Abstract
The Neotropics represent a hotspot for freshwater biodiversity with vast number of fish species of scarce ecological knowledge. This holds true for the Uruguay River, where fish assemblages and their diets remain unexplored. Fish assemblages were surveyed in 14 sites along the river main course, from headwaters to mouth (approximately 1800 km), with the aim to identify the trophic roles of fishes and to describe trophic structure of these assemblages, following standardized sampling campaigns and laboratory procedures. One hundred species (2309 gut contents) were analysed and classified into four trophic groups subdivided into eight lower-level groups: Piscivore, piscivore-invertivore, detritivore, omnivore-detritivore, omnivore-invertivore, omnivore-planktivore and omnivore-herbivore. The trophic structure of the assemblages varied along the river, with the relative species richness of fish consuming terrestrial invertebrates increasing towards the middle river section, probably driven by the large floodplains in that areas, supporting global theories such as the flood pulse concept. This study describes the feeding habits of fish along the Uruguay River, being the first dietary description for 29 species. This knowledge is essential for management and conservation, serving as baseline in the context of future environmental changes while generating novel evidence on the functioning of ecosystems in this scarcely studied climatic region.
1. Introduction
The knowledge about trophic structure of communities is essential to understand some of the main relationships among species in ecosystems [1,2,3]. Information of the feeding habits of species permits a holistic understanding of ecosystem functioning [4]. In aquatic ecosystems, fish are used to describe food webs since they occupy a great diversity of trophic niches and circulate matter and energy from basal resources to the highest levels of the web [5,6]. They are also capable to move between different habitats within the water body and even connect different ecosystems through feeding interactions, for example by feeding on allochthonous material from the riverbanks and riparian zones [5,6,7] or by migrating between rivers and the sea. The analysis of fish diets is also important to better understand the behaviour of the species [8,9]. Large-scale trophic groups’ classification is the basis to understand the trophic structure of assemblages and their natural spatial or temporal variability (e.g., [10,11]). In fluvial ecosystems, longitudinal gradients in fish assemblage trophic structure are often found, where the relative importance of different trophic groups shifts from headwaters to mouth, possibly following changes in energy availability and habitat structure (e.g., [12,13,14,15,16,17]). Some evidence suggests that the trophic structure of the fish assemblages changes from dominance of small compressed-bodied benthivorous fishes in headwaters towards higher importance of omnivores-herbivores, planktivorous and piscivorous strategists in the lower sections [12,13,14,15]. However, most of this evidence comes from streams and low order river ecosystems (e.g., river orders 1–5), but longitudinal patterns in fish trophic structure in large river ecosystems remain largely unexplored.
Moreover, most of the theories that aim to explain river functioning have been generated in temperate regions of the northern hemisphere. Despite that the Neotropical region represents one of the largest hotspots for freshwater fish biodiversity [18,19], the functioning of its riverine ecosystems and the biology of the vast majority of the species remains understudied [20,21]. Besides, while most Neotropical fish assemblage studies focus on tropical and subtropical rivers, with marked flow seasonality (e.g., Amazonas River, Parana River, Orinoco River [22,23,24,25]), less research effort has been made in large irregular flow rivers of southern subtropical areas (see [26,27,28]). Particularly within the La Plata River basin, most studies describing aspects of fish biology focused on the large Parana River (e.g., [29,30,31]) while its smaller tributary—a 1800 km long and 6000 m3/s river bearing at least 10 species of long-distance migratory fish of commercial importance—the irregular-flow subtropical Uruguay River remains largely unstudied in its total extension [32]. Research on this region is highly necessary given that, as most freshwaters in South America, it faces a growing biodiversity loss rate [19,29,33].
The knowledge about South America’s fish assemblages is based almost exclusively on taxonomical records and species distribution analyses [19,34]. The scarce information available for the Uruguay River is not the exception, consisting mostly on scientific notes reporting length-weight relationships [10,35], or new records of a few rare species [36,37]. Moreover, most fish ecology research made in the Uruguay River has been focused on few commercially important migratory species such as sabalo (Prochilodus lineatus), boga (Megaleporinus obtusidens) and dorado (Salminus brasilinsis) (e.g., [26,38]). Most of these migratory species migrate between Paraná, Río de la Plata and Uruguay Rivers to use different feeding and reproduction grounds along the fluvial gradient; but several local species exist along the river as well [26]. Regarding fish trophic ecology, and to the extent of our knowledge, only few studies describing the diet of limited key species exist (e.g., [39,40,41,42]).
The objective of this study was to report the fish species present in the Uruguay River, describing their diets with the aim of reaching a standardized and objective classification in trophic groups. Furthermore, this study was also aimed to describe the spatial variation in the trophic structure of assemblages from upper to lower river sections, facilitating the comprehension of the structure and functioning of the unstudied fish assemblages in this large subtropical river serving as baseline information for management purposes.
2. Materials and Methods
2.1. Study Area and Fish Sampling
This research was conducted in the Uruguay River, the second largest tributary of the La Plata River drainage basin. This river rises at the confluence of the Pelotas River and the Canoas River in Brazil, and extends for 1800 km to its mouth in the La Plata estuary shared between Uruguay and Argentina [43]. The drainage basin covers three countries: Brazil, Argentina and Uruguay, with the largest area in the states of Santa Catarina and Rio Grande do Sul, Brazil [44]. According to geological characteristics, the Uruguay River could be divided into three main regions; upper, middle and lower sections. The Yucuma Falls in Brazil represent the division between the upper and middle section, while the Salto Grande Dam (Uruguay) divides the middle from the lower section of the river [45]. The hydrology of the Uruguay River is determined by the precipitation patterns in the upper two-thirds of the catchment (upper and middle sections) and, as opposite to the Parana River, does not show a seasonal pattern in flow, being highly irregular [46]. The hydrological conditions differ between the three sections, with a steeper slope and faster current velocities in the upper than in the middle section. On the other hand, the river flow in the lower section is constrained by the hydroelectric dam of Salto Grande [26]. The dominant substrates in the upper and middle region of the river are typically hard rocks, whereas in the lower section, sandstone substrate prevails [43]. In all the extension of the Uruguay River four hydroelectric dams were built (three in the upper section, and one in the lower section). The middle section remains largely hydrologically undisturbed.
