Emission Pattern of Biogenic Volatile Organic Compounds from Wetland Vegetation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Plant Species
2.2. BVOC Sampling and Analysis
2.3. BVOC Emission Rates
2.4. Physiological Parameters
2.5. Statistical Analysis
3. Results
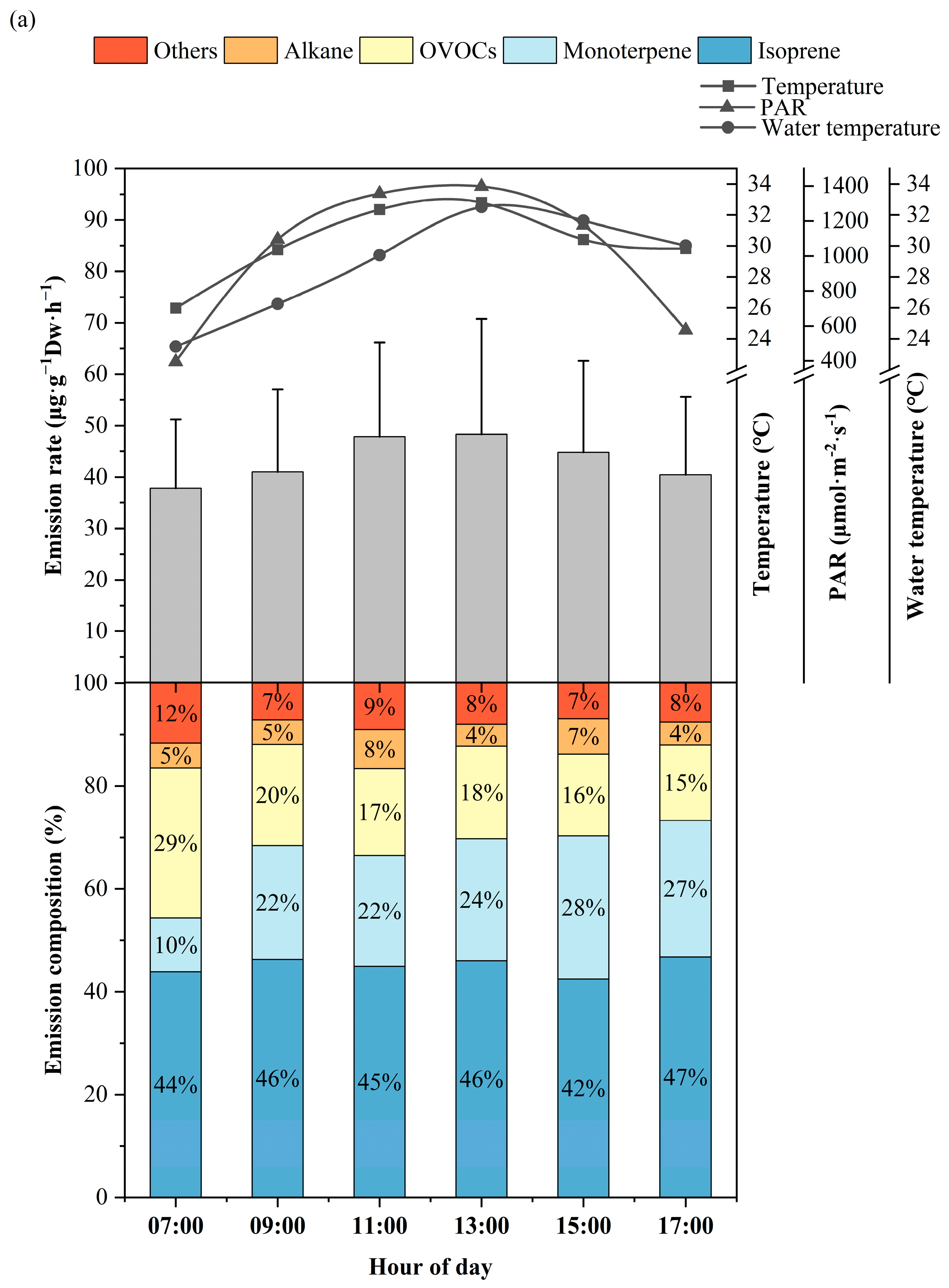
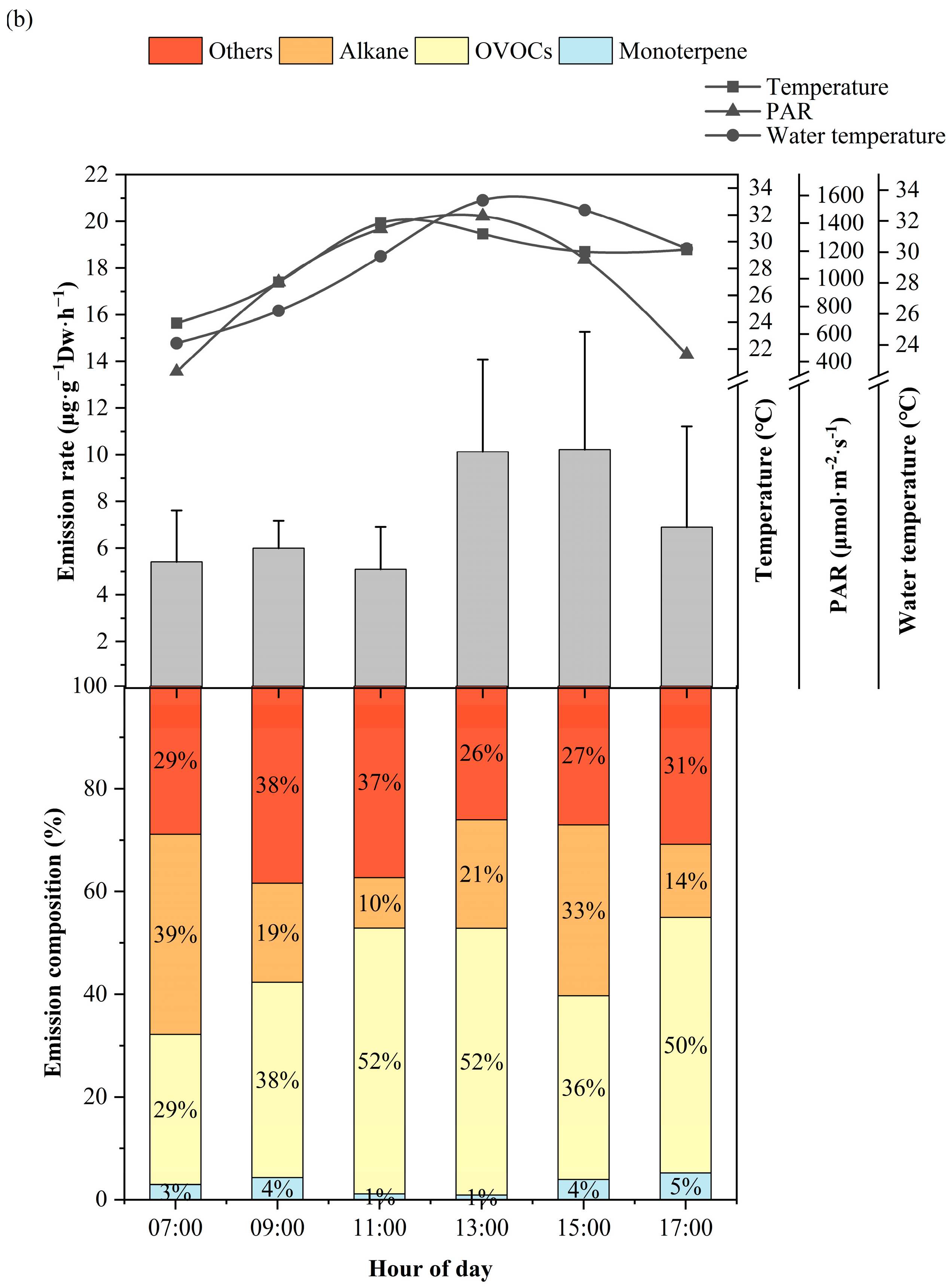
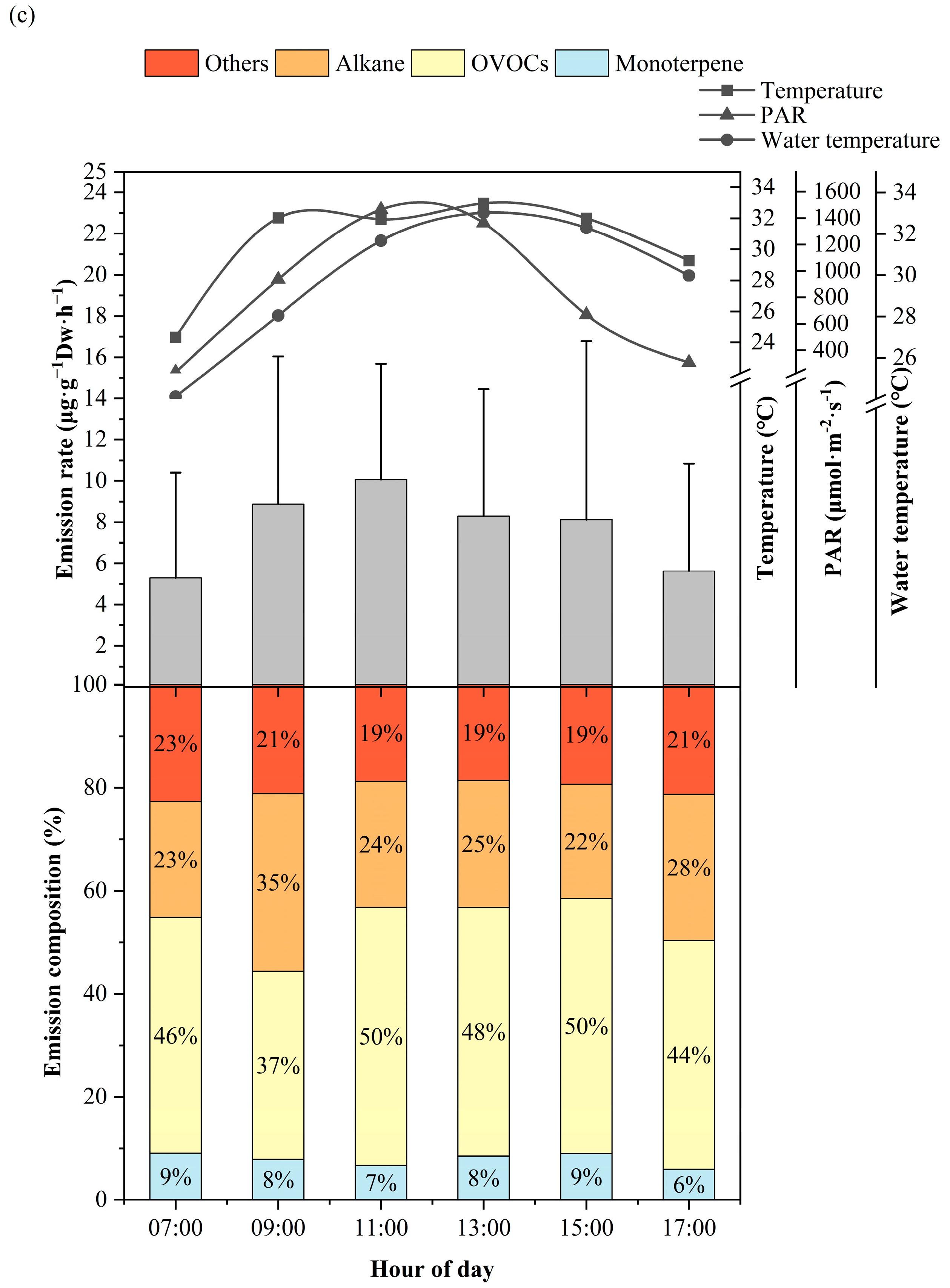
3.1. Diurnal Variation of BVOCs
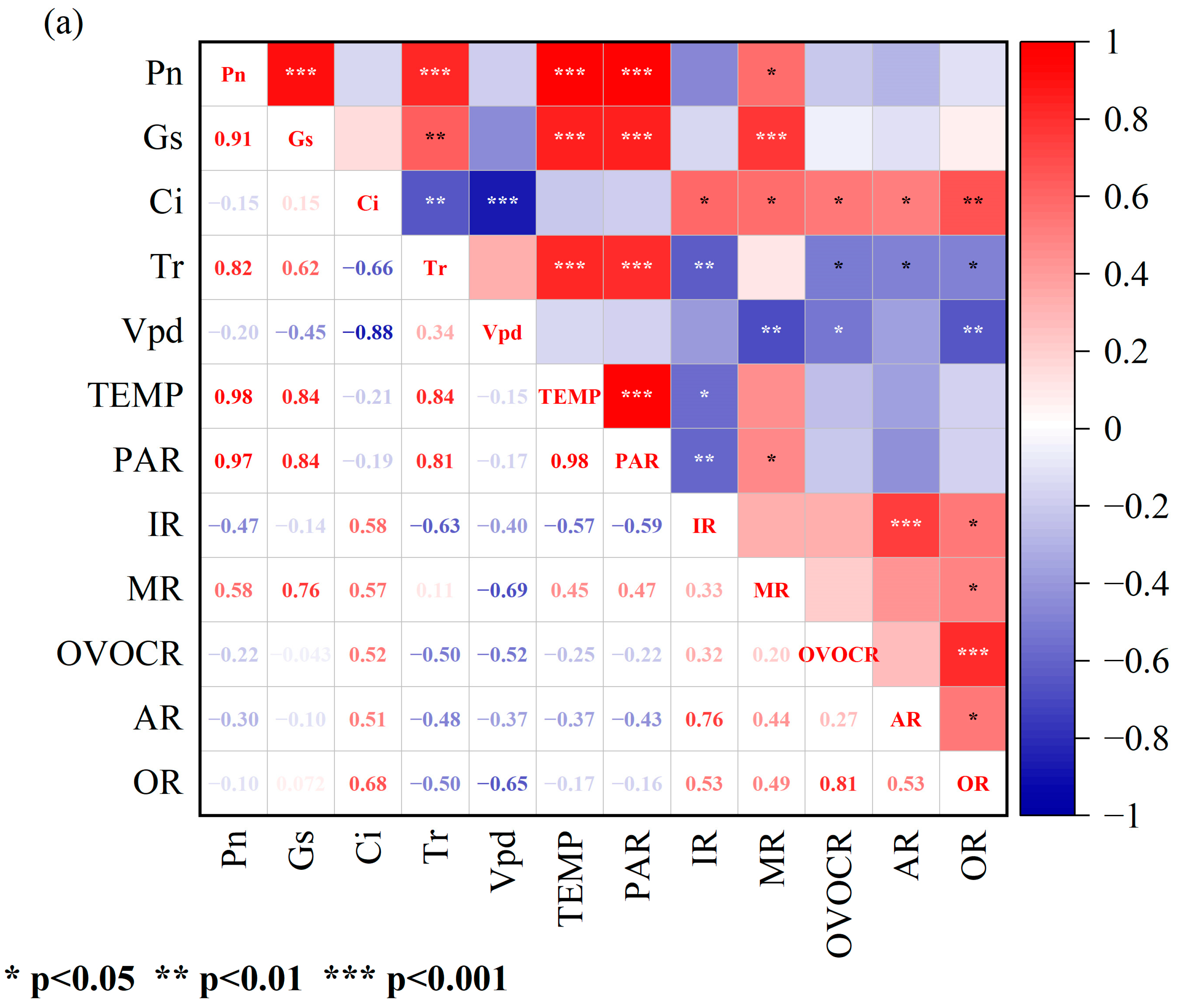
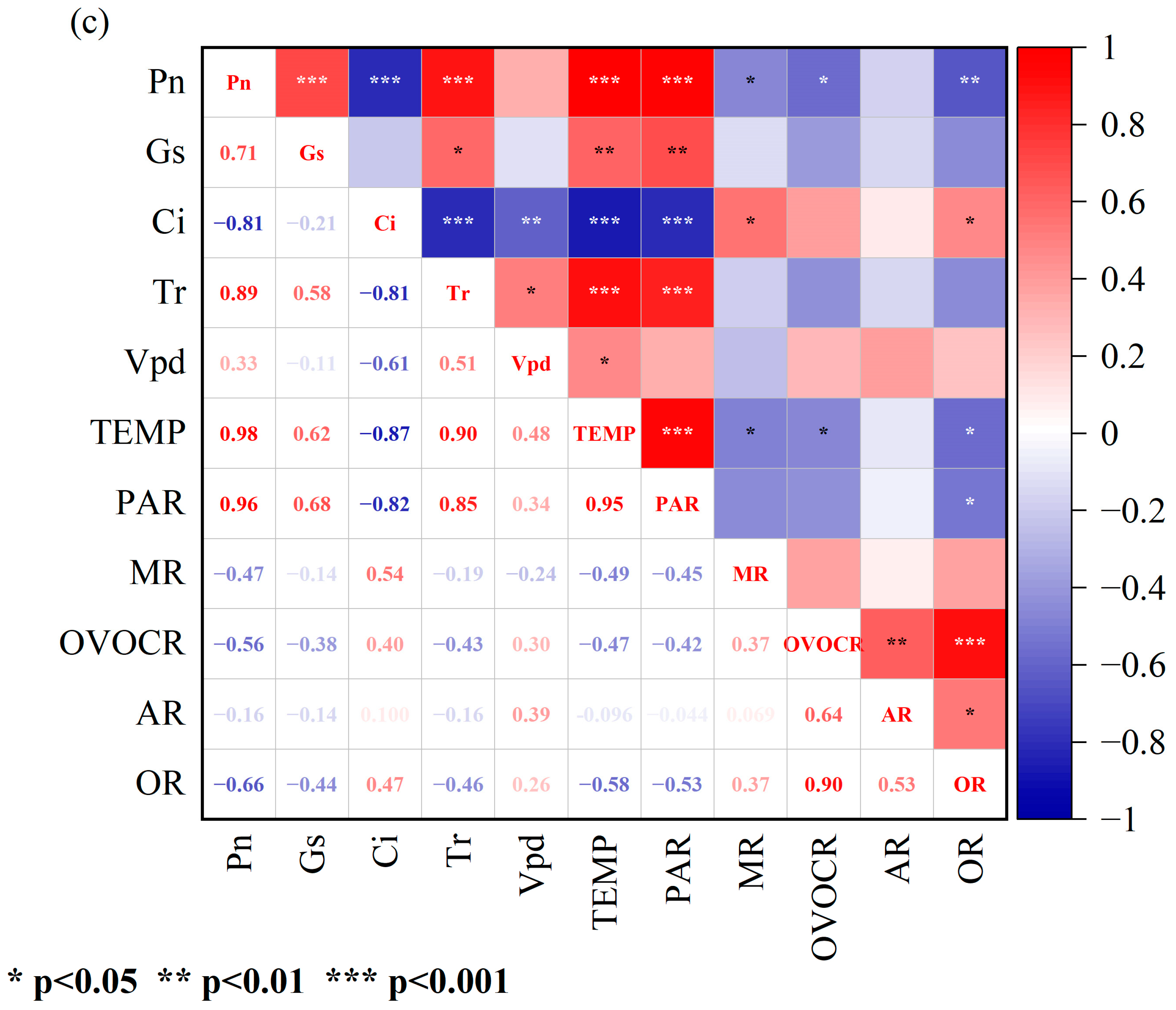
3.2. Correlations between Physiological Parameters and BVOC Emission Rates
4. Discussion
4.1. BVOC Emission Characteristics: Comparison with Other Plant Species
4.2. The Relationship between Isoprenoid Emission and Physiological Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Joutsensaari, J.; Loivamäki, M.; Vuorinen, T.; Miettinen, P.; Nerg, A.M.; Holopainen, J.K.; Laaksonen, A. Nanoparticle formation by ozonolysis of inducible plant volatiles. Atmos. Chem. Phys. 2005, 5, 1489–1495. [Google Scholar] [CrossRef]
- Sindelarova, K.; Markova, J.; Simpson, D.; Huszar, P.; Karlicky, J.; Darras, S.; Granier, C. High-resolution biogenic global emission inventory for the time period 2000–2019 for air quality modeling. Earth Syst. Sci. Data 2022, 14, 251–270. [Google Scholar] [CrossRef]
- Li, L.; Yang, W.; Xie, S.; Wu, Y. Estimations and uncertainty of biogenic volatile organic compound emission inventory in China for 2008–2018. Sci. Total Environ. 2020, 733, 139301. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ho, S.S.H.; Li, X.; Guo, L.; Chen, A.; Hu, L.; Yang, Y.; Chen, D.; Lin, A.; Fang, X. A comprehensive review on anthropogenic volatile organic compounds (VOCs) emission estimates in China: Comparison and outlook. Environ. Int. 2021, 156, 106710. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Bei, N.; Huang, R.-J.; Cao, J.; Zhang, Q.; Zhou, W.; Tie, X.; Liu, S.; Zhang, T.; Su, X.; et al. Summertime ozone formation in Xi’an and surrounding areas, China. Atmos. Chem. Phys. 2016, 16, 4323–4342. [Google Scholar] [CrossRef]
- Sheng, J.; Zhao, D.; Ding, D.; Li, X.; Huang, M.; Gao, Y.; Quan, J.; Zhang, Q. Characterizing the level, photochemical reactivity, emission, and source contribution of the volatile organic compounds based on PTR-TOF-MS during winter haze period in Beijing, China. Atmos. Res. 2018, 212, 54–63. [Google Scholar] [CrossRef]
