpha-pinene. Environ. Sci. Technol. 2006, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.P.; Lin, C.C.; Yang, S.C.; Zhao, P. Enhancement effect of relative humidity on the formation and regional respiratory deposition of secondary organic aerosol. J. Hazard. Mater. 2011, 191, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.N.; Flueckiger, A.C.; Petrucci, G.A. Relative Humidity Impact on Organic New Particle Formation from Ozonolysis of α- and β-Pinene at Atmospherically Relevant Mixing Ratios. Atmosphere 2023, 14, 173. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, K.F.; Ho, S.S.H.; Lee, S.C.; Yau, P.S.; Cheng, Y. Physical parameters effect on ozone-initiated formation of indoor secondary organic aerosols with emissions from cleaning products. J. Hazard. Mater. 2011, 192, 1787–1794. [Google Scholar] [CrossRef]
- Jonsson, Å.M.; Hallquist, M.; Ljungström, E. The effect of temperature and water on secondary organic aerosol formation from ozonolysis of limonene, Δ3-carene and α-pinene. Atmos. Chem. Phys. 2008, 8, 6541–6549. [Google Scholar] [CrossRef]
- Bousiotis, D.; Brean, J.; Pope, F.D.; Dall’Osto, M.; Querol, X.; Alastuey, A.; Perez, N.; Petaja, T.; Massling, A.; Nojgaard, J.K.; et al. The effect of meteorological conditions and atmospheric composition in the occurrence and development of new particle formation (NPF) events in Europe. Atmos. Chem. Phys. 2021, 21, 3345–3370. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Le, C.; Xu, Q.; Peng, W.H.; Jiang, H.H.; Lin, Y.H.; Cocker, D.R.; Zhang, H.F. Compositional Evolution of Secondary Organic Aerosol as Temperature and Relative Humidity Cycle in Atmospherically Relevant Ranges. ACS Earth Space Chem. 2019, 3, 2549–2558. [Google Scholar] [CrossRef]
- Liang, L.L.; Engling, G.; Cheng, Y.; Zhang, X.Y.; Sun, J.Y.; Xu, W.Y.; Liu, C.; Zhang, G.; Xu, H.; Liu, X.Y.; et al. Influence of High Relative Humidity on Secondary Organic Carbon: Observations at a Background Site in East China. J. Meteorol. Res. 2019, 33, 905–913. [Google Scholar] [CrossRef]
- Li, X.X.; Chee, S.; Hao, J.M.; Abbatt, J.P.D.; Jiang, J.K.; Smith, J.N. Relative humidity effect on the formation of highly oxidized molecules and new particles during monoterpene oxidation. Atmos. Chem. Phys. 2019, 19, 1555–1570. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Fu, H.B.; Chen, J.M. Effect of relative humidity and the presence of aerosol particles on the alpha-pinene ozonolysis. J. Environ. Sci. 2018, 71, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hinks, M.L.; Montoya-Aguilera, J.; Ellison, L.; Lin, P.; Laskin, A.; Laskin, J.; Shiraiwa, M.; Dabdub, D.; Nizkorodov, S.A. Effect of relative humidity on the composition of secondary organic aerosol from the oxidation of toluene. Atmos. Chem. Phys. 2018, 18, 1643–1652. [Google Scholar] [CrossRef]