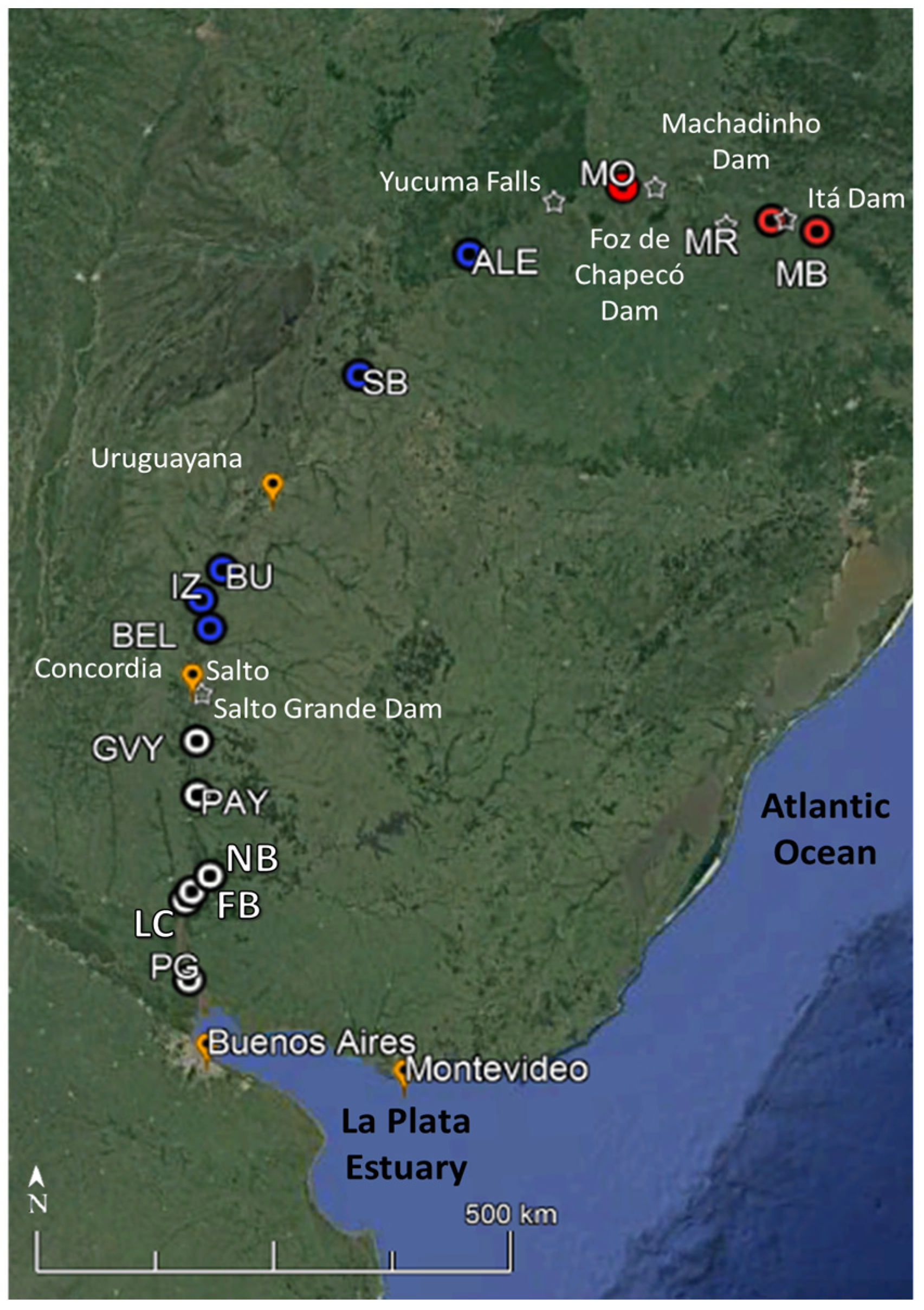
Fish samplings were performed during austral autumn of 2017 (May–early June) in 14 sites of the main course of Uruguay River, from its headwaters (States of Santa Catarina and Rio Grande do Sul, Brazil) to the mouth (Colonia, Uruguay). Three locations were sampled in the upper river section, five in the middle section, and six in the lower section (Figure 1, Tables S1 and S2). Sampling locations were chosen considering available monitoring programs along the river easing logistics for this study. At all sites, littoral habitats of depths from 1–4 m were sampled to cover for a similar range of environmental variability in each area. In large river ecosystems, littoral areas usually host the highest biodiversity. Furthermore, the autumn season was chosen to sample because during that season, a higher diversity of fish size ranges might be expected as the spawning of most species of the region usually occur in spring-summer and then, both juvenile and adults of most species could be collected during autumn.
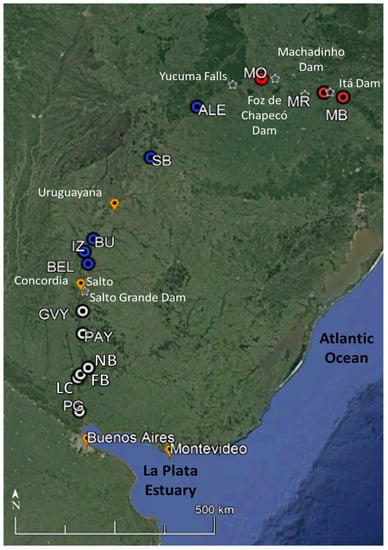
Figure 1.
Location of the 14 sampling sites of the Uruguay River. The Uruguay River sampling sites are coloured according to the different sections; red: Upper; blue: Middle, and white: Lower. MB: Barracão, at the confluence of Canoas River and Pelotas River; MR: Marcelino Ramos; MO: Mondaí; Ale: Alecrim; SB: São Borja; BU: Bella Unión; IZ: Isla del Zapallo island; Bel: Belén; GVY: Guaviyú; Pays: Paysandú; NB: Nuevo Berlín; FB: Fray Bentos; LC: Las Cañas, and PG: Punta Gorda, at the mouth of the Uruguay River. Major towns (orange) and waterfalls and dams (white star) are represented in the figure.
In the middle and lower sections of the river, fish collections were carried out using multi-mesh Nordic gillnets. In each site, four sets of benthic gillnets were placed in the littoral zone (1.5–2.0 m deep areas at 50–100 m away from the shore) and four in a deeper zone (2–5.0 m deep) about 500 m away from the shoreline. Each Nordic gillnet was 30 m long by 1.5 m high and were composed of 12 mesh sizes (5.0, 6.25, 8.0, 10.0, 12.5, 15.5, 19.5, 24.0, 29.0, 35.0, 43.0, and 55 mm knot to knot). Gillnets were set from sunset to sunrise (c.a. 12 h). The same sampling effort was performed in each site. Fish sampling and handling procedures were approved by the Honorary Commission of Animal Experimentation (CHEA) in Uruguay (Permit ID 309).
In the upper river section, due to a different standardization of the ongoing monitoring programs subsidizing this study, a set of gill and trammel nets were used, with mesh sizes ranging from 15.0 to 80.0 mm knot to knot, instead of Nordic gillnets. However, time of net set was comparable as net were also set overnight. Gillnets ranged from 20 to 120 m in length and from 1.6 to 8.0 m in height; while trammel nets varied between 30 to 40 m in length with 1.8 m height. Both set of nets were placed in the littoral zone in the evening and removed in the following morning, being set for approximately 12 h. At each site, additional sampling was performed with seine nets and cast nets (both with mesh size of 8.0 mm) in the littoral zone.
In addition to our own sampling campaigns, some commercially important large fish specimens were also obtained from local fishermen in the middle and lower sections of the river, as to complete the sampling wherever these species (known to be present along all the river) were not captured. Furthermore, to complement the diet description of some rare species (i.e., with less than five individuals collected during sampling) gut content data from two previous sampling campaigns arrayed in spring 2014 and autumn 2016 in the lower section of Uruguay River was used (Table S1).
In the field, fishes were identified to the lowest taxonomic level possible (i.e., species level in most cases), measured (total and standard length in cm) and weighed (total fresh biomass in g). For the gut content analysis, the stomach and intestines of 15 individuals per species and site, considering a wide size range (or all individuals obtained, when <15 were caught) were removed and preserved in 10% formalin for posterior laboratory analysis. A previous study in Uruguayan streams using prey species accumulation curves has established that 15 individuals usually suffice to represent well the richness of diet items [47]. Individuals were selected to cover all length classes obtained at each site (Table S1).
Gut content analysis (GCA) was performed in the laboratory. The occurring food items were classified broadly into eight item types as follows: Detritus, plankton (zooplankton and phytoplankton), periphyton (diatoms and filamentous algae), aquatic macroinvertebrates (insects, molluscs, and macrocrustaceans), terrestrial macroinvertebrates (terrestrial insects and arachnids), fish remains (entire fish, scales, fins and fish remains) aquatic macrophytes, and terrestrial vegetal matter (seeds, fruits and vegetal tissues). Zooplankton and phytoplankton were pooled because phytoplankton was only present in few individuals along with large amounts of zooplankton. The absolute volume of each food item was measured using standardized Hyslop’s indirect volumetric method. With this information, the relative contribution of each food item type to the diet of individuals was calculated [48].
The frequency of occurrence was calculated as the number of occurrences of a food item in the guts of a given species divided by the total number of individuals analysed. Then, the Index of Relative Importance (IRI) of each item for each species was calculated, considering the unit volume of food items weighted by its frequency of occurrence and expressed as percentage [49]:
where: Vi = volume of the food item i and Fi = frequency of occurrence of the food item i. Data from empty guts and those that only had indeterminate prey items were excluded from the analysis.
For the trophic classification of species, data from each individual belonging to a species from the different river sections was pooled. This procedure was applied in order to obtain a broader view of diet plasticity and to minimize the potential effect of the short time scale and the strong habitat specificity typically considered by GCA [50]. This procedure was followed to use variability in space along the whole river as a proxy of the potential variability across time and different habitat scenarios for a given species. For the classification purpose, the term “omnivores” was used to define species feeding at contrasting trophic levels, such as primary producers and consumers of any kind. This is a pragmatic use of the definition that allows a rather conservative but unequivocal visualization of this feeding strategy [11], but acknowledging that omnivores are strictly those feeding on more than one trophic level [51,52].