- Wang, P.; Ying, Q.; Zhang, H.; Hu, J.; Lin, Y.; Mao, H. Source apportionment of secondary organic aerosol in China using a regional source-oriented chemical transport model and two emission inventories. Environ. Pollut. 2018, 237, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Medori, M.; Michelini, L.; Nogues, I.; Loreto, F.; Calfapietra, C. The impact of root temperature on photosynthesis and isoprene emission in three different plant species. Sci. World J. 2012, 2012, 525827. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Brilli, F.; Nogues, I.; Velikova, V.; Tsonev, T.; Dagli, S.; Loreto, F. Isoprene emission and primary metabolism in Phragmites australis grown under different phosphorus levels. Plant Biol. 2008, 10, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ge, Z.; Zhou, X.; Li, S.; Li, X.; Tang, J. Conversion of coastal wetlands, riparian wetlands, and peatlands increases greenhouse gas emissions: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 1638–1653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yu, J.; Guan, B.; Li, Y.; Yu, M.; Qu, F.; Zhan, C.; Lv, Z.; Wu, H.; Wang, Q.; et al. A Comparison of the Development of Wetland Restoration Techniques in China and Other Nations. Wetlands 2020, 40, 2755–2764. [Google Scholar] [CrossRef]
- Mao, D.; Wang, Z.; Du, B.; Li, L.; Tian, Y.; Jia, M.; Zeng, Y.; Song, K.; Jiang, M.; Wang, Y. National wetland mapping in China: A new product resulting from object-based and hierarchical classification of Landsat 8 OLI images. ISPRS J. Photogramm. Remote Sens. 2020, 164, 11–25. [Google Scholar] [CrossRef]
- Quin, A.; Jaramillo, F.; Destouni, G. Dissecting the ecosystem service of large-scale pollutant retention: The role of wetlands and other landscape features. Ambio 2015, 44, S127–S137. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, W.J.; Mander, Ü. Wetlands and carbon revisited. Ecol. Eng. 2018, 114, 1–6. [Google Scholar] [CrossRef]
- Zhou, J.B.; Wu, J.; Gong, Y.Z. Valuing wetland ecosystem services based on benefit transfer: A meta-analysis of China wetland studies. J. Clean. Prod. 2020, 276, 11. [Google Scholar] [CrossRef]
- Sun, R.H.; Chen, A.L.; Chen, L.D.; Lü, Y.H. Cooling effects of wetlands in an urban region: The case of Beijing. Ecol. Indic. 2012, 20, 57–64. [Google Scholar] [CrossRef]
