Thermodynamic and Economic Analyses of a Novel Cooling, Heating and Power Tri-Generation System with Carbon Capture
Abstract
:1. Introduction
- (1)
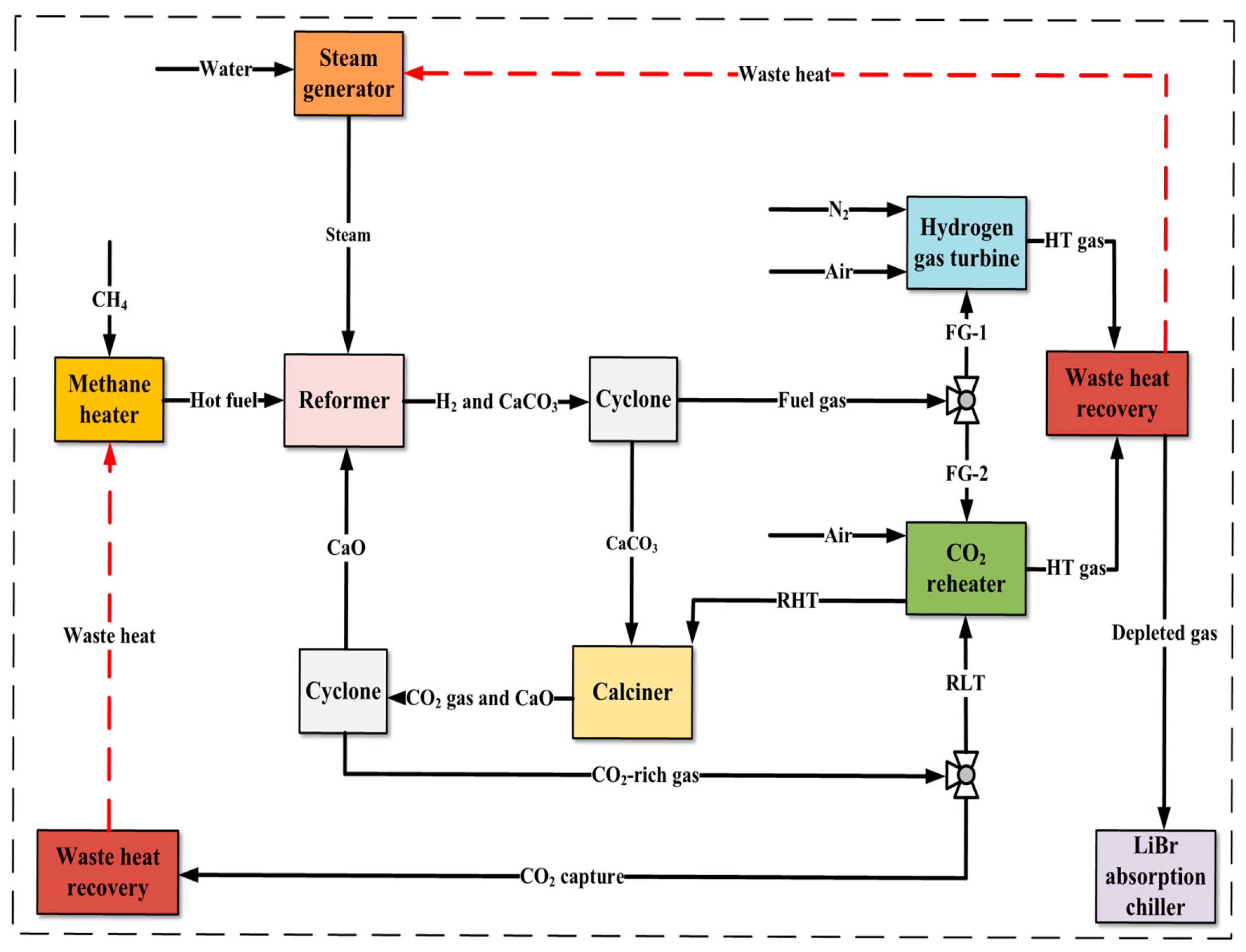
- The energy penalty for CO2 capture is small. This is because calcium looping in the dual fluidized bed reactor is utilized, and the heat consumption for the calcination reaction and the heat release for the carbonation reaction can be balanced in theory.
- (2)
- No additional heat source is required. This is because a CO2 reheater is designed to heat the circulating CO2 flow via burning a small portion of the hydrogen-rich gas generated by the system.
- (3)