- Lewandowski, M.; Jaoui, M.; Offenberg, J.H.; Krug, J.D.; Kleindienst, T.E. Atmospheric oxidation of isoprene and 1,3-butadiene: Influence of aerosol acidity and relative humidity on secondary organic aerosol. Atmos. Chem. Phys. 2015, 15, 3773–3783. [Google Scholar] [CrossRef]
- Kristensen, K.; Cui, T.; Zhang, H.; Gold, A.; Glasius, M.; Surratt, J.D. Dimers in alpha-pinene secondary organic aerosol: Effect of hydroxyl radical, ozone, relative humidity and aerosol acidity. Atmos. Chem. Phys. 2014, 14, 4201–4218. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, G.H.; Cao, J.J.; Wang, X.M.; Zhang, R.J. Observation of biogenic secondary organic aerosols in the atmosphere of a mountain site in central China: Temperature and relative humidity effects. Atmos. Chem. Phys. 2013, 13, 11535–11549. [Google Scholar] [CrossRef]
- Emanuelsson, E.U.; Watne, A.K.; Lutz, A.; Ljungstrom, E.; Hallquist, M. Influence of Humidity, Temperature, and Radicals on the Formation and Thermal Properties of Secondary Organic Aerosol (SOA) from Ozonolysis of beta-Pinene. J. Phys. Chem. A 2013, 117, 10346–10358. [Google Scholar] [CrossRef]
- Hamed, A.; Korhonen, H.; Sihto, S.L.; Joutsensaari, J.; Jarvinen, H.; Petaja, T.; Arnold, F.; Nieminen, T.; Kulmala, M.; Smith, J.N.; et al. The role of relative humidity in continental new particle formation. J. Geophys. Res. Atmos. 2011, 116, D03202. [Google Scholar] [CrossRef]
- von Hessberg, C.; von Hessberg, P.; Poschl, U.; Bilde, M.; Nielsen, O.J.; Moortgat, G.K. Temperature and humidity dependence of secondary organic aerosol yield from the ozonolysis of beta-pinene. Atmos. Chem. Phys. 2009, 9, 3583–3599. [Google Scholar] [CrossRef]
- Pommer, L.; Fick, J.; Andersson, B.; Nilsson, C. The influence of O-3, relative humidity, NO and NO2 on the oxidation of alpha-pinene and Delta(3)-carene. J. Atmos. Chem. 2004, 48, 173–189. [Google Scholar] [CrossRef]
- Boy, M.; Kulmala, M. Nucleation events in the continental boundary layer: Influence of physical and meteorological parameters. Atmos. Chem. Phys. 2002, 2, 1–16. [Google Scholar] [CrossRef]
- Flueckiger, A.C.; Snyder, C.N.; Petrucci, G.A. Nontrivial Impact of Relative Humidity on Organic New Particle Formation from Ozonolysis of cis-3-Hexenyl Acetate. Air 2023, 1, 222–236. [Google Scholar] [CrossRef]