- Seifollahi-Aghmiuni, S.; Nockrach, M.; Kalantari, Z. The Potential of Wetlands in Achieving the Sustainable Development Goals of the 2030 Agenda. Water 2019, 11, 609. [Google Scholar] [CrossRef]
- Wang, Z.H.; Bai, Y.H.; Zhang, S.Y. A biogenic volatile organic compounds emission inventory for Beijing. Atmos. Environ. 2003, 37, 3771–3782. [Google Scholar] [CrossRef]
- Jing, X.; Lun, X.; Fan, C.; Ma, W. Emission patterns of biogenic volatile organic compounds from dominant forest species in Beijing, China. J. Environ. Sci. 2020, 95, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhou, W.; Wang, W.; Yao, Y.; Xu, L. Tree species classification improves the estimation of BVOCs from urban greenspace. Sci. Total Environ. 2024, 914, 169762. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, J.; Yu, X. Environ.mental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ. Pollut. 2020, 259, 113955. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Long, J.; Liu, H.; Sun, G.; Li, J.; Xu, L.; Xu, C. Biogenic volatile organic compounds from 14 landscape woody species: Tree species selection in the construction of urban greenspace with forest healthcare effects. J. Environ. Manag. 2021, 300, 113761. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lun, X.; Wu, J.; Wang, Q.; Tao, J.; Dou, X.; Zhang, Z. Investigation of biogenic volatile organic compounds emissions in the Qinghai-Tibetan Plateau. Sci. Total Environ. 2023, 902, 165877. [Google Scholar] [CrossRef] [PubMed]
- Abis, L.; Kalalian, C.; Lunardelli, B.; Wang, T.; Zhang, L.W.; Chen, J.M.; Perrier, S.; Loubet, B.; Ciuraru, R.; George, C. Measurement report: Biogenic volatile organic compound emission profiles of rapeseed leaf litter and its secondary organic aerosol formation potential. Atmos. Chem. Phys. 2021, 21, 12613–12629. [Google Scholar] [CrossRef]
- Deng, X.J.; Peng, J.Y.; Luo, B.; Wei, M.; Hu, W.L.; Du, J.W. A direct quantitative analysis method for monitoring biogenic volatile organic compounds released from leaves of Pelargonium hortoram in situ. Anal. Bioanal. Chem. 2004, 380, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.C.; Drewer, J.; Heal, M.R. A comparison of isoprene and monoterpene emission rates from the perennial bioenergy crops short-rotation coppice willow andMiscanthusand the annual arable crops wheat and oilseed rape. GCB Bioenergy 2016, 8, 211–225. [Google Scholar] [CrossRef]