- Highly pure CO2 can be generated as a by-product. Circulating CO2 is used as a heat carrier for the calcination process; thus, the produced CO2 is nearly pure and can be commercially used for other purposes.
2. Modeling Methods
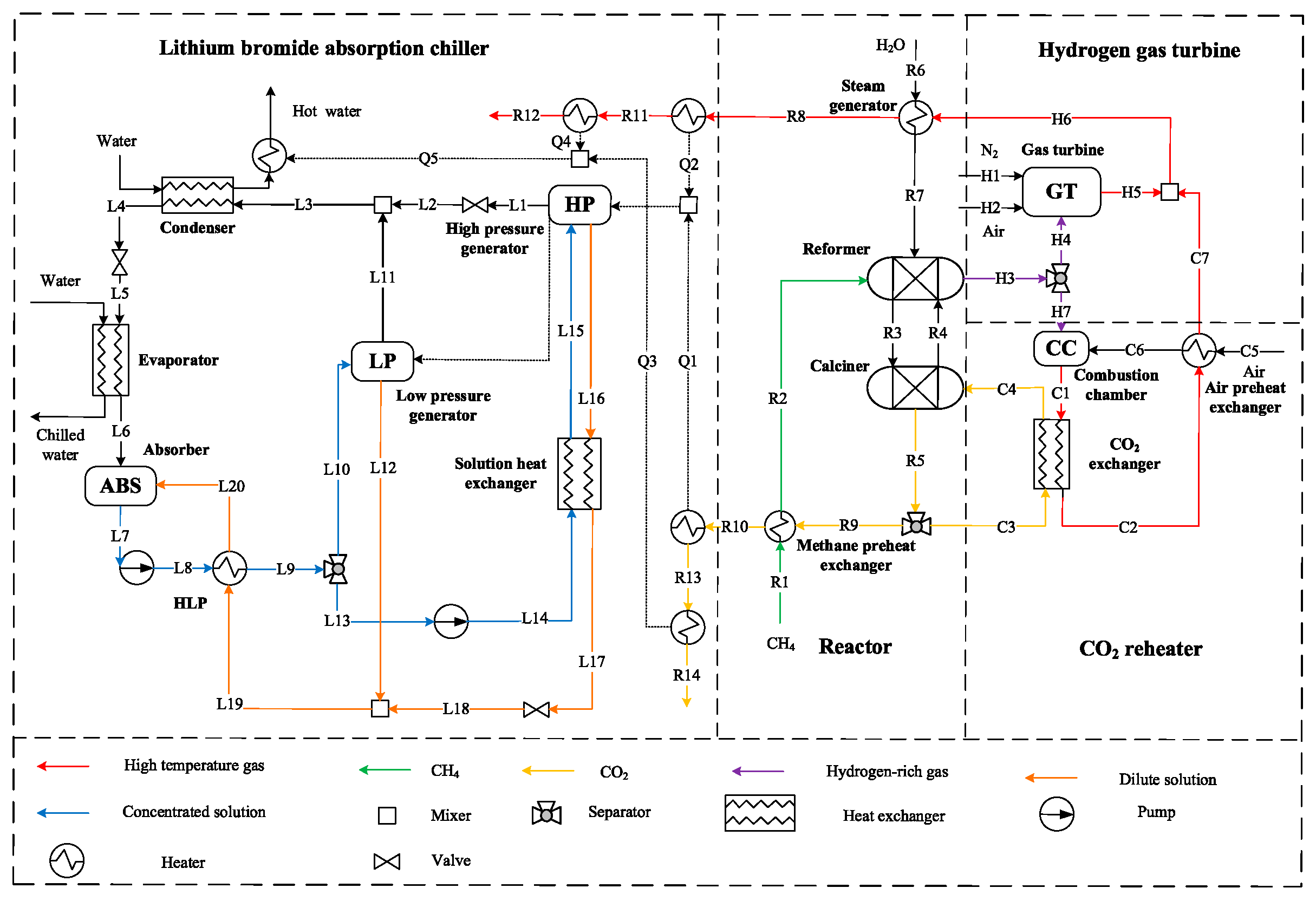
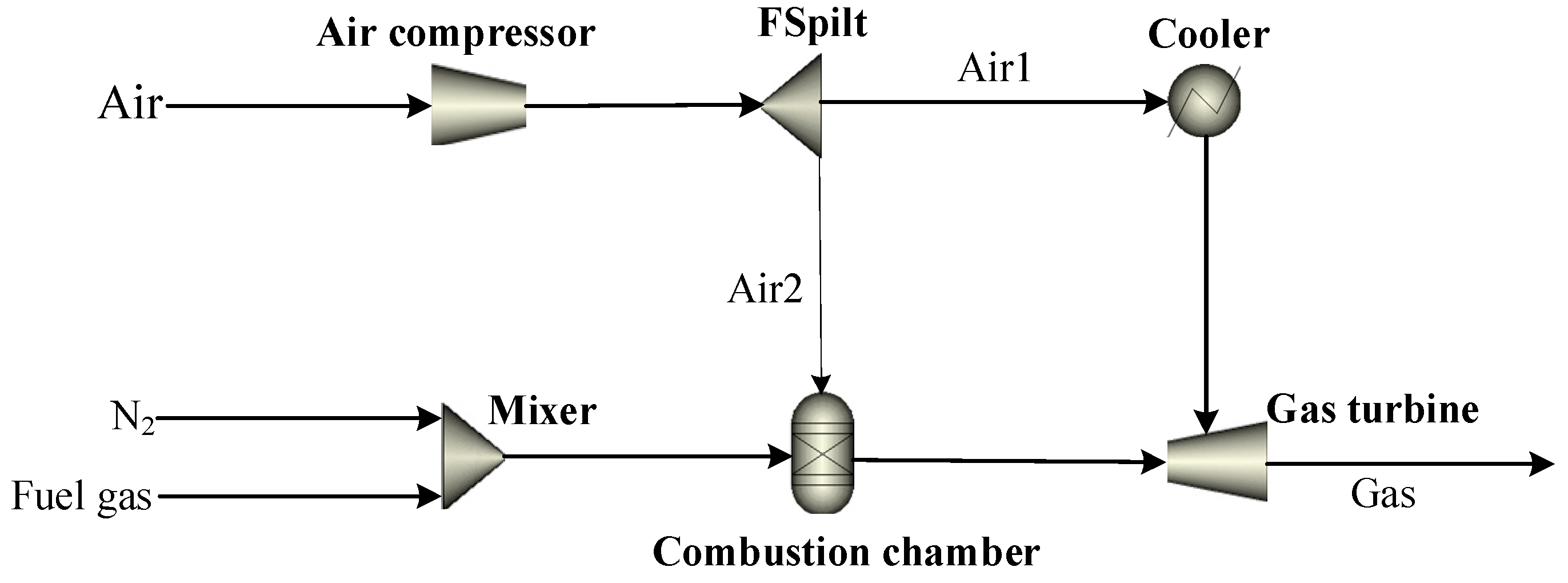
2.1. General Description
- (1)
- The system operates under steady-state conditions.
- (2)
- The mole fractions of O2 and N2 in air are 21% and 79%.
- (3)
- The system piping and material loss is ignored in the conveying process.
- (4)
- Pressure is constant in the condenser and the evaporator.
- (5)
- The pump efficiency is assumed to be 0.85.
- (6)
- The isotropic and mechanical efficiencies of turbine are 0.85 and 0.95.