2.2. Data Analysis
Fish species were grouped and diets were compared using a cluster analysis, following the Bray–Curtis ordination method and Euclidian distance as an index of dissimilitude. This kind of group analysis is commonly used in studies of trophic ecology (e.g., [53]). To complement the cluster analysis, the data was visualized in a principal component analysis (PCA). To test for significant differences in the diet composition between the groups that emerged from the cluster analysis, a non-parametric Permutational Multivariate Analysis of Variance (PERMANOVA; Bray Curtis index; with 999 random permutations) was performed [54]. PCA analysis and the PERMANOVA test without data from detritivore and piscivore fish groups were also run to better visualize and classify the omnivore fish groups. A special focus on this group was made because of the known high relative richness of omnivore species in subtropical and tropical systems [11]. All the statistical analyses were conducted using the free statistical software PAST and the “vegan” package in R (R Development Core Team [55]).
Afterwards, the relative biomass, abundance and species richness of each trophic group was estimated for each sampled site within each river section. In this way, an aim to describe potential changes in trophic structure of assemblages between the upper, middle and lower river sections was made. The relative abundance, biomass and species richness data was used instead of total numbers, to avoid a potential bias given by the slightly different sampling methodologies (different distribution of net mesh sizes) displayed in the upper river section. To analyze potential changes in trophic structure between these sections we performed PERMANOVA tests (α = 0.05; Bonferroni-corrected P-values), using metrics for each trophic groups as response variables (i.e., relative biomass, relative abundance, and relative species richness), and the sampling sites within a river section as a replicates. Furthermore, changes in the relative biomass, abundance and species richness of each particular trophic group among river section were tested using Analysis of Variance (One way ANOVA) or Kruskall Wallis, depending on the accomplishments of data homoscedasticity and normality.
To compare the generality of the results, a bibliographic review of dietary descriptions for the same species in other locations was performed using the Google Scholar search engine. For the dietary review of each species, the terms “species name” + “feeding” + “diet” were used as keywords, and we considered the first ten results obtained. This information can help identify the diet plasticity of many species and also the gaps of information for certain species (Table 1).

Table 1.
Diet and trophic classification of fish species sampled along a longitudinal gradient in Uruguay River. The values for each dietary item type in each species represent the index of relative importance, which combines the frequency of occurrence and the relative volume of each dietary item to describe the diet of a species. For n and size ranges analysed see Table S2. Previous trophic classification of species for other systems surveyed from literature is shown in the last column and in References Table 1. NA = No data available. The species with * have not been grouped due to unique dietary characteristics.
3. Results
One hundred species were recorded in the main course of Uruguay River belonging to nine orders, with the Characiformes and Siluriformes being the most represented (42% and 41% of all the species, respectively) (Table S1). Most were native species, with the record of only one exotic species (Oreochromis niloticus, Nile tilapia) collected in the upper river section (Table 1).
From a total of 2309 stomachs analysed, 1890 (82%) were used in the feeding groups classification. The remaining stomachs were empty or with indeterminate dietary content.
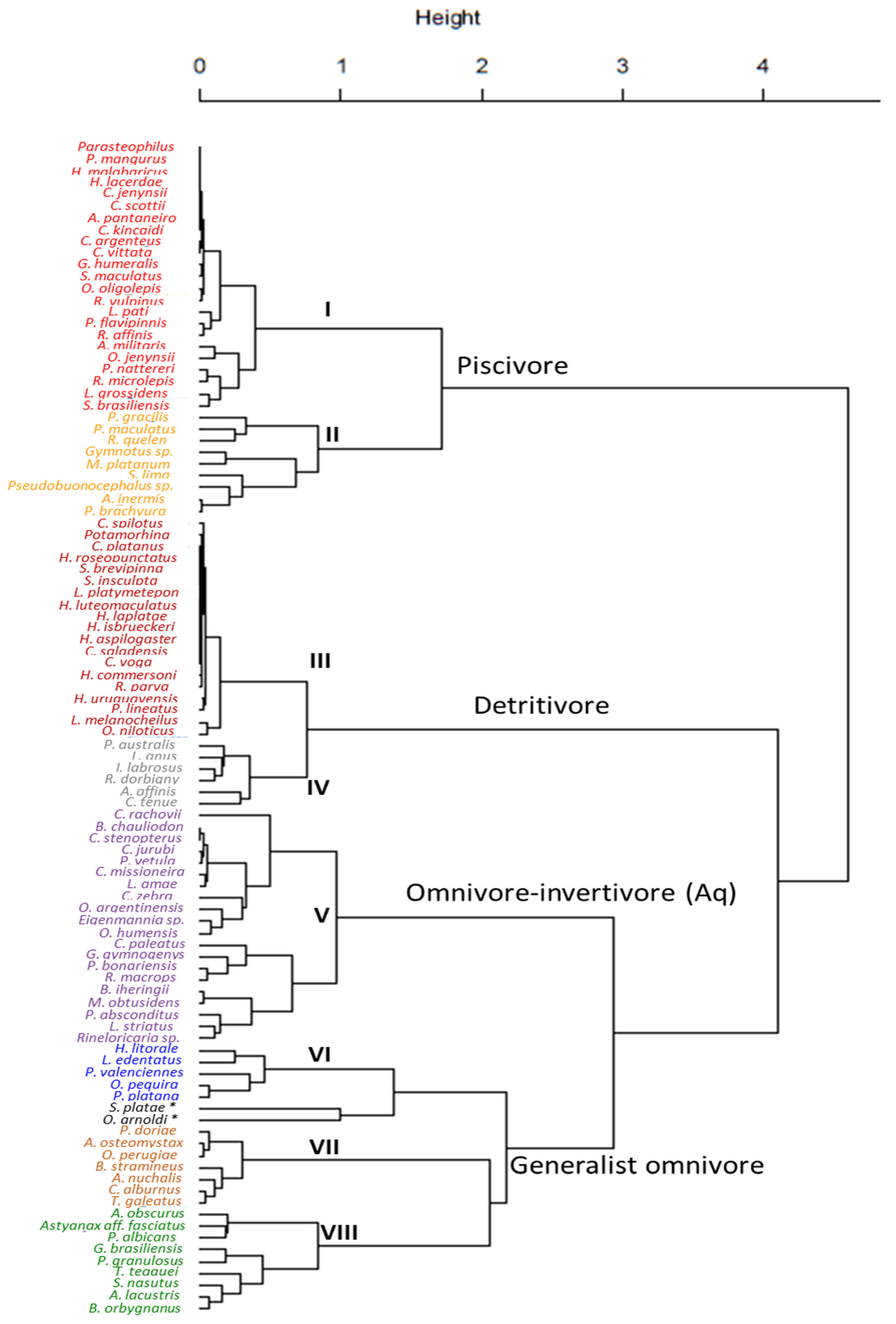
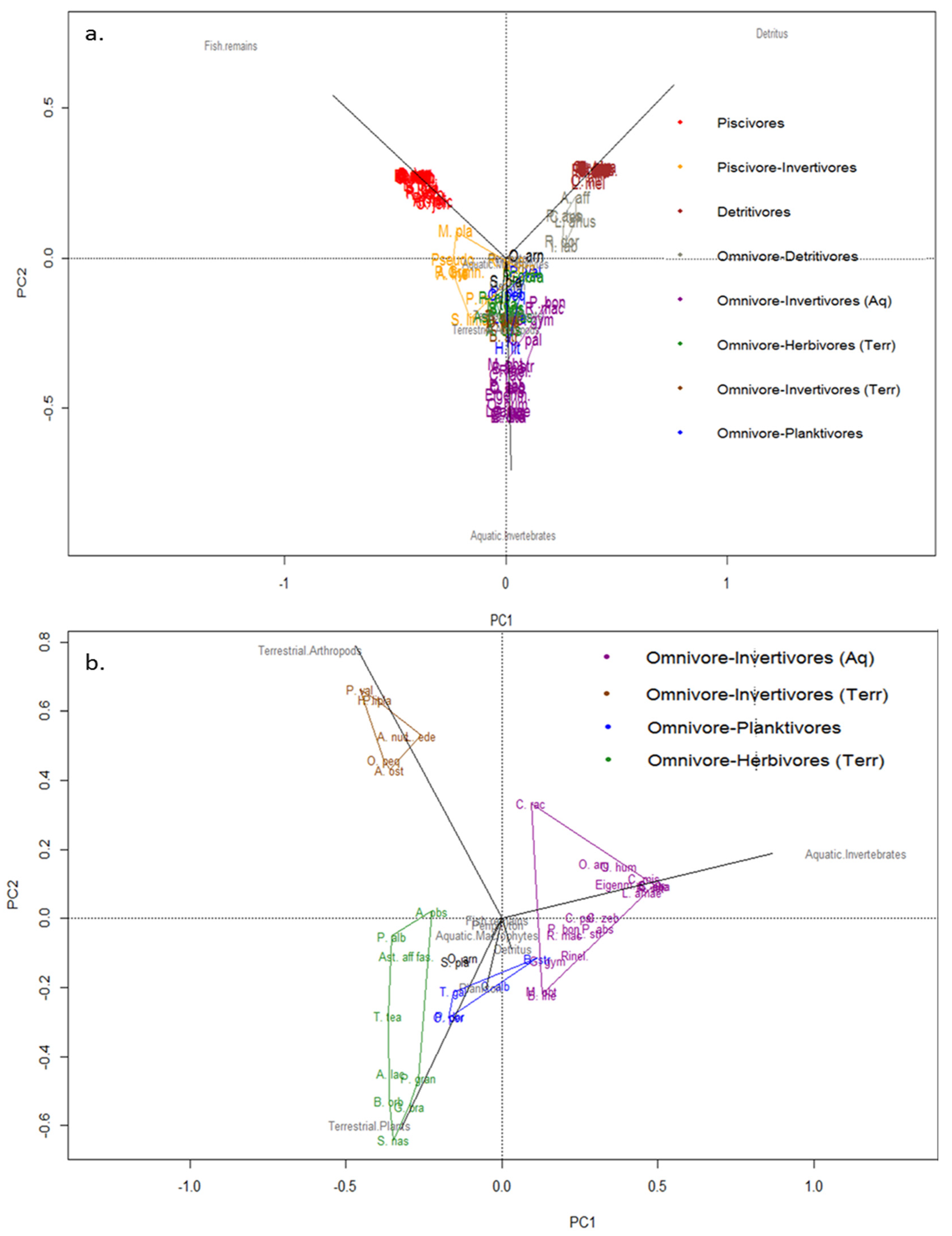