- Li, S.; Yuan, X.; Xu, Y.; Li, Z.; Feng, Z.; Yue, X.; Paoletti, E. Biogenic volatile organic compound emissions from leaves and fruits of apple and peach trees during fruit development. J. Environ. Sci. 2021, 108, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hartmann, H.; Hellen, H.; Wisthaler, A.; Perreca, E.; Weinhold, A.; Rucker, A.; van Dam, N.M.; Gershenzon, J.; Trumbore, S.; et al. New Perspectives on CO(2), Temperature, and Light Effects on BVOC Emissions Using Online Measurements by PTR-MS and Cavity Ring-Down Spectroscopy. Environ. Sci. Technol. 2018, 52, 13811–13823. [Google Scholar] [CrossRef] [PubMed]
- Van Meeningen, Y.; Schurgers, G.; Rinnan, R.; Holst, T. Isoprenoid emission response to changing light conditions of English oak, European beech and Norway spruce. Biogeosciences 2017, 14, 4045–4060. [Google Scholar] [CrossRef]
- Son, Y.-S.; Hwang, Y.-S.; Sung, J.-H.; Kim, J.-C. Variations of BVOCs Emission Characteristics according to Increasing PAR. J. Korean Soc. Atmos. Environ. 2012, 28, 77–85. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Tan, Y.; Tan, Y.; Bai, J.; Gu, D.; Ma, Z.; Du, J.; Han, Z. Effects of light on the emissions of biogenic isoprene and monoterpenes: A review. Atmos. Pollut. Res. 2022, 13, 101397. [Google Scholar] [CrossRef]
- Ashworth, K.; Chung, S.H.; McKinney, K.A.; Liu, Y.; Munger, J.W.; Martin, S.T.; Steiner, A.L. Modelling bidirectional fluxes of methanol and acetaldehyde with the FORCAsT canopy exchange model. Atmos. Chem. Phys. 2016, 16, 15461–15484. [Google Scholar] [CrossRef]
- Pazouki, L.; Niinemets, U. Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance. Front. Plant Sci. 2016, 7, 1019. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harley, P.C.; Niinemets, U. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Xiong, F.; Sun, N.; Li, Y.; Liu, C.; Yin, S. Photosynthesis and Related Physiological Parameters Differences Affected the Isoprene Emission Rate among 10 Typical Tree Species in Subtropical Metropolises. Int. J. Environ. Res. Public Health 2021, 18, 954. [Google Scholar] [CrossRef] [PubMed]