2.2. Exergy Analyses Methodology
2.3. Economic Analyses Methodology
3. Modeling and Validation
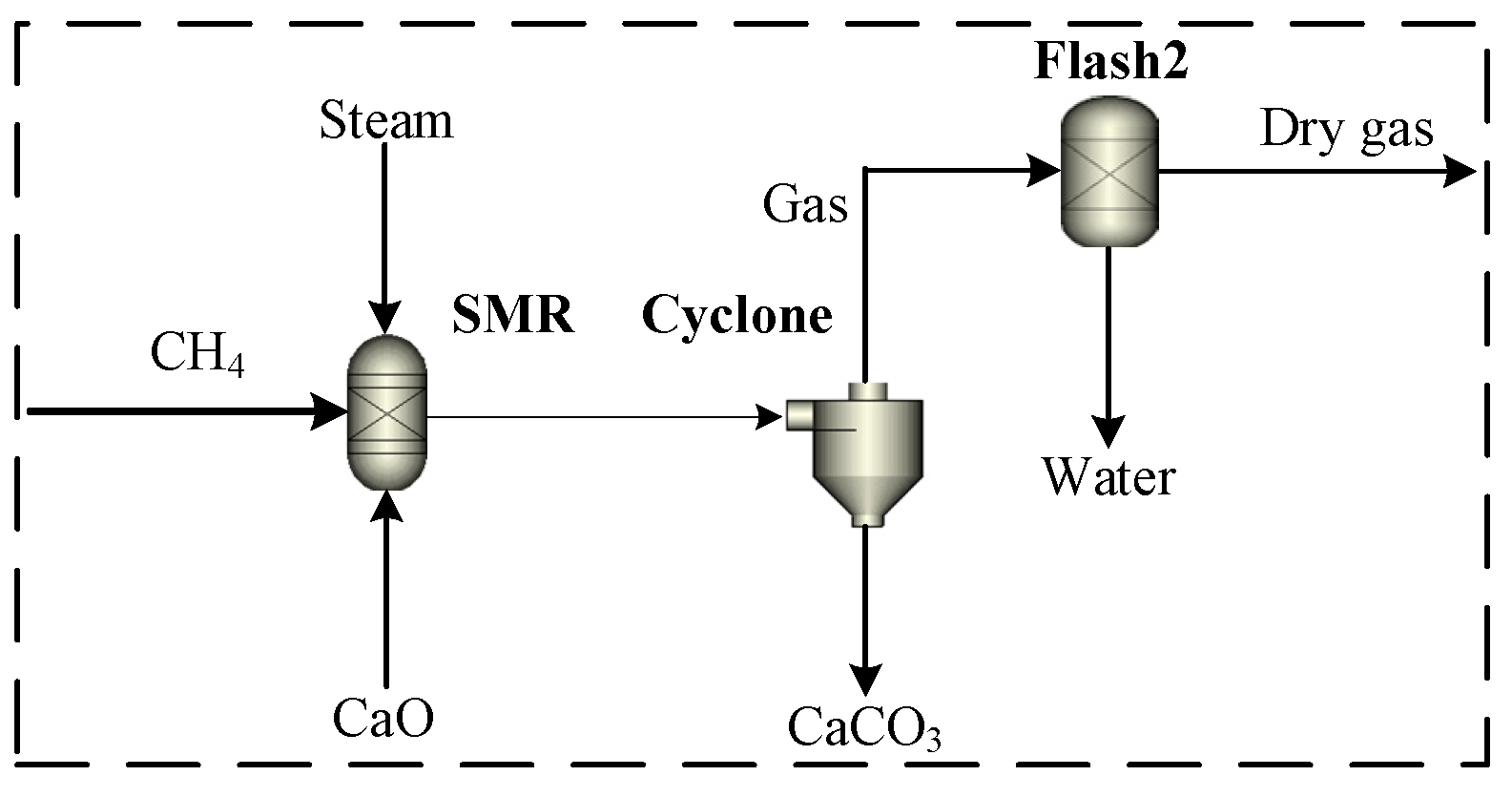
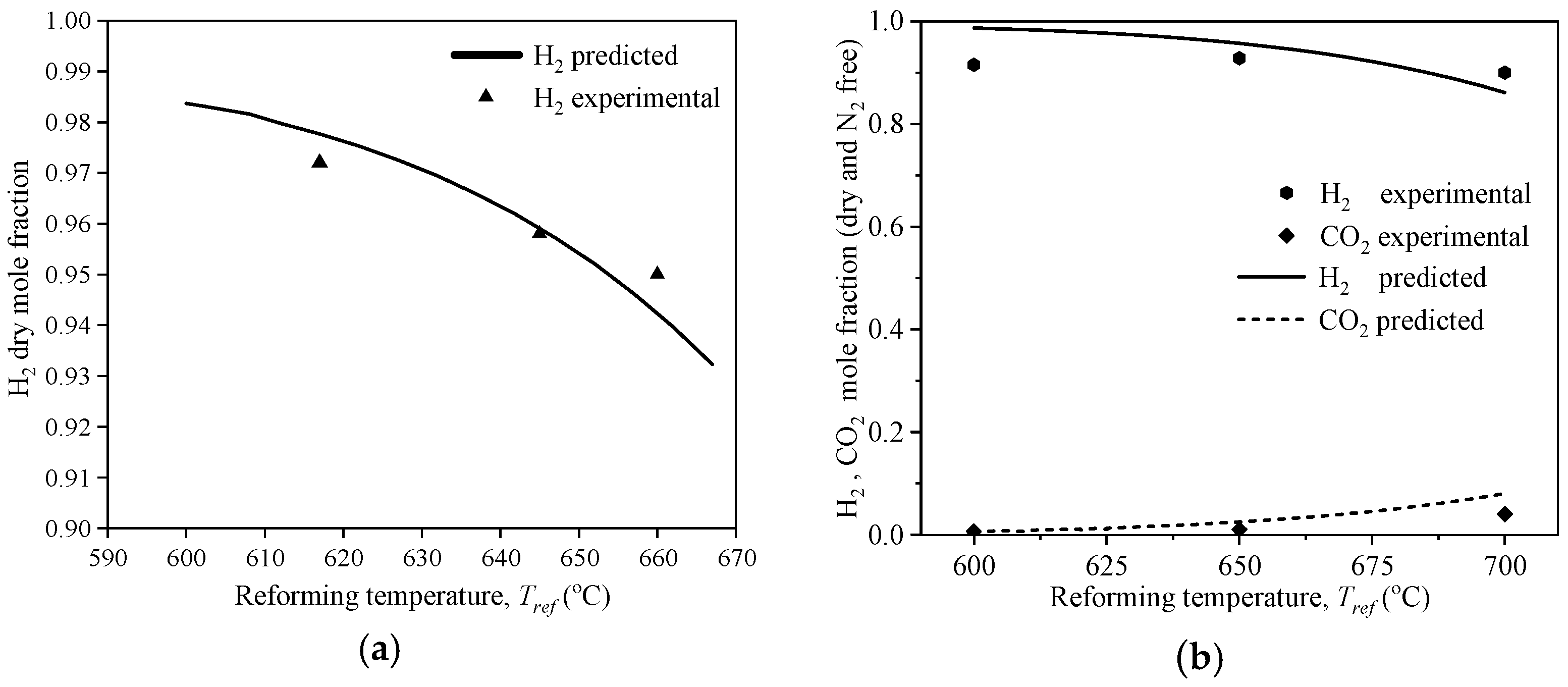
3.1. Sub-Model Validation for Sorption-Enhanced Steam Methane Reforming (SE-SMR)
3.2. Sub-Model Validation for the Hydrogen Gas Turbine
3.3. Sub-Model Validation for the Lithium Bromide Absorption Chiller
3.4. Economic Model Validation
4. Results and Discussion
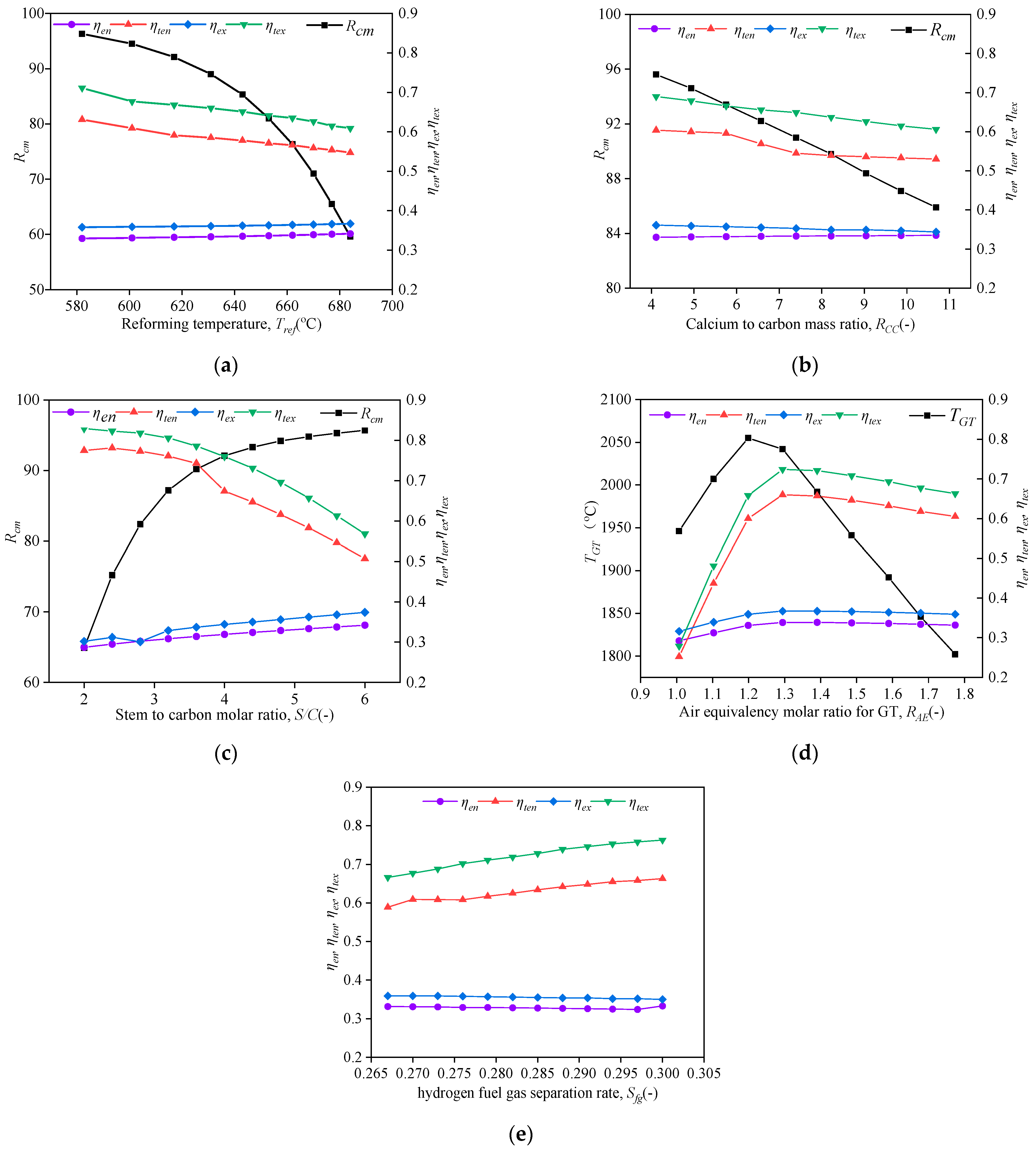
4.1. Sensitivity Analyses