- Flueckiger, A.; Petrucci, G.A. Methodological advances to improve repeatability of SOA generation in enivronmental chambers. Aerosol Sci. Technol. 2023, 57, 925–933. [Google Scholar] [CrossRef]
- Chu, C.W.; Zhai, J.H.; Han, Y.M.; Ye, J.H.; Zaveri, R.A.; Martin, S.T.; Hung, H.M. New Particle Formation and Growth Dynamics for alpha-Pinene Ozonolysis in a Smog Chamber and Implications for Ambient Environments. ACS Earth Space Chem. 2022, 6, 2826–2835. [Google Scholar] [CrossRef]
- Tillmann, R.; Hallquist, M.; Jonsson, Å.M.; Kiendler-Scharr, A.; Saathoff, H.; Iinuma, Y.; Mentel, T.F. Influence of relative humidity and temperature on the production of pinonaldehyde and OH radicals from the ozonolysis of α-pinene. Atmos. Chem. Phys. 2010, 10, 7057–7072. [Google Scholar] [CrossRef]
- Tajuelo, M.; Rodriguez, D.; Rodriguez, A.; Escalona, A.; Viteri, G.; Aranda, A.; Diaz-de-Mera, Y. Secondary organic aerosol formation from the ozonolysis and oh-photooxidation of 2,5-dimethylfuran. Atmos. Environ. 2021, 245, 118041. [Google Scholar] [CrossRef]
- Bianchi, F.; Junninen, H.; Bigi, A.; Sinclair, V.A.; Dada, L.; Hoyle, C.R.; Zha, Q.; Yao, L.; Ahonen, L.R.; Bonasoni, P.; et al. Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols. Nat. Geosci. 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Sulo, J.; Sarnela, N.; Kontkanen, J.; Ahonen, L.; Paasonen, P.; Laurila, T.; Jokinen, T.; Kangasluoma, J.; Junninen, H.; Sipila, M.; et al. Long-term measurement of sub-3 nm particles and their precursor gases in the boreal forest. Atmos. Chem. Phys. 2021, 21, 695–715. [Google Scholar] [CrossRef]
- Artaxo, P.; Hansson, H.C.; Andreae, M.O.; Back, J.; Alves, E.G.; Barbosa, H.M.J.; Bender, F.; Bourtsoukidis, E.; Carbone, S.; Chi, J.S.; et al. Tropical and Boreal Forest Atmosphere Interactions: A Review. Tellus Ser. B-Chem. Phys. Meteorol. 2022, 74, 24–163. [Google Scholar] [CrossRef]
- Junninen, H.; Ahonen, L.; Bianchi, F.; Quéléver, L.; Schallhart, S.; Dada, L.; Manninen, H.E.; Leino, K.; Lampilahti, J.; Mazon, S.B.; et al. Terpene emissions from boreal wetlands can initiate stronger atmospheric new particle formation than boreal forests. Commun. Earth Environ. 2022, 3, 00406–00409. [Google Scholar] [CrossRef]
- Kammer, J.; Flaud, P.M.; Chazeaubeny, A.; Ciuraru, R.; Le Menach, K.; Geneste, E.; Budzinski, H.; Bonnefond, J.M.; Lamaud, E.; Perraudin, E.; et al. Biogenic volatile organic compounds (BVOCs) reactivity related to new particle formation (NPF) over the Landes forest. Atmos. Res. 2020, 237, 104869. [Google Scholar] [CrossRef]