The combination of the IRI values of each dietary item (detailed in Table 1) used in the cluster analysis allowed classifying species into four coarse-level trophic groups: Piscivore (32% of the species), detritivore (24% of the species), omnivore-invertivore (aquatic) (20% of the species, being omnivores mostly feeding on aquatic macroinvertebrates) and generalist-omnivore (23% of the species being omnivores mostly feeding on terrestrial material) groups (Figure 2, Table 1). When visualising this data in principal component analysis (PCA) the separation of this same four broad trophic groups was as clearly evident as in the cluster analysis of Figure 2, with the first two axis explaining 67% of the variation in the data (PC1 = 40% and PC2 = 27%) (Figure 3a). The PERMANOVA test gave strong statistical support to this broad level classification into four groups, showing significant differences in the IRI index value for the multiple dietary items between every group (F3, 96 = 58.22; P = 0.001).
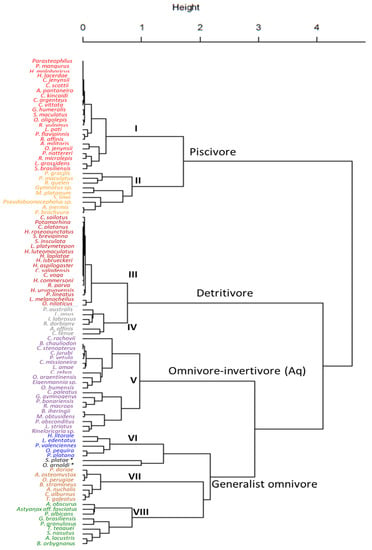
Figure 2.
Cluster analysis showing trophic classification of 100 fish species along the Uruguay River. The text in the main tree branches corresponds to the broad scale trophic classification into four large trophic groups. Within each group, a statistically significant separation into more detailed sub-groups is made and marked with numbers and different text font colours. The final eight trophic groups are: I: Piscivore; II: Piscivore-invertivore; III: Detritivore; IV: Omnivore-detritivore; V: Omnivore-invertivore—(Aq.); VI: Omnivore-planktivore; VII: Omnivore-invertivore—(Terr.); and VIII: Omnivore-herbivore—(Terr.). The two species with * were excluded from groups due to their unique diet composition. Species abbreviations are shown, for full species names and detailed dietary characterization see Table 1.
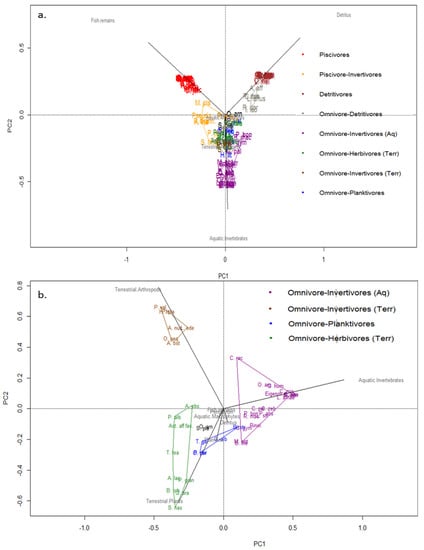
Figure 3.
Principal component analysis: (a) showing all fish species assemblages in trophic groups according main food item in diet; PCA axes 1 and 2 explained 40% and 27% of the variance, respectively; (b) PCA (without the piscivore and detritivore groups) showing omnivorous fish specialization and grouped in trophic groups; PCA axes 1 and 2 explained 43% and 24% of the variance, respectively. Each colored polygon represents one trophic group. To see the full name of the species, see Figure 2 and/or Table 1.
Furthermore, data exploration using both PCA and cluster analysis suggested the suitability of increasing the resolution of the four broad trophic groups. For instance, piscivore and detritivore groups could be separated into two groups each (Figure 2 and Figure 3a, Table 1), including the strictly piscivorous and detritivorous groups of species, and those that while feeding mostly on fish and detritus respectively also include other diet items to a lesser extent (Figure 3, Table 1). To perform this finer scale classification a PCA using exclusively the omnivorous groups was made to better resolve and classify them into four trophic subgroups (Figure 3b). In this case, 67% of the variation in the data was explained (PC1 = 43% and PC2 = 24%). The PERMANOVA test also showed consistent statistical support to this finer separation of omnivores into four subgroups (F1, 41 = 16.16; P = 0.001).
Altogether, the ordination methods supported the separation into eight trophic groups:
- Piscivore: Diet dominated by entire fishes, fish remains, scales and fins.
- Piscivore-invertivore: Diet dominated by fishes, fish remains, scales and fins, with inclusion of aquatic macroinvertebrates and terrestrial arthropods.
- Detritivore: Diet dominated by detritus.
- Omnivore-detritivore: A combination of vegetal and animal sources, with dominance of detritus.
- Omnivore-invertivore (Aquatic): A combination of species with either a diet largely dominated by aquatic macroinvertebrates and generally a minor inclusion of vegetal components.
- Omnivore-planktivore: Combination of vegetal and animal sources, with dominance of planktonic items (mostly zooplankton).
- Omnivore-invertivore (Terrestrial): A combination of species with either a diet largely dominated by terrestrial arthropods and generally a minor inclusion of vegetal components.
- Omnivore-herbivores (Terrestrial): Diet dominated by terrestrial seeds and fruits, but with minor inclusion of terrestrial arthropods.