4.2. Economic Predictions
4.3. Sub-Unit Optimization
5. Conclusions
- (1)
- The whole system’s ηten, ηtex, and Rcm can reach ~61%, ~68%, and ~96%, respectively. The SE-SMR reaction temperature and steam-to-carbon mole ratio have major impacts on Rcm, which can be over 90% as Tref < 620 °C and S⁄C > 4.0.
- (2)
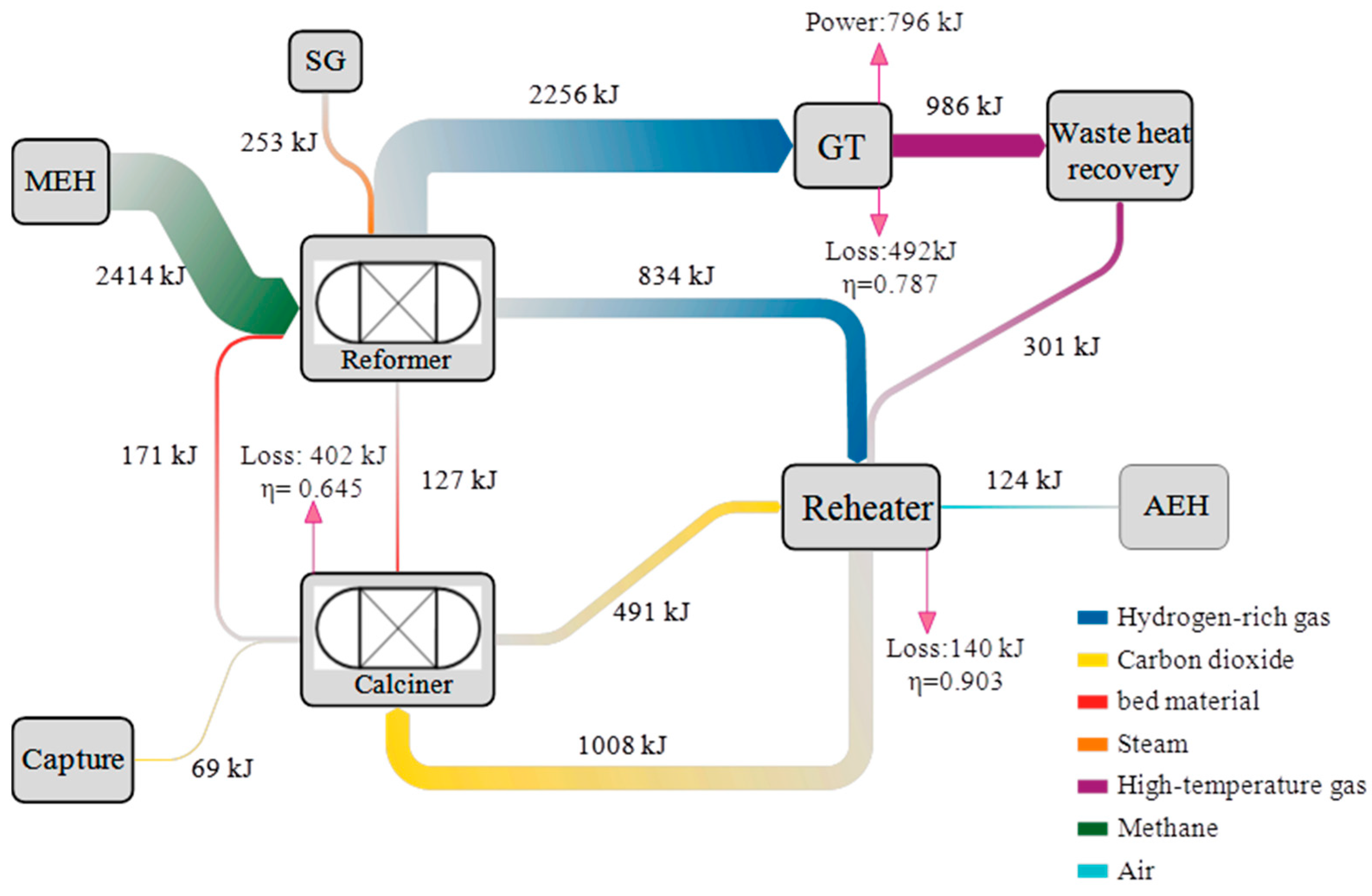
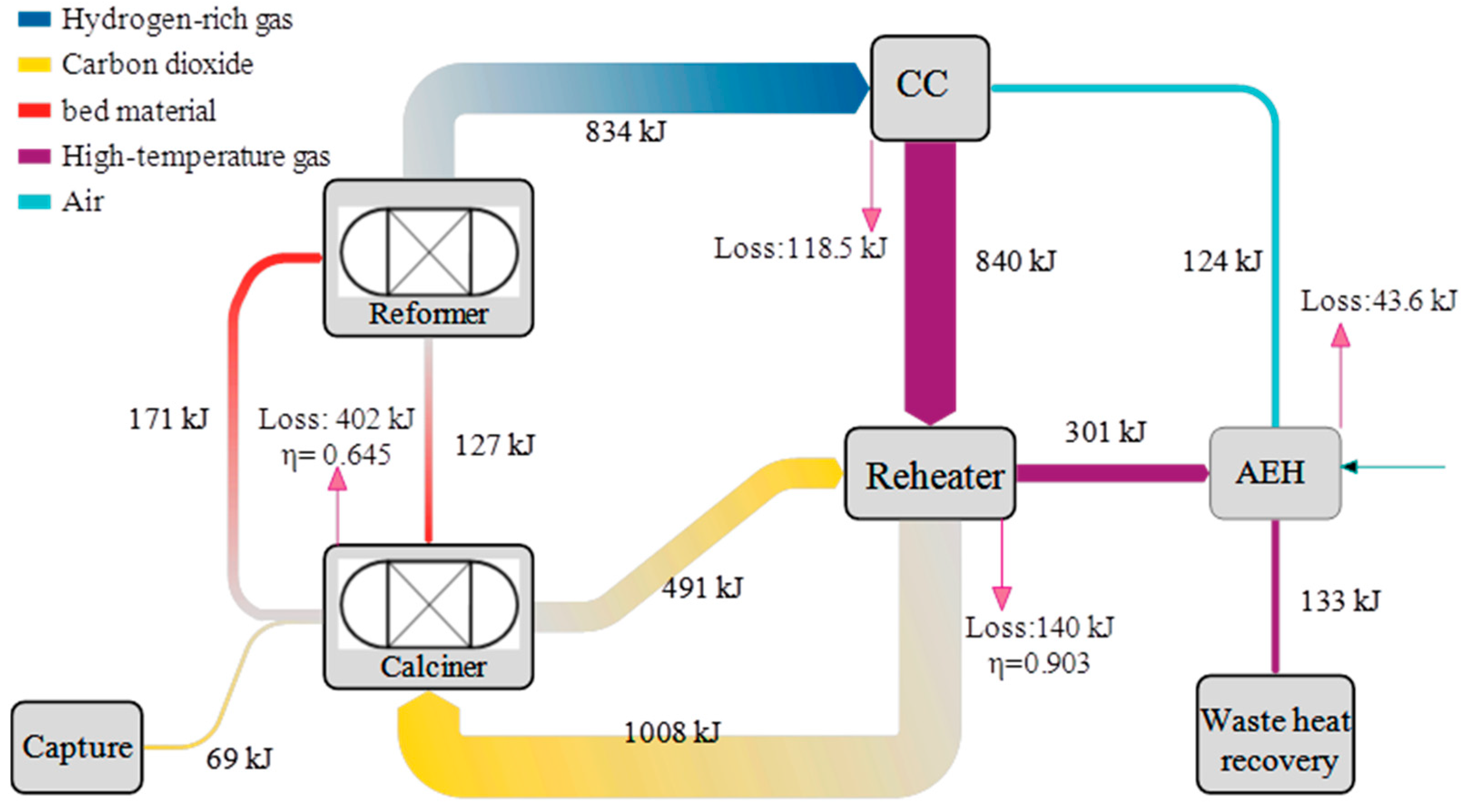
- The minimum exergy efficiency is 64.5%, existing at the calciner component. Although the GT has the highest exergy destruction of 492 kW, it has a higher exergy efficiency of 78.7%. The main cause of minimum exergy efficiency is the large temperature difference between the calciner’s internal environment and the circulating CO2 flows. It can be improved by increasing the CO2 recirculation flow rate and decreasing the reheating temperature.
- (3)
- The chiller can keep a high value of ηr and COP with the increase in RHL in the range of 0.50 to 0.60. The maximum values of ηr and COP are 0.52 and 1.33, respectively, as RHL is 0.57.