- Liu, Y.F.; Su, H.; Wang, S.W.; Wei, C.; Tao, W.; Pöhlker, M.L.; Pöhlker, C.; Holanda, B.A.; Krüger, O.O.; Hoffmann, T.; et al. Strong particle production and condensational growth in the uppertroposphere sustained by biogenic VOCs from the canopy of the Amazon Basin. Atmos. Chem. Phys. 2023, 23, 251–272. [Google Scholar] [CrossRef]
- Riipinen, I.; Pierce, J.R.; Yli-Juuti, T.; Nieminen, T.; Häkkinen, S.; Ehn, M.; Junninen, H.; Lehtipalo, K.; Petäjä, T.; Slowik, J.; et al. Organic condensation: A vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations. Atmos. Chem. Phys. 2011, 11, 3865–3878. [Google Scholar] [CrossRef]
- Trostl, J.; Chuang, W.K.; Gordon, H.; Heinritzi, M.; Yan, C.; Molteni, U.; Ahlm, L.; Frege, C.; Bianchi, F.; Wagner, R.; et al. The role of low-volatility organic compounds in initial particle growth in the atmosphere. Nature 2016, 533, 527–531. [Google Scholar] [CrossRef]
- Dada, L.; Stolzenburg, D.; Simon, M.; Fischer, L.; Heinritzi, M.; Wang, M.; Xiao, M.; Vogel, A.L.; Ahonen, L.; Amorim, A.; et al. Role of sesquiterpenes in biogenic new particle formation. Sci. Adv. 2023, 9, eadi5297. [Google Scholar] [CrossRef] [PubMed]
- Quelever, L.L.J.; Kristensen, K.; Jensen, L.N.; Rosati, B.; Teiwes, R.; Daellenbach, K.R.; Perakyla, O.; Roldin, P.; Bossi, R.; Pedersen, H.B.; et al. Effect of temperature on the formation of highly oxygenated organic molecules (HOMs) from alpha-pinene ozonolysis. Atmos. Chem. Phys. 2019, 19, 7609–7625. [Google Scholar] [CrossRef]
- Yu, S.S.; Jia, L.; Xu, Y.F.; Zhang, H.L.; Zhang, Q.; Pan, Y.P. Wall losses of oxygenated volatile organic compounds from oxidation of toluene: Effects of chamber volume and relative humidity. J. Environ. Sci. 2022, 114, 475–484. [Google Scholar] [CrossRef]
- Cai, R.L.; Häkkinen, E.; Yan, C.; Jiang, J.K.; Kulmala, M.; Kangasluoma, J. The effectiveness of the coagulation sink of 3-10 nm atmospheric particles. Atmos. Chem. Phys. 2022, 22, 11529–11541. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Bergin, M.H.; Dibb, J.E.; Weber, R.J. Enhanced secondary organic aerosol formation due to water uptake by fine particles. Geophys. Res. Lett. 2008, 35, L18801. [Google Scholar] [CrossRef]
- Prisle, N.L.; Engelhart, G.J.; Bilde, M.; Donahue, N.M. Humidity influence on gas-particle phase partitioning of alpha-pinene + O-3 secondary organic aerosol. Geophys. Res. Lett. 2010, 37, L01802. [Google Scholar] [CrossRef]