- Borsdorf, H.; Bentele, M.; Müller, M.; Rebmann, C.; Mayer, T. Comparison of Seasonal and Diurnal Concentration Profiles of BVOCs in Coniferous and Deciduous Forests. Atmosphere 2023, 14, 1347. [Google Scholar] [CrossRef]
- Kammer, J.; Flaud, P.M.; Chazeaubeny, A.; Ciuraru, R.; Le Menach, K.; Geneste, E.; Budzinski, H.; Bonnefond, J.M.; Lamaud, E.; Perraudin, E.; et al. Biogenic volatile organic compounds (BVOCs) reactivity related to new particle formation (NPF) over the Landes forest. Atmos. Res. 2020, 237, 11. [Google Scholar] [CrossRef]
- Mu, Z.; Llusià, J.; Zeng, J.; Zhang, Y.; Asensio, D.; Yang, K.; Yi, Z.; Wang, X.; Peñuelas, J. An Overview of the Isoprenoid Emissions From Tropical Plant Species. Front. Plant Sci. 2022, 13, 833030. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhou, W.; Xu, L.; Zheng, Z. A meta-analysis on plant volatile organic compound emissions of different plant species and responses to Environ.mental stress. Environ. Pollut. 2023, 318, 120886. [Google Scholar] [CrossRef] [PubMed]
- Salvador, C.M.; Chou, C.C.K.; Ho, T.-T.; Tsai, C.-Y.; Tsao, T.-M.; Tsai, M.-J.; Su, T.-C. Contribution of Terpenes to Ozone Formation and Secondary Organic Aerosols in a Subtropical Forest Impacted by Urban Pollution. Atmosphere 2020, 11, 1232. [Google Scholar] [CrossRef]
- Liu, L.; Seyler, B.C.; Liu, H.; Zhou, L.; Chen, D.; Liu, S.; Yan, C.; Yang, F.; Song, D.; Tan, Q.; et al. Biogenic volatile organic compound emission patterns and secondary pollutant formation potentials of dominant greening trees in Chengdu, southwest China. J. Environ. Sci. 2022, 114, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C. Where, When, and Why Do Plant Volatiles Mediate Ecological Signaling? The Answer Is Blowing in the Wind. Annu. Rev. Plant Biol. 2023, 74, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.G.; Gajbhiye, T.; Pandey, S.K. Plant specific emission pattern of biogenic volatile organic compounds (BVOCs) from common plant species of Central India. Environ. Monit. Assess. 2018, 190, 631. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, Q.; Jiang, M.; Wang, T.; Li, X.; Chen, Q.; Luo, Z.; Lv, B. Seasonal Variation in the Thermal Environ.ment and Health-Related Factors in Two Clustered Recreational Bamboo Forests. Forests 2023, 14, 1894. [Google Scholar] [CrossRef]