Finally, a one-way PERMANOVA performed with all eight subgroups supported the trophic classification, showing significant between each group (F8, 91 = 101.42; P = 0.001). Two species were excluded (although appeared related to the Omnivore-planktivore group in the cluster analysis) due to their unique diet: Otocinclus arnoldi, that fed mostly on periphyton with minor inclusion of detritus, and Schizodon platae, with a diet almost entirely composed of aquatic macrophytes (Table 1).
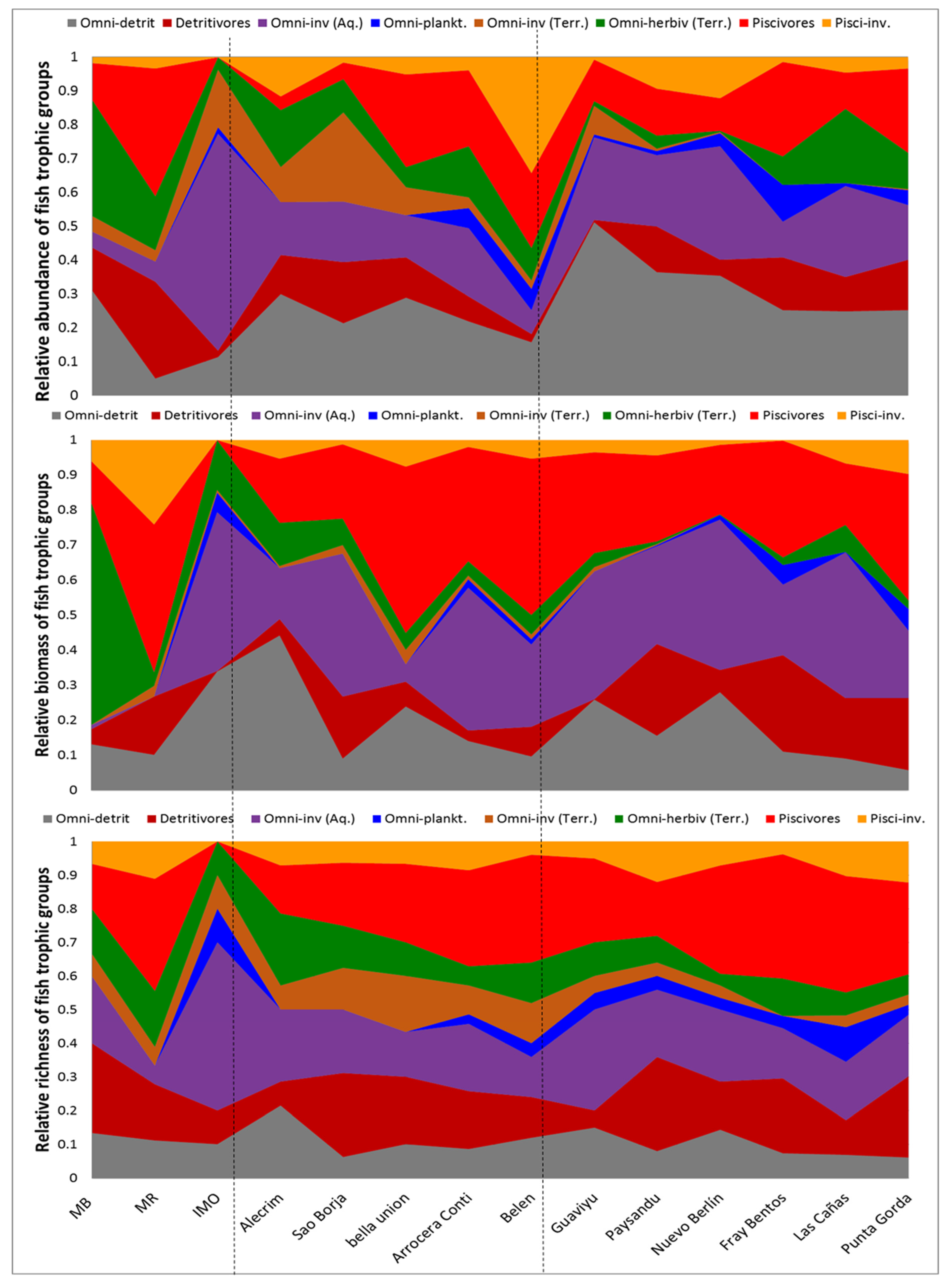
The trophic composition of the assemblages did not differ significantly between the three river sections in term of relative biomass (PERMANOVA F7, 111 = 1.4, P = 0.18), relative abundance (PERMANOVA F7, 111 = 1.03, P = 0.41) or relative species richness (PERMANOVA F7, 111 = 1.18, P=0.31) of trophic groups. The three sites in the upper portion of the river were particularity variable in its trophic composition in terms of relative abundance and biomass (Table S1, Figure 4). Moreover, no significant difference in the relative biomass, abundance or species richness of any of the trophic groups was found between the three river sections; the only exception being the relative species richness of the omnivores species feeding on terrestrial invertebrates, which was greater in the middle than in the lower Uruguay River section (ANOVA F2, 13 = 12.6; P = 0.001; 6 species in the middle vs. 3 in the lower section).
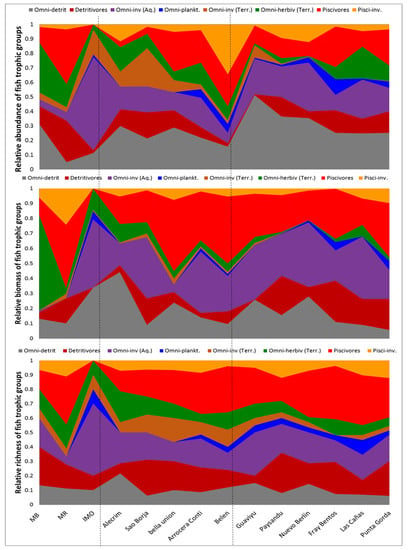
Figure 4.
Longitudinal variability in trophic structure of the fish assemblages in sampled locations of Uruguay River from upper (MB, MR, IMO) to middle (Alecrim, Sao Borja, Bella Unión, Isla del Zapallo, Belén) and lower (Guaviyú, Paysandú, Nuevo Berlín, Fray Bentos, Las Cañas and Punta Gorda) river sections. Dashed lines represent the division between river sections. Above: Relative biomass; middle panel: Relative abundance; and below relative richness of each trophic group.
In terms of relative abundance of individuals, the assemblages along the river were generally dominated by omnivore-detritivore (26 ± 11% of total abundance, mean and Standard Deviation), followed by the omnivore-invertivore feeding mostly on aquatic prey (20 ± 15%, mean and SD) and the piscivores (16 ± 11%, mean and SD). Meanwhile, detritivore and omnivore-herbivore groups represented about 10% of the total abundance each, whereas the remaining trophic groups represented less than 5% of the total abundance (Table S1, Figure 4).
In contrast, both in terms of relative biomass and relative species richness, the trophic structure of the assemblages was clearly dominated by the piscivore group (representing 27 ± 13% of total biomass and 24 ± 10% of total richness), followed by the omnivore-invertivore group that feed on aquatic macroinvertebrates (representing 26 ± 16% of the total biomass and 20 ± 10% of the total richness). The omnivore-detritivore conformed the third most important group (18 ± 11% of total biomass), while the remaining groups represented 10% or less of the total biomass. In terms of relative species richness, omnivore-detritivore groups occupied the third place in importance, representing 20 ± 10% of all the species present on average. Each of the remaining trophic groups hosted about 10% of the total species number or less (Table S1, Figure 4). Remarkably, the trophic group with less relative biomass, density and species richness was the omnivore-planktivores—composed by five species feeding on copepods, cladocerans and/or ostracods mostly (see Table 1)—present in only one third of the upper and middle river section localities, but being always present in the lower river section.
4. Discussion
A total of one hundred species were recorded in a single sampling campaign comprising 14 localities spread along the main course of Uruguay River. This elevated taxa number illustrates the high biodiversity of the river, especially because this is a 12-h gillnet sampling in each site (in comparison with larger studies), but approximates to the total number of species historically registered for the river [43,165]. Moreover, the species richness seems to be at a similar level than that found for tropical rivers of comparable discharge. For example a study performed within a river stretch of a similar length in the Teles Pires River, located in Central Brazil and with similar characteristics to the Uruguay River (1600 km extension, c.a. 4000 m3/s of average discharge) in a year of sampling, 90 species were collected [82]. Another example is the Miranda River, a tropical river located in Pantanal, Brazil, where 101 species were recorded over two years of sampling [166].