- Surdu, M.; Lamkaddam, H.; Wang, D.S.; Bell, D.M.; Xiao, M.; Lee, C.P.; Li, D.; Caudillo, L.; Marie, G.; Scholz, W.; et al. Molecular Understanding of the Enhancement in Organic Aerosol Mass at High Relative Humidity. Environ. Sci. Technol. 2023, 57, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Gong, Z.H.; Ye, J.H.; Liu, P.F.; McKinney, K.A.; Martin, S.T. Quantifying the Role of the Relative Humidity-Dependent Physical State of Organic Particulate Matter in the Uptake of Semivolatile Organic Molecules. Environ. Sci. Technol. 2019, 53, 13209–13218. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.X.; Jorga, S.D.; Pierce, J.R.; Donahue, N.M.; Pandis, S.N. Particle wall-loss correction methods in smog chamber experiments. Atmos. Meas. Tech. 2018, 11, 6577–6588. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Erdakos, G.B.; Asher, W.E.; Pankow, J.F. Modeling the formation of secondary organic aerosol (SOA). 2. The predicted effects of relative humidity on aerosol formation in the alpha-pinene-, beta-pinene-, sabinene-, Delta(3)-Carene-, and cyclohexene-ozone systems. Environ. Sci. Technol. 2001, 35, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Jathar, S.H.; Mahmud, A.; Barsanti, K.C.; Asher, W.E.; Pankow, J.F.; Kleeman, M.J. Water uptake by organic aerosol and its influence on gas/particle partitioning of secondary organic aerosol in the United States. Atmos. Environ. 2016, 129, 142–154. [Google Scholar] [CrossRef]
- Pankow, J.F.; Marks, M.C.; Barsanti, K.C.; Mahmud, A.; Asher, W.E.; Li, J.Y.; Ying, Q.; Jathar, S.H.; Kleeman, M.J. Molecular view modeling of atmospheric organic particulate matter: Incorporating molecular structure and co-condensation of water. Atmos. Environ. 2015, 122, 400–408. [Google Scholar] [CrossRef]
- Qin, Y.; Ye, J.; Ohno, P.; Zhai, J.; Han, Y.; Liu, P.; Wang, J.; Zaveri, R.A.; Martin, S.T. Humidity Dependence of the Condensational Growth of α-Pinene Secondary Organic Aerosol Particles. Environ. Sci. Technol. 2021, 55, 14360–14369. [Google Scholar] [CrossRef]
- Pankow, J.F. Organic particulate material levels in the atmosphere: Conditions favoring sensitivity to varying relative humidity and temperature. Proc. Natl. Acad. Sci. USA 2010, 107, 6682–6686. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.H.; Han, Y.M.; Liu, P.F.; Ye, J.H.; Keutsch, F.N.; McKinney, K.A.; Martin, S.T. Influence of Particle Physical State on the Uptake of Medium-Sized Organic Molecules. Environ. Sci. Technol. 2018, 52, 8381–8389. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, R.A.; Shilling, J.E.; Zelenyuk, A.; Liu, J.M.; Bell, D.M.; D’Ambro, E.L.; Gaston, C.; Thornton, J.A.; Laskin, A.; Lin, P.; et al. Growth Kinetics and Size Distribution Dynamics of Viscous Secondary Organic Aerosol. Environ. Sci. Technol. 