- Zeng, J.; Song, W.; Zhang, Y.; Mu, Z.; Pang, W.; Zhang, H.; Wang, X. Emissions of isoprenoids from dominant tree species in subtropical China. Front. For. Glob. Chang. 2022, 5, 1089676. [Google Scholar] [CrossRef]
- Pikkarainen, L.; Nissinen, K.; Ghimire, R.P.; Kivimaenpaa, M.; Ikonen, V.P.; Kilpelainen, A.; Virjamo, V.; Yu, H.; Kirsikka-Aho, S.; Salminen, T.; et al. Responses in growth and emissions of biogenic volatile organic compounds in Scots pine, Norway spruce and silver birch seedlings to different warming treatments in a controlled field experiment. Sci. Total Environ. 2022, 821, 153277. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, H.K.; Sevanto, S.; Bäck, J.; Ruuskanen, T.M.; Kolari, P.; Taipale, R.; Rinne, J.; Kulmala, M.; Hari, P. Day-time concentrations of biogenic volatile organic compounds in a boreal forest canopy and their relation to Environ.mental and biological factors. Atmos. Chem. Phys. 2009, 9, 5447–5459. [Google Scholar] [CrossRef]
- Pegoraro, E.; Potosnak, M.J.; Monson, R.K.; Rey, A.; Barron-Gafford, G.; Osmond, C.B. The effect of elevated CO2, soil and atmospheric water deficit and seasonal phenology on leaf and ecosystem isoprene emission. Funct. Plant Biol. 2007, 34, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Lerdau, M.; Gray, D. Ecology and evolution of light-dependent and light-independent phytogenic volatile organic carbon. New Phytol. 2003, 157, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Pétron, G.; Harley, P.; Greenberg, J.; Guenther, A. Seasonal temperature variations influence isoprene emission. Geophys. Res. Lett. 2001, 28, 1707–1710. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Mu, Z.; Pang, W.; Zhang, H.; Wu, Z.; Song, W.; Wang, X. Temperature and light dependency of isoprene and monoterpene emissions from tropical and subtropical trees: Field observations in south China. Appl. Geochem. 2023, 155, 105757. [Google Scholar] [CrossRef]
- Sabillón, D.; Cremades, L.V. Diurnal and seasonal variation of monoterpene emission rates for two typical Mediterranean species (Pinus pinea and Quercus ilex) from field measurements-relationship with temperature and PAR. Atmos. Environ. 2001, 35, 4419–4431. [Google Scholar] [CrossRef]