- (4)
- The specific cost of the product is 0.024 USD/kWh. The system annualized return on investment is about 12%, and the payback period of investment is about 9 years.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Guo, J. Analysis of multi-cascade CCHP system with gas turbine bypass extraction air energy storage. Appl. Therm. Eng. 2023, 232, 121021. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Lin, F.; Yang, K. Thermodynamic analysis of a novel combined cooling, heating, and power system consisting of wind energy and transcritical compressed CO2 energy storage. Energy Convers. Manag. 2022, 260, 119648. [Google Scholar] [CrossRef]
- Xia, Y.; Lei, H.; Wu, X.; Hu, G.; Pan, H.; Fang, B. Design of new test system for proton exchange membrane fuel cell. Energies 2023, 16, 833. [Google Scholar] [CrossRef]
- Feng, L.; Dai, X.; Mo, J.; Shi, L. Analysis of energy-matching performance and suitable users of conventional CCHP systems coupled with different energy storage systems. Energy Convers. Manag. 2019, 200, 112093. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, X.; Tu, Z. 4E analysis of a SOFC-CCHP system with a LiBr absorption chiller. Energy Rep. 2022, 8, 5284–5295. [Google Scholar] [CrossRef]
- Gao, L.; Hwang, Y.; Cao, T. An overview of optimization technologies applied in combined cooling, heating and power systems. Renew. Sustain. Energy Rev. 2019, 114, 109344. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, L.; Zheng, N.; Lu, Z. 4E Analysis of a novel combined cooling, heating and power system coupled with solar thermochemical process and energy storage. Energy 2023, 275, 127498. [Google Scholar] [CrossRef]
- Wang, X.; Duan, L.; Zheng, N. Thermodynamic analysis of a novel tri-generation system integrated with a solar energy storage and solid oxide fuel cell—Gas turbine. Appl. Therm. Eng. 2023, 219, 119648. [Google Scholar] [CrossRef]
- Ji, J.; Wen, W.; Xie, Y.; Xia, A.; Wang, W.; Xie, J.; Yin, Q.; Ma, M.; Huang, H.; Huang, X.; et al. Optimization and uncertainty analysis of Co-combustion ratios in a semi-isolated green energy combined cooling, heating, and power system (SIGE-CCHP). Energy 2024, 302, 131784. [Google Scholar] [CrossRef]
- Wang, L.; Bo, G.; Gao, R.; Ayadi, M.; Chammam, W.; Ooi, J.B.; Qin, M. Thermoeconomic assessment of an innovative combined cooling, heating, and power system based on biomass combustion, TCO2 cycle, absorption chiller, and desalination. Process Saf. Environ. Prot. 2024, 184, 151–169. [Google Scholar] [CrossRef]
- Jiang, R.; Yang, X. Performance analysis and application of a novel combined cooling, heating and power system integrated with multi-energy storage system. J. Energy Storage 2024, 86, 111276. [Google Scholar] [CrossRef]
- Lucarelli, G.; Genovese, M.; Florio, G.; Fragiacomo, P. 3E (energy, economic, environmental) multi-objective optimization of CCHP industrial plant: Investigation of the optimal technology and the optimal operating strategy. Energy 2023, 278, 127837. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; You, H. Exergoeconomic analysis and multi-objective optimization of a CCHP system based on SOFC/GT and transcritical CO2 power/refrigeration cycles. Appl. Therm. Eng. 2023, 230, 120686. [Google Scholar] [CrossRef]
- Eddine, G.D.; Meriem, A.; Khaled, L.; Umberto, D.; Mohand, T. Multi-objective optimization of CCHP system with hybrid chiller under new electric load following operation strategy. Energy 2021, 219, 119574. [Google Scholar]
- Mahmood, C.; Reza, N.; Majid, H.S.; Rahmat, M.; Seyedesmail, H. Energy, exergy, economic, and environmental (4E) analyses and optimization of a CCHP system with steam turbine. Energy Sci. Eng. 2021, 9, 867–915. [Google Scholar]
- Song, Z.; Liu, T.; Liu, Y.; Jiang, X.; Lin, Q. Study on the optimization and sensitivity analysis of CCHP systems for industrial park facilities. Int. J. Electr. Power Energy Syst. 2020, 120, 105984. [Google Scholar] [CrossRef]
- Fang, B.; Daniel, L.; Bonakdarpour, A.; Wilkinson, D. Upgrading the state-of-the-art electrocatalysts for PEM fuel cell applications. Adv. Mater. Interfaces 2022, 9, 2200349. [Google Scholar] [CrossRef]
- Fang, B.; Daniel, L.; Bonakdarpour, A.; Govindarajan, R.; Sharman, J.; Wilkinson, D. Dense Pt nanowire electrocatalysts for improved fuel cell performance using a graphitic carbon nitride-decorated hierarchical nanocarbon support. Small 2021, 17, 2102288. [Google Scholar] [CrossRef] [PubMed]
- Darabadi, Z.A.A.; Mortaza, Y.; Hossein, N.; Farzad, M. Low-carbon hydrogen, power and heat production based on steam methane reforming and chemical looping combustion. Energy Convers. Manag. 2023, 279, 116752. [Google Scholar]
- Di Giuliano, A.; Gallucci, K. Sorption enhanced steam methane reforming based on nickel and calcium looping: A review. Chem. Eng. Process.-Process Intensif. 2018, 130, 240–252. [Google Scholar] [CrossRef]
- García-Lario, A.L.; Aznar, M.; Martinez, I.; Grasa, G.S.; Murillo, R. Experimental study of the application of a NiO/NiAl2O4 catalyst and a CaO-based synthetic sorbent on the sorption enhanced reforming process. Int. J. Hydrogen Energy 2015, 40, 219–232. [Google Scholar] [CrossRef]
- Meyer, J.; Mastin, J.; Pinilla, C.S. Sustainable Hydrogen Production from Biogas Using Sorption-Enhanced Reforming. Energy Procedia 2014, 63, 6800–6814. [Google Scholar] [CrossRef]
- Yan, L.; Li, K.; Sui, H.; He, B.; Geng, C.; Fang, B. Simulation of Sorption-Enhanced Steam Methane Reforming over Ni-Based Catalyst in a Pressurized Dual Fluidized Bed Reactor. Inorganics 2023, 11, 107. [Google Scholar] [CrossRef]
- Yan, Y.; Manovic, V.; Anthony, E.J.; Clough, P.T. Techno-economic analysis of low-carbon hydrogen production by sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 226, 113530. [Google Scholar] [CrossRef]