2018, 52, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, R.A.; Shilling, J.E.; Zelenyuk, A.; Zawadowicz, M.A.; Suski, K.; China, S.; Bell, D.M.; Veghte, D.; Laskin, A. Particle-Phase Diffusion Modulates Partitioning of Semivolatile Organic Compounds to Aged Secondary Organic Aerosol. Environ. Sci. Technol. 2020, 54, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Kerminen, V.M.; Lehtinen, K.E.J.; Anttila, T.; Kulmala, M. Dynamics of atmospheric nucleation mode particles: A timescale analysis. Tellus B Chem. Phys. Meteorol. 2004, 56, 135–146. [Google Scholar] [CrossRef]
- Kürten, A.; Williamson, C.; Almeida, J.; Kirkby, J.; Curtius, J. On the derivation of particle nucleation rates from experimental formation rates. Atmos. Chem. Phys. 2015, 15, 4063–4075. [Google Scholar] [CrossRef]
- Charan, S.M.; Huang, Y.L.; Seinfeld, J.H. Computational Simulation of Secondary Organic Aerosol Formation in Laboratory Chambers. Chem. Rev. 2019, 119, 11912–11944. [Google Scholar] [CrossRef]

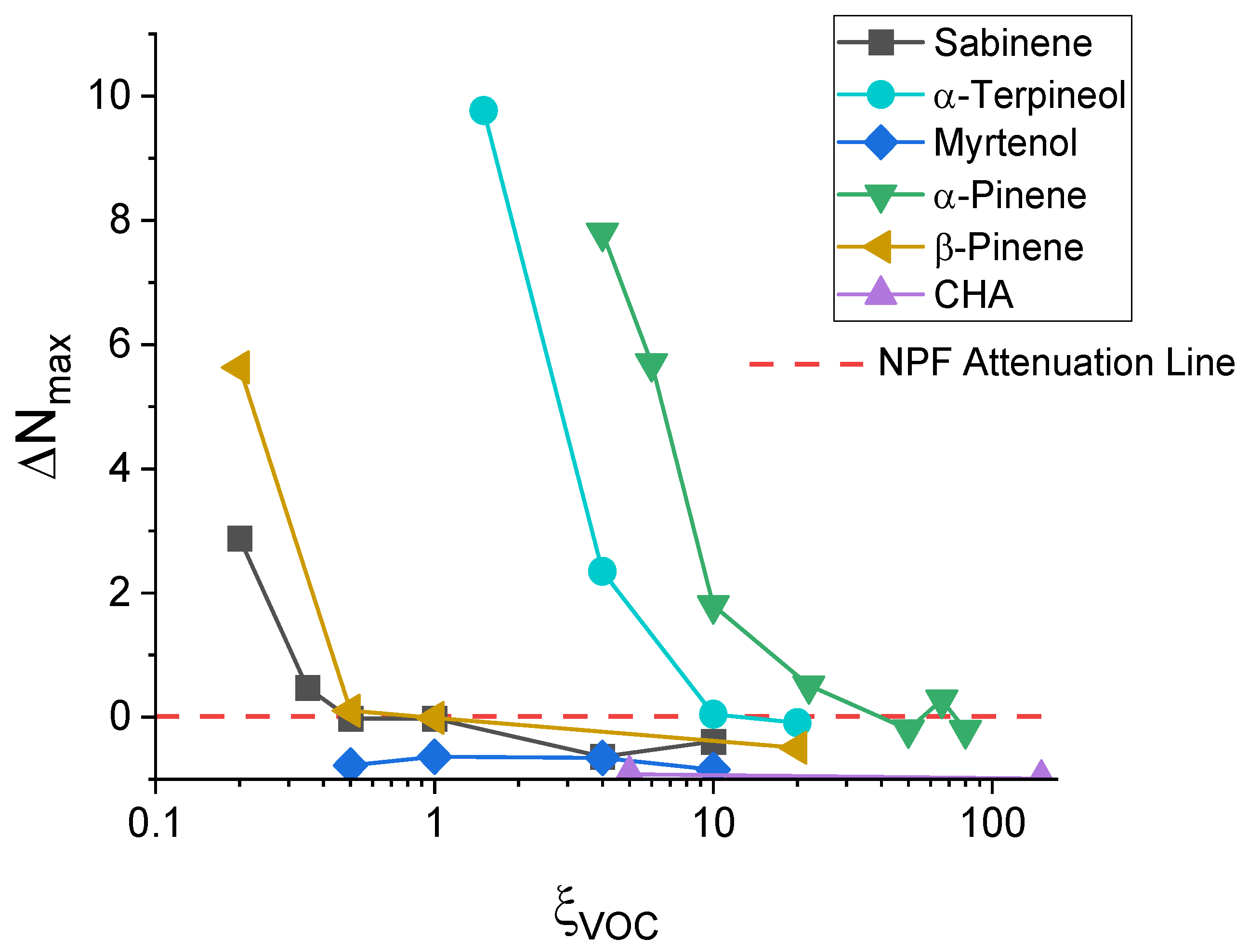
| ξVOC (ppbv) | Sabinene | α-Terpineol | Myrtenol |
|---|---|---|---|
| 0.20 (±0.02) | 2.88 (±0.25) | -- | -- |
| 0.35 (±0.03) | 0.47 (±0.04) | -- | -- |
| 0.50 (±0.03) | −0.02 (±0.001) | -- | −0.77 (±0.06) |
| 1.00 (±0.03) | −0.02 (±0.001) | -- | −0.64 (±0.02) |
| 1.5 (±0.03) | -- | 9.77 (±0.86) | -- |
| 4.0 (±0.1) | −0.64 (±0.02) | 2.35 (±0.07) | −0.66 (±0.02) |
| 10.0 (±0.1) | −0.39 (±0.01) | 0.04 (±0.001) | −0.85 (±0.03) |
| 20.0 (±0.3) | -- | −0.09 (±0.003) | -- |
| VOC | Solubility in H2O (mg/L) * |
|---|---|
| Sabinene | 2.5 |
| α-Terpineol | 7100 |
| Myrtenol | 427 |
| α-Pinene | Insoluble |
| β-Pinene | Insoluble |
| CHA | 900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flueckiger, A.C.; Petrucci, G.A. Effect of Relative Humidity on the Rate of New Particle Formation for Different VOCs. Atmosphere 2024, 15, 480. https://doi.org/10.3390/atmos15040480
Flueckiger AC, Petrucci GA. Effect of Relative Humidity on the Rate of New Particle Formation for Different VOCs. Atmosphere. 2024; 15(4):480. https://doi.org/10.3390/atmos15040480
Chicago/Turabian StyleFlueckiger, Austin C., and Giuseppe A. Petrucci. 2024. "Effect of Relative Humidity on the Rate of New Particle Formation for Different VOCs" Atmosphere 15, no. 4: 480. https://doi.org/10.3390/atmos15040480
APA StyleFlueckiger, A. C., & Petrucci, G. A. (2024). Effect of Relative Humidity on the Rate of New Particle Formation for Different VOCs. Atmosphere, 15(4), 480. https://doi.org/10.3390/atmos15040480