- Taylor, T.C.; Smith, M.N.; Slot, M.; Feeley, K.J. The capacity to emit isoprene differentiates the photosynthetic temperature responses of tropical plant species. Plant Cell Environ. 2019, 42, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, I.; Inafuku, M.; Saitoh, S.; Iwasaki, H.; Fukuta, M.; Watanabe, K.; Oku, H. Temperature controls on the basal emission rate of isoprene in a tropical tree Ficus septica: Exploring molecular regulatory mechanisms. Plant Cell Environ. 2016, 39, 2260–2275. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Preiser, A.L.; Weraduwage, S.M.; Gog, L. Source of 12C in Calvin-Benson cycle intermediates and isoprene emitted from plant leaves fed with 13CO2. Biochem. J. 2020, 477, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Fall, R. Isoprene emission from aspen leaves: Influence of Environ.ment and relation to photosynthesis and photorespiration. Plant Physiol. 1989, 90, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Guidolotti, G.; Pallozzi, E.; Gavrichkova, O.; Scartazza, A.; Mattioni, M.; Loreto, F.; Calfapietra, C. Emission of constitutive isoprene, induced monoterpenes, and other volatiles under high temperatures in Eucalyptus camaldulensis: A (13) C labelling study. Plant Cell Environ. 2019, 42, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.S.; Wu, Z.F.; Liao, H.; Ren, Y. Interaction Processes of Environ.ment and Plant Ecophysiology with BVOC Emissions from Dominant Greening Trees. Forests 2023, 14, 523. [Google Scholar] [CrossRef]
- De Souza, V.F.; Niinemets, Ü.; Rasulov, B.; Vickers, C.E.; Duvoisin, S.; Araújo, W.L.; Gonçalves, J.F.D. Alternative Carbon Sources for Isoprene Emission. Trends Plant Sci. 2018, 23, 1081–1101. [Google Scholar] [CrossRef]
- Staudt, M.; Daussy, J.; Ingabire, J.; Dehimeche, N. Growth and actual leaf temperature modulate CO2 responsiveness of monoterpene emissions from holm oak in opposite ways. Biogeosciences 2022, 19, 4945–4963. [Google Scholar] [CrossRef]
- Ladd, S.N.; Daber, L.E.; Bamberger, I.; Kübert, A.; Kreuzwieser, J.; Purser, G.; Ingrisch, J.; Deleeuw, J.; van Haren, J.; Meredith, L.K.; et al. Leaf-level metabolic changes in response to drought affect daytime CO2 emission and isoprenoid synthesis pathways. Tree Physiol. 2023, 43, 1917–1932. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Penuelas, J.; Llusia, J. Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. New Phytol. 