Moreover, the abundance and the movement of migrating species is controlled by seasonality, spatial and temporal environmental variability, and the hydrological regime [167]; therefore, it is not likely that all species that inhabit the main course of the river would be collected at the same time. However, according to previous sampling experience (e.g., [28]) and general literature for the region (e.g., [168,169]) we argue that our sampling was representative of the most common and frequent species in the river.
This study represents the first standardized fish assemblage description published and trophic classification of the species of the entire Uruguay River. Regarding the fish species present registered, it becomes of particular interest to highlight the presence of one exotic invasive fish species that represents a global threat to native biodiversity in the upper Uruguay River: The Nile Tilapia (Oreochromis niloticus). This species is one of the most commonly used in freshwater pisciculture production worldwide [170], and often generates great negative ecological consequences, particularly competing with native species [170]. The proliferation of these and other exotic species could affect local biodiversity by predation and competition with native species that share the same trophic niche.
Furthermore, this is the first dietary description for 29 fish species, despite that some of them are of elevated importance in fisheries (e.g., Luciopimelodus pati, being one of the most captured species by artisanal-commercial fisheries in the region) [171,172,173] and aquaculture (e.g., Hoplias lacerdae with lack of published field diet studies) (e.g., [174]). The other species with a previously unknown diet are rare species that are not usually collected in large numbers (e.g., Otocinclus arnoldi and most of the Hypostomus species). All this new information contributes to the knowledge of the trophic structure of fish assemblage. Moreover, when reviewing literature of the previously studied species, it most generally falls within a similar trophic classification; but one (Leporinus striatus) shows contrasting diet differences. L. striatus analyzed in this study lie well within the omnivore-invertivore trophic group, with important contribution of aquatic invertebrates (mostly invasive golden mussel, Limnoperna fortunei) to its diet. However, previous studies describe the species as an herbivore. This evidence suggest that the trophic classification of this species should be reassigned in the Uruguay River following our study. The reason behind this change might be the contrasting food availability between study sites (Amazon River Basin vs. Uruguay River) after the invasion of the golden mussel into the Uruguay River. The invasive golden mussel is nowadays known to represent a key dietary item in some Anostomid fish species (e.g., M. obtusidens and L. striatus), formerly classified as herbivorous ([175], González-Bergonzoni et al., in Prep).
Regarding the general trophic classification made here, it must be held in mind that the Uruguay River has a great spatial and temporal variability along its length, which could mean a high intra-specific variability in diet—particularly in the species with feeding plasticity—responding to flood pulses, seasonality, or local habitat conditions (e.g., [176,177]). This kind of spatial and individual size variability was not considered in the current analyses, because the main objective of this study consisted in a broad-scale classification for each species that surpassed local particularities or a particular life stage. Although diet analysis of some rare species that only presented one or few individuals was also performed, those were still kept into the analyses because their diets were sometimes completely unknown in the region. The aspects outlined above must be taken into account if an objective to describe food webs at a fine resolution or at a local level is to be addressed. However, a broad classification of fishes into feeding groups such as this one is an important tool in ecology, allowing comparisons among different environments, river basins or regions, based on fish assemblage structure [178].
The trophic structure of fish assemblages did not generally differ among the three river sections, being the piscivores dominant in terms of relative biomass and richness and the omnivore-detritivore dominant in terms of abundance. This partly reflects the contrasting size structure of species within those trophic groups, being the piscivores usually larger and with higher biomass in the assemblage. Much of the dominance in abundance of the omnivorous-detritivorous group responds to the high frequency and abundance of the Iheringichthys labrosus species, sampled along of most of the river length. This ubiquitous species is highly plastic in its diet [40] and digestive morphological features [179], being a constantly dominant species across the entire river.
The observed significantly higher relative species richness of omnivorous species feeding on terrestrial invertebrates towards the middle section of the river may correspond to the dominant environmental characteristics of that zone. In particular, the middle section hosts several large floodplains in which the river channel contacts grasslands and forest areas during floods where terrestrial invertebrates become highly available (e.g., [180]). In this context, it needs to be mentioned that sampling took place during a high river flow scenario, with significant floods, particularly in the middle and lower stretches. Most of the species within this trophic group have morphological adaptations to feed on the water surface (e.g., supra-terminal mouth), where arthropods derived from the land drift in the water surface. This evidence generally agrees with large river theories (e.g. “The flood pulse concept”) in which increased land-water contact increases terrestrial subsidies for fish biomass [180]. Moreover, it matches well with the observed in studies arrayed at diverse scales, where the terrestrial food intake of fish increase whenever the land-water interphase increases, e.g., towards flooded forests (e.g., [181]), or towards stream ecosystems with riparian forests [182]. Thus, this study finding probably remarks that terrestrial carbon input and flow in aquatic ecosystem food webs might be increased in regions with high terrestrial-aquatic habitat connectivity.
The relative importance of trophic groups such as piscivore and omnivore-herbivore did not increase downstream as previously evidenced for smaller fluvial ecosystems (at least at the coarse level defined here) [12,13,14]. The change in the scale of analysis (large river vs. middle size rivers and streams in the evidence fueling most river theories) may account for the absence of strong changes in the fish assemblage trophic structure from headwaters to mouth, probably because, even in the upper section, the system may be already large and productive enough to sustain high trophic diversity. However, and remarkably, the omnivorous-planktivorous fish trophic group was far more frequent in the lower than in the middle and upper sections, probably reflecting that the river velocity and turbulent flow decrease downstream as the river widens up allowing establishment of planktonic communities (as postulated by Horwitz 1978, and Vannote 1980 [12,13]).
Several anthropogenic factors may affect fish assemblages, such as the agrochemical inputs from the basin, fisheries, industrial and domestic sewage [183] and habitat fragmentation caused by hydroelectric dams [27,184]. This anthropogenic intervention in freshwater ecosystems typically results in the reduction of local biodiversity and affected community structure, particularly of fish [185,186]. For example, the low species richness and high spatial variability in the relative representation of different trophic groups in the upper Uruguay River might well be attributed to the presence of three hydroelectric dams between sampling sites (being this, a well-known impact of dams) [27,184,187]. Unfortunately, as there is a lack of baseline information on fish trophic structure it became impossible to disentangle the anthropogenic and natural effects driving fish trophic structure along the Uruguay River gradient. In a global scenario of increased anthropogenic pressure to aquatic ecosystems, and particularly of river fragmentation by dam construction [186,188,189,190] there is an increasing need for the generation of appropriate information about the ecology and biology of fishes, particularly in South America, to achieve better understanding of the ecosystems and improve management plans for the entire continent [34].
This research contributes with basic knowledge that allows interpreting how food webs are structured within this ecosystem, enabling predictions about the roles of particular trophic groups and fish species in the system. Moreover, a proper management of natural resources (such as many of this species that are target for fisheries) demands baseline knowledge on trophic interactions between species, previously inexistent along the entire Uruguay River. Future standardized monitoring programs along the river longitudinal gradient may increase the understanding of these observed patterns across seasons and long temporal scales including the effects of climate variability. Furthermore, in a global scale, the information about trophic classification of fishes generated in this study contributes to the knowledge of ecosystem functioning in this scarcely studied region, and may allow for comparisons with other climate regions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/11/7/1374/s1, Table S1: Fish assemblage trophic structure along a longitudinal gradient in Uruguay River. Sites are arranged from headwaters to mouth from left to right columns in the table. Values represent the relative abundance / relative biomass (%) of each species and trophic group (as the sum of all species within a group) at each study site. Values are only shown for the species collected in standardized samplings; species presence is marked with “X” in the case of individuals obtained from local fishermen or in previous samplings. The species with * have not been grouped due to unique dietary characteristics, Table S2: Fish species sampled along a longitudinal gradient in the Uruguay River. Taxonomic identification, minimum-maximum standard length (and number of guts analyzed) for each species and site are shown. Sites are arranged from headwaters to mouth from right to left columns in the table. Note that for some species the number of fish is very low and were kept in the analysis for being rare species from which information is highly novel. Use that information with special care.