- Yan, Y.; Thanganadar, D.; Clough, P.T.; Mukherjee, S.; Patchigolla, K.; Manovic, V.; Anthony, E.J. Process simulations of blue hydrogen production by upgraded sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 222, 113144. [Google Scholar] [CrossRef]
- Aspen Technology, Inc. Aspen Plus User Models; Aspen Technology, Inc.: Bedford, MA, USA, 2015. [Google Scholar]
- Modi, B.; Mudgal, A.; Patel, B. Energy and Exergy Investigation of Small Capacity Single Effect Lithium Bromide Absorption Refrigeration System. Energy Procedia 2017, 109, 203–210. [Google Scholar] [CrossRef]
- Zare, A.D.; Saray, R.K.; Mirmasoumi, S.; Bahlouli, K. Optimization strategies for mixing ratio of biogas and natural gas co-firing in a cogeneration of heat and power cycle. Energy 2019, 181, 635–644. [Google Scholar] [CrossRef]
- Darabadi, Z.A.A.; Mortaza, Y.; Farzad, M.; Hossein, N.; Umberto, D. Thermodynamic and exergoeconomic analysis of a multi- generation gas-to-X system based on fuel-rich combustion to produce power, hydrogen, steam and heat. Sustain. Cities. Soc. 2019, 86, 104139. [Google Scholar]
- Eveloy, V.; Karunkeyoon, W.; Rodgers, P.; Alili, A.A. Energy, exergy and economic analysis of an integrated solid oxide fuel cell—Gas turbine—Organic Rankine power generation system. Int. J. Hydrogen Energy 2016, 41, 13843. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Sayyad, S.; Zonouz, M.J. Energy, exergy and sensitivity analyses of a hybrid combined cooling, heating and power (CCHP) plant with molten carbonate fuel cell (MCFC) and Stirling engine. J. Clean. Prod. 2017, 148, 283–294. [Google Scholar] [CrossRef]
- Yumrutas, R.; Kunduz, M.; Kanoğlu, M. Exergy analysis of vapor compression refrigeration systems. Int. J. Exergy 2002, 2, 266. [Google Scholar] [CrossRef]
- Bereche, R.P.; Gonzales, R.; Nebra, S.A. Exergy calculation of lithium bromide–water solution and its application in the exergetic evaluation of absorption refrigeration systems LiBr-H2O. Int. J. Energy Res. 2012, 36, 166–181. [Google Scholar] [CrossRef]
- Yan, L.B.; Cao, Y.; He, B.S. Energy, exergy and economic analyses of a novel biomass fueled power plant with carbon capture and sequestration. Sci. Total Environ. 2019, 690, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.B.; Cao, Y.; Wang, Z.L.; He, B.S. On a novel carbon-negative IGCC system with cascade CO2 combined cycle. Energy Convers. Manag. 2020, 221, 113202. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, P.; Yao, J.; Zhang, S.; Ren, J.; Yang, F.; Zhang, Z. Combined biomass gasification, SOFC, IC engine, and waste heat recovery system for power and heat generation: Energy, exergy, exergoeconomic, environmental (4E) evaluations. Appl. Energy 2020, 279, 115794. [Google Scholar] [CrossRef]
- Mohammad, O.; Faryar, S.; Mojtaba, O. A solar-biomass system associated with CO2 capture, power generation and waste heat recovery for syngas production from rice straw and microalgae: Technological, energy, exergy, exergoeconomic and environmental assessments. Appl. Energy 2023, 340, 120999. [Google Scholar]
- Moghimi, M.; Emadi, M.; Ahmadi, P.; Moghadasi, H. 4E analysis and multi-objective optimization of a CCHP cycle based on gas turbine and ejector refrigeration. Appl. Therm. Eng. 2018, 141, 516–530. [Google Scholar] [CrossRef]
- You, H.; Han, J.; Liu, Y.; Chen, C.; Ge, Y. 4E analysis and multi-objective optimization of a micro poly-generation system based on SOFC/MGT/MED and organic steam ejector refrigerator. Energy 2020, 206, 118122. [Google Scholar] [CrossRef]
- Yuan, Z.; Liang, T.; Yang, K. An integrated energy storage system consisting of Compressed Carbon dioxide energy storage and Organic Rankine Cycle: Exergoeconomic evaluation and multi-objective optimization. Energy 2022, 247, 123566. [Google Scholar]
- Wang, S.; Fu, Z. Thermodynamic and economic analysis of solar assisted CCHP-ORC system with DME as fuel. Energy Convers. Manag. 2019, 186, 535–545. [Google Scholar] [CrossRef]
- Cormos, C.C.; Vatopoulos, K.; Tzimas, E. Assessment of the consumption of water and construction materials in state-of-the-art fossil fuel power generation technologies involving CO2 capture. Energy 2013, 51, 37–49. [Google Scholar] [CrossRef]
- Di Carlo, A.; Bocci, E.; Zuccari, F.; Dell’Era, A. Numerical Investigation of Sorption Enhanced Steam Methane Reforming Process Using Computational Fluid Dynamics Eulerian−Eulerian Code. Ind. Eng. Chem. Res. 2010, 49, 1561–1576. [Google Scholar] [CrossRef]
- Abanades, J.C. The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3. Chem. Eng. J. 2017, 90, 303–306. [Google Scholar] [CrossRef]
- Lan, W.; Chen, G.; Zhu, X.; Wang, X.; Liu, C.; Xu, B. Biomass gasification-gas turbine combustion for power generation system model based on ASPEN PLUS. Sci. Total Environ. 2018, 628, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.E.; Radermacher, R.; Klein, S.A. Absorption Chillers and Heat Pumps; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Xu, L.; Huang, Y.; Li, G. Simulation and analysis of dual-effect absorption refrigeration units based on Aspen Plus. Build. Therm. Vent. Air Cond. 2021, 40, 6. [Google Scholar]



| Design Parameters | Value |
|---|---|
| Ambient pressure (kPa) | 101.3 |
| Ambient temperature (°C) | 25 |
| Steam-to-carbon mole ratio (−) | 2–6 |
| Gas turbine back pressure (kPa) | 107 |
| Isentropic efficiency of compressor (%) | 0.85 |
| Isentropic efficiency of pump (%) | 0.9 |
| Lower heating value of CH4 (kJ/kg) | 49,303 |
| Lower heating value of H2 (kJ/kg) | 11,900 |
| AC pressure ratio (−) | 17 |
| Maximum effectiveness of heat exchangers (%) | 95 |
| The life time (year) | 30 |
| Annual plant operating hours (h) | 8000 |
| Design Parameters | Value |
|---|---|
| GEN-HP operation pressure (kPa) | 66 |
| GEN-LP operation pressure (kPa) | 4 |
| Concentration of concentrated solution (%) | 60 |