2006, 169, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Harley, P.; Eller, A.; Guenther, A.; Monson, R.K. Observations and models of emissions of volatile terpenoid compounds from needles of ponderosa pine trees growing in situ: Control by light, temperature and stomatal conductance. Oecologia 2014, 176, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, S.; Seco, R.; Kim, S.; Guenther, A. Heat stress strongly induces monoterpene emissions in some plants with specialized terpenoid storage structures. Agric. For. Meteorol. 2023, 333, 13. [Google Scholar] [CrossRef]
- Tissier, A.; Morgan, J.A.; Dudareva, N. Plant Volatiles: Going ’In’ but not ’Out’ of Trichome Cavities. Trends Plant Sci. 2017, 22, 930–938. [Google Scholar] [CrossRef]
- Foster, A.J.; Aloni, R.; Fidanza, M.; Gries, R.; Gries, G.; Mattsson, J. Foliar phase changes are coupled with changes in storage and biochemistry of monoterpenoids in western redcedar (Thuja plicata). Trees-Struct. Funct. 2016, 30, 1361–1375. [Google Scholar] [CrossRef]
- Chen, J.; Bi, H.; Yu, X.; Fu, Y.; Liao, W. Influence of physiological and Environ.mental factors on the diurnal variation in emissions of biogenic volatile compounds from Pinus tabuliformis. J. Environ. Sci. 2019, 81, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.J.; Dong, X.M.; Li, Q.; Chen, Z.X.; Liu, L. An update on the function and regulation of methylerythritol phosphate and mevalonate pathways and their evolutionary dynamics. J. Integr. Plant Biol. 2021, 63, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 64, pp. 665–700. [Google Scholar]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’etre for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, L.; Wu, J.; Lun, X.; Wang, X.; Li, X. Emission Pattern of Biogenic Volatile Organic Compounds from Wetland Vegetation. Atmosphere 2024, 15, 651. https://doi.org/10.3390/atmos15060651
Chen W, Wang L, Wu J, Lun X, Wang X, Li X. Emission Pattern of Biogenic Volatile Organic Compounds from Wetland Vegetation. Atmosphere. 2024; 15(6):651. https://doi.org/10.3390/atmos15060651
Chicago/Turabian StyleChen, Wenbin, Luxi Wang, Ju Wu, Xiaoxiu Lun, Xiaoyue Wang, and Xiaoyi Li. 2024. "Emission Pattern of Biogenic Volatile Organic Compounds from Wetland Vegetation" Atmosphere 15, no. 6: 651. https://doi.org/10.3390/atmos15060651