Author Contributions
Conceptualization, A.L.-R. and I.G.-B.; data curation, A.L.-R., I.S. and I.G.-B.; formal analysis, A.L.-R., I.S., S.d.Á.-S., S.S., R.B., J.P., G.T. and I.G.-B.; funding acquisition, F.T.d.M., A.D. and I.G.-B.; investigation, A.L.-R. and I.G.-B.; methodology, A.L.-R., I.S., S.d.Á.-S., S.S., R.B., M.V.M., J.P., G.T., F.T.d.M., A.D., N.V., M.M., D.A.R.-T., E.Z.-F. and I.G.-B.; project administration, A.D. and I.G.-B.; resources, F.T.d.M., A.D. and I.G.-B.; supervision, F.T.d.M., A.D., N.V., M.M. and I.G.-B.; visualization, A.L.-R., I.S. and I.G.-B.; writing—original draft, A.L.-R. and I.G.-B.; writing—review and editing, A.L.-R., I.S., S.d.Á.-S., S.S., R.B., M.V.M., J.P., G.T., F.T.d.M., A.D., N.V., M.M., D.A.R.-T., E.Z.-F. and I.G.-B.
Funding
This research project was funded partly by Scientific Research Sectorial Commission (Uruguay) project CSIC I+D_2016_577-348 and the National Agency for Innovation and Research (ANII) project ANII FCE_ 2_2016_1_126780. ALR and IS received financial support from the Postgraduate Academic Commission (CAP) scholarship programme. R.B, M.V.M and D.A.R.-T. received financial support by FAPERGS: Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul. IGB, AD, NV, MM and FTM received financial support by the ANII National System of Researchers (SNI), and IGB also from the ANII scholarship ANII PD_NAC_2015_1_108121.
Acknowledgments
We gratefully thank the many students and researchers that helped along with fish sampling campaigns and sample processing such as Nicolas Boullosa, Patricia Correa, Renata Maria Guereschi, Samara Hermes-Silva, Ronaldo da Silva and Pedro Iaczinski. We also thank artisanal fishermen Lito López, Elbio Russo, Jorge Fagúndez, Mario Britos, Rogelio Zunini, J. Tajes, Pablo Conti, Jorge Franchini, and the wildlife park ranger from Nuevo Berlín: Ángel Rosano for their constant support and collaboration with fish samplings. We also thank three anonymous reviewers whose contribution improved the manuscript considerably.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elton, C.S. Animal Ecology; Sigwick and Jackson: London, UK, 1927. [Google Scholar]
- Paine, R.T. Food Webs: Linkage, Interactions Strength and Community Infrastructure. J. Anim. Ecol. 1980, 49, 666–685. [Google Scholar] [CrossRef]
- Thompson, R.M.; Dunne, J.A.; Woodward, G. Freshwater food webs: Towards a more fundamental understanding of biodiversity and community dynamics. Freshw. Biol. 2012, 57, 1329–1341. [Google Scholar] [CrossRef]
- Woodward, G. Biodiversity, ecosystem functioning and food webs in fresh waters: assembling the jigsaw puzzle. Freshw. Biol. 2009, 54, 2174–2187. [Google Scholar] [CrossRef]
- Power, M.E. Effects of fish in river food webs. Science 1990, 250, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Delong, M.; Thorp, J.M.; Thons, M.S.; Mcintosh, L. Trophic niche dimensions of fish communities as a function of historical hydrological conditions in a Plains river. River Syst. 2011, 19, 177–187. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 2002, 83, 2152–2161. [Google Scholar] [CrossRef]
- Vidotto-Magnoni, A.P.; Carvalho, E.D. Aquatic insects as the main food resource of fish the community in a Neotropical reservoir. Neotrop. Icthyol. 2009, 7, 701–708. [Google Scholar] [CrossRef]
- Tonella, L.H.; Fugi, R.; Vitorino, O.B.; Suzuki, H.I.; Gomes, L.C.; Agostinho, A.A. Importance of feeding strategies on the long-term success of fish invasions. Hydrobiologia 2018, 817, 239–252. [Google Scholar] [CrossRef]
- Teixeira-de Mello, F.; Vidal, N.; González-Bergonzoni, I.; Iglesias, C. Length–weight relationships of eight fish species from the lower section of the Uruguay River (Río Negro, Uruguay). J. Appl. Ichthyol. 2009, 25, 128–129. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Meerhoff, M.; Davidson, T.A.; Teixeira-de Mello, F.; Baattrup-Pedersen, A.; Jeppesen, E. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems 2012, 15, 492–503. [Google Scholar] [CrossRef]
- Horwitz, R.J. Temporal variability patterns and the distributional patterns of stream fishes. Ecol. Monogr. 1978, 48, 307–321. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Ibañez, C.; Belliard, J.; Hughes, R.M.; Irz, P.; Kamdem-Toham, A.; Lamorouroux, N.; Tedesco, P.A.; Oberdoff, T. Convergence of temperate and tropical stream fish assemblages. Ecography 2009, 32, 658–670. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Hoeinghaus, D.J.; Pease, A.A.; Esselman, P.C.; Honeycutt, R.L.; Gbanaador, D.; Carrera, E.; Payne, J. Stable isotope analysis reveals food web structure and watershed impacts along the fluvial gradient of a Mesoamerican coastal river. Riv. Res. Applic. 2010, 27, 791–803. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Sato, T.; Zou, L.; Jiang, K.; Yahara, T.; Kano, Y. Distribution pattern, threats and conservation of fish biodiversity in the East Tiaoxi, China. Environm. Biol. Fish. 2013, 96, 519–533. [Google Scholar] [CrossRef]
- Pease, A.A.; González-Díaz, A.A.; Rodiles-Hernández, R.; Winemiller, K.O. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Lévêque, C.; Oberdorff, T.; Paugy, D.; Stiassny, M.L.J.; Tedesco, P.A. Global diversity of fish (Pisces) in freshwater. In Freshwater Animal Diversity Assessment; Bailan, E.V., Lévêque, C., Segers, H., Martens, K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 545–567. [Google Scholar]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarones, M.M.; Petry, P.; Rocha, L.A. Fish biodiversity and conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef]
- Reis, R.E.; Kullander, S.O.; Ferraris, C.J., Jr. Check List of the Freshwater Fishes of South and Central America; Edipucrs: Porto Alegre, Brazil, 2003; ISBN 85-7430-361-5. [Google Scholar]
- Reynalte-Tataje, D.A.; Agostinho, A.A.; Bialetzki, A.; Hermes-Silva, S.; Fernandes, R.; Zaniboni-Filho, E. Spatial and temporal variation of the ichthyoplankton in a subtropical river in Brazil. Environ. Biol. Fishes 2012, 94, 403–419. [Google Scholar] [CrossRef]
- Petry, P.; Bayley, P.B.; Markle, D.F. Relationship between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. J. Fish. Biol. 2003, 63, 547–579. [Google Scholar] [CrossRef]
- Röpke, C.P.; Amadio, S.; Zuanon, J.; Ferreira, E.J.; De Deus, C.P.; Pires, T.H.; Winemiller, K.O. Simultaneous abrupt shifts in hydrology and fish assemblage structure in a floodplain lake in the central Amazon. Sci. Rep. 2017, 7, 40170. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, A.A.; Pelicice, F.M.; Petry, A.C.; Gomes, L.C.; Júlio, H.F., Jr. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat. Ecosyst. Health 2007, 10, 174–186. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Winemiller, K.O.; Lewis, W.M., Jr.; Taphorn Baechle, D.C. The freshwater habitats, fishes, and fisheries of the Orinoco River basin. Aquat. Ecosyst. Health 2007, 10, 140–152. [Google Scholar] [CrossRef]