| Concentration of dilute solution (%) | 55 |
| Pressure of condenser (kPa) | 4 |
| Pressure of evaporator (kPa) | 1 |
| Pressure of absorber (kPa) | 1 |
| High-pressure stage solution flow rate (kg/s) | 0.51 |
| Low-pressure stage solution flow rate (kg/s) | 0.70 |
| Mass flow rate ratio of GEN-LP and GEN-HP (−) | 0.578 |
| TIC Calculation | Methods |
|---|---|
| Equipment cost CE | See Table 4 |
| Utilities and offsite units installed cost (A) | 25% of main equipment cost |
| Installed cost (IC) | |
| Owners’ cost and contingency (B) | 15% of IC |
| Land purchase, permitting, surveying, etc. (C) | 5% of IC |
| Total investment costs (TIC) | |
| O&M calculation | Methods |
| VOM costs (D) | SUM (1:8) |
| 1 Raw material (excluding fuel) | See Table 5 |
| 2 Fuel cost | See Table 5 |
| 3 Maintenance | 3% of TIC |
| 4 Operating supplies | 1% of TIC |
| 5 Patents and royalties | 3% of O&M costs |
| 6 Operating labor | 4 laborers [34] |
| 7 Supervision/clerical | 10% of operating labor |
| 8 Laboratory charges | 10% of operating labor |
| Fixed charges (F1) | SUM (9:11) |
| 9 Capital charge | β*TIC |
| 10 Local taxes | 2% of TIC |
| 11 Insurance | 1% of TIC |
| Plant overhead (F2) | 5% of O&M costs |
| General expenses (F3) | SUM (12:14) |
| 12 Marketing, sales, distribution | 2% of O&M costs |
| 13 Administrative | 2% of O&M costs |
| 14 R&D | 5% of O&M costs |
| FOM costs () | - |
| CO2 emission penalty (G) | CO2 emission in flue gases * ETS CO2 price |
| O&M costs | D + F + G |
| Components | Equipment Cost Equations | Year | Source |
|---|---|---|---|
| Reformer | 2014 | [36] | |
| Calciner | 2015 | [37] | |
| GT | 2020 | [38,39] | |
| AEH | 2014 | [40,41] | |
| MEH | 2014 | [7,40] | |
| CBH | 2014 | [7,40] | |
| SG | 2014 | [7,40] | |
| Chiller | 2019 | [41] | |
| Heating | 2014 | [7,40] |
| Items | Assumptions | Source |
|---|---|---|
| CaO cost | 5.534 USD/t | [37] |
| CH4 cost | 9.51 USD/GJ | [29] |
| Ni cost | 21,800 USD/t | - |
| LiBr cost | 690.7 USD/t | - |
| Cooling water price | 0.014 USD/t | [42] |
| Labor employee cost | 11,634.2 USD/year | [34] |
| ETS CO2 price | 21.59 USD/t | [35] |
| CO2 price | 6.11 USD/t | [35] |
| Economical plant life | 20 years | [13] |
| Parameters | Design Value | Model Prediction | Deviation % |
|---|---|---|---|
| Compression ratio | 17 | 17 | - |
| Expansion ratio | 17 | 17 | - |
| Net electric power (MW) | 270 | 274.5 | 1.70 |
| Gas turbine inlet temperature (°C) | 1400 | 1409.5 | 0.70 |
| Gas turbine outlet temperature (°C) | 586 | 571.0 | 2.60 |
| Air mass flow rate (kg/s) | 651 | 649.5 | 0.20 |
| Net power efficiency (%) | 38.2 | 38.5 | 0.80 |
| Parameters | Literature Data | Simulation Results | Deviation % |
|---|---|---|---|
| GENHP load (kW) | 265.4 | 266 | −0.23 |
| GENHP to GENLP energy (kW) | 201.9 | 199.5 | 1.19 |
| Condenser load (kW) | 183.6 | 180.7 | 1.58 |
| Absorber load (kW) | 442.5 | 435.2 | 1.65 |
| Evaporator load (kW) | 360.6 | 349.8 | 2.99 |
| Component | Unit | Literature Data | Economic Data | Deviation % | Source |
|---|---|---|---|---|---|
| Reformer | USD/kmol | 0.172 | 0.164 | 4.70 | [36] |
| Calciner | USD/t | 1.663 | 1.585 | 4.90 | [37] |
| Gas turbine | USD/h | 3.527 | 3.624 | 2.80 | [38,39] |
| Components | Exergy Destruction (kW) | η (%) | Proportion (%) |
|---|---|---|---|
| Calciner | 402 | 64.5 | 38.9 |
| GT | 492 | 78.7 | 47.6 |
| Reheater | 140 | 90.3 | 13.5 |
| TIC Calculation | Value (kUSD) |
|---|---|
| Equipment cost CE | 1023.3 |
| Utilities and offsite units installed cost (A) | 255.8 |
| Installed cost (IC) | 1279.1 |
| Owners’ cost and contingency (B) | 191.9 |
| Land purchase, permitting, surveying, etc. (C) | 63.9 |
| Total investment costs (TIC) | 1534.9 |
| O&M calculation | Value (kUSD) |
| VOM costs (D) | 900.7 |
| 1 Raw material (excluding fuel) | 83.8 |
| 2 Fuel cost | 662.2 |
| 3 Maintenance | 46.0 |
| 4 Operating supplies | 15.3 |
| 5 Patents and royalties | 37.7 |
| 6 Operating labor | 46.5 |
| 7 Supervision/clerical | 4.6 |
| 8 Laboratory charges | 4.6 |
| Fixed charges (F1) | 144.2 |
| 9 Capital charge | 98.2 |
| 10 Local taxes | 30.7 |
| 11 Insurance | 15.3 |
| Plant overhead (F2) | 60.7 |
| General expenses (F3) | 109.3 |
| 12 Marketing, sales, distribution | 24.3 |
| 13 Administrative | 24.3 |
| 14 R&D | 60.7 |
| FOM costs () | 314.2 |
| CO2 emission penalty (G) | 3.8 |
| O&M costs | 1218.7 |
| Components | C0 (kUSD) |
|---|---|
| Reformer | 284.2 |
| Calciner | 46.2 |
| GT | 579.8 |
| AEH | 3.5 |
| MEH | 1.8 |
| CBH | 7.8 |
| SG | 10.3 |
| Chiller | 74.8 |
| Heating | 14.9 |
| Items | Cost (kUSD) |
|---|---|
| CaO cost | 37.8 |
| CH4 cost | 662.2 |
| Ni cost | 24.8 |
| LiBr cost | 16.3 |
| Cooling water price | 4.9 |
| Labor employee cost | 290.8 |
| ETS CO2 price | 3.8 |
| CO2 price | 22.1 |
| Model | RAEC | Rcir | Tcir (°C) | η (%) | Exergy Destruction (kW) |
|---|---|---|---|---|---|
| A | 1.40 | 7.03 | 1477 | 64.4 | 404 |
| B | 1.40 | 8.72 | 1327 | 65.9 | 409 |
| C | 1.94 | 12.3 | 1227 | 72.5 | 403 |
| D | 1.94 | 8.81 | 1377 | 67.6 | 410 |
| E | 1.94 | 14.0 | 1177 | 74.4 | 401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Jia, Z.; Liu, Y.; Geng, C.; He, B. Thermodynamic and Economic Analyses of a Novel Cooling, Heating and Power Tri-Generation System with Carbon Capture. Atmosphere 2024, 15, 836. https://doi.org/10.3390/atmos15070836
Yan L, Jia Z, Liu Y, Geng C, He B. Thermodynamic and Economic Analyses of a Novel Cooling, Heating and Power Tri-Generation System with Carbon Capture. Atmosphere. 2024; 15(7):836. https://doi.org/10.3390/atmos15070836
Chicago/Turabian StyleYan, Linbo, Ziyue Jia, Yang Liu, Cong Geng, and Boshu He. 2024. "Thermodynamic and Economic Analyses of a Novel Cooling, Heating and Power Tri-Generation System with Carbon Capture" Atmosphere 15, no. 7: 836. https://doi.org/10.3390/atmos15070836