- Zaniboni-Filho, E.; Ribolli, J.; Hermes-Silva, S.; Nuñer, A.P.O. Wide reproductive period of a long-distance migratory fish in a subtropical river, Brazil. Neotrop. Ichthyol. 2017, 15, e160135. [Google Scholar] [CrossRef]
- Schork, G.; Zaniboni-Filho, E. Structure dynamics of a fish community over ten years of formation in the reservoir of the hydroelectric power plant in upper Uruguay River. Braz. J. Biol. 2017, 77, 710–723. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; D’Anatro, A.; Vidal, N.; Stebniki, S.; Tesitore, G.; Silva, I.; Teixeira-de Mello, F. Origin of fish biomass in a diverse subtropical river: An allochthonic-supported biomass increase following flood pulses. Ecosystems 2019. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Gomes, L.C.; Santos, N.C.L.; Ortega, J.C.G.; Pelicice, F.M. Fish assemblages in Neotropical reservoirs: Colonization patterns, impacts and management. Fish. Res. 2016, 173, 26–36. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Baumgartner, M.T.; Gomes, L.C.; Agostinho, A.A. Long-term effects of flow regulation by dams simplify fish functional diversity. Freshw. Biol. 2018, 63, 293–305. [Google Scholar] [CrossRef]
- Pelicice, F.M.; Azevedo-Santos, V.M.; Esguicero, A.L.H.; Agostinho, A.A.; Arcifa, M.S. Fish diversity in the cascade of reservoirs along the Paranapanema River, southeast Brazil. Neotrop. Ichthyol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Ávila-Simas, S.; Reynalte-Tataje, D.A.; Zaniboni-Filho, E. Pools and rapids as spawning and nursery areas for fish in a river stretch without floodplains. Neotrop. Ichthyol. 2014, 12, 611–622. [Google Scholar] [CrossRef]
- Reynalte-Tataje, D.A.; Zaniboni-Filho, E.; Bialetzki, A.; Agostinho, A.A. Temporal variability of fish larvae assemblages: influence of natural and anthropogenic disturbances. Neotrop. Ichthyol. 2012, 10, 837–846. [Google Scholar] [CrossRef][Green Version]
- Barletta, M.; Jaureguizar, A.J.; Baigun, C.; Fontoura, N.F.; Agostinho, A.A.; Almeida-Val, V.M.; Val, A.L.; Torres, R.A.; Jimenes-Segura, L.F.; Giarrizzo, T.; et al. Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J. Fish. Biol. 2010, 76, 2118–2176. [Google Scholar] [CrossRef]
- Nuñer, A.P.O.; Zaniboni-Filho, E. Length-weight relationships of fish species caught in the Upper Uruguay River, Brazil. J. Appl. Ichthyol. 2009, 25, 362–364. [Google Scholar] [CrossRef]
- Zarucki, M.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Duarte, A.; Serra, S.; Quintans, F.; Loureiro, M. New records of freshwater fish for Uruguay. Check List. 2010, 6, 191–194. [Google Scholar] [CrossRef]
- Serra, W.S.; Teixeira-de Mello, F.; DÁnatro, A.; Vidal, N.; González-Bergonzoni, I.; García, D.; Masdeu, M.; Lenzi, J.; Tana, J. New records and distribution extension of Pimelodus absconditus Azpelicueta, 1995 (Siluriformes: Pimelodidae) and Triportheus nematurus (Kner, 1985) (Characiformes: Triportheidae). Bol. Soc. Zool. Uruguay (2a época) 2017, 26, 16–20. [Google Scholar]
- Reynalte-Tataje, D.A.; Barcellos, R.P.; Hartmann, P.B.; Scherer, J.B.; Martine, G.; Vlieger, I.T.; Zaniboni-Filho, E.; Hermes-Silva, S.; Pelicice, F.M. O médio rio Uruguai como importante área de reprodução do surubim-pintado Pseudoplatystoma corruscans (Siluriformes: Pimelodidae). Bol. Soc. Ictiol. Londrina 2017, 122, 10–15. [Google Scholar]
- González-Bergonzoni, I.; Teixeira-de Mello, F.; Vidal, N.; D’Anatro, A.; Masdeu, M. Reappearence and diet of juvenile armado catfish (Pterodoras granulosus) in Lower Uruguay River, (Río Negro, Uruguay). Bol. Soc. Zool. Uruguay (2a época) 2010, 19, 42–46. [Google Scholar]
- Masdeu, M.; Teixeira-de Mello, F.; Loureiro, M.; Arim, M. Feeding habits and morphometry of Iheringichthys labrosus (Lütken, 1874) in the Uruguay River (Uruguay). Neotrop. Ichthyol. 2011, 9, 657–664. [Google Scholar] [CrossRef]
- Meurer, S.; Zaniboni-Filho, E. Reproductive and feeding biology of Acestrorhynchus pantaneiro Menezes, 1992 (Osteichthyes: Acestrorhynchidae) in areas under the influence of dams in the upper Uruguay River, Brazil. Neotrop. Ichthyol. 2012, 10, 159–166. [Google Scholar] [CrossRef]
- Ferriz, R.A.; Arrieta, P.M.; Dmánico, A.A. Trophic characterization of nine species of fishes in Middle and Lower Uruguay River. In Historia Natural; Azara, Fundación de Historia Natural, Universidad Maimónides: Buenos Aires, Argentina, 2016; Volume 6, pp. 41–53. [Google Scholar]
- Di Persia, D.H.; Neiff, J.J.; Olazarri, J. The Uruguay River System. In The Ecology of River Systems; Davies, B.R., Walker, K.F., Eds.; Monographiae Biologicae, Springer: Dordrecht, The Netherlands, 1986; pp. 599–629. [Google Scholar]
- Bischoff, S.A.; García, N.O.; Vargas, W.M.; Jones, P.D.; Conway, D. Climatic variability and Uruguay River Flows. Water Int. 2000, 25, 446–456. [Google Scholar] [CrossRef]
- Zaniboni-Filho, E.; Schulz, U.H. Migratory fishes of the Uruguay River. In Migratory Fishes of the South America: Biology, Fisheries and Conservation Status; Carolsfeld, J., Harvey, B., Ross, C., Baer, A., Eds.; World Fisheries Trust: Victoria, BC, Canada; World Bank: Washington, DC, USA; IDRC: Otawa, ON, Canada, 2003; pp. 135–168, e-ISBN 1552501140. [Google Scholar]
- Krepper, C.M.; Garcia, N.O.; Jones, P.D. Interannual variability in the Uruguay River basin. Intern. J. Climatol. 2003, 23, 103–115. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Jeppesen, E.; Vidal, N.; Teixeira-de Mello, F.; Goyenola, G.; López-Rodríguez, A.; Meerhoff, M. Potential drivers of seasonal shifts in fish omnivory in a subtropical stream. Hydrobiologia 2016, 768, 183–196. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food habits of Albacore Bluefin Tuna and Bonito in California Waters. Calif. Fish Game. 1971, 152, 1–105. [Google Scholar]
- Nielsen, J.M.; Clare, E.L.; Hayden, B.; Brett, M.T.; Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods Ecol. Evol. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Pouilly, M.; Yunoki, T.; Rosales, C.; Torres, L. Trophic structure of fish assemblages from Mamoré River floodplain lakes (Bolivia). Ecol. Freshw. Fish 2004, 13, 245–257. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Pimm, S.L. Food webs. In Food Webs; Springer: Dordrecht, The Netherlands, 1982; pp. 1–11. [Google Scholar]
- Polis, G.A. Food webs, trophic cascades and community structure. Aust. J. Ecol. 1994, 19, 121–136. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J. The Vegan Package. Comm. Ecol. Pack. 2007, 10, 631–637. [Google Scholar]
- Cantanhêde, G.; Fugi, R.; Hahn, N.S. Variation in prey selection of a piscivores fish after the impoundment of a neotropical reservoir: Prey size and type. J. Fish Biol. 2009, 75, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Krinski, D. Diet of the dog fish Acestrorhynchus pantaneiro Menezes, 1992 (Characidae: Acestrorhynchinae) from Pantanal of Poconé, Mato Grosso State, Brazil. Biosci. J. 2010, 26, 287–295. [Google Scholar]
- Catella, A.C.; Petrere, M. Body-shape and food habits of fish from Baía da Onçca, a Pantanal flood plain lake, Brazil. Verh. Int. Verein. Limnol. 1998, 26, 2203–2208. [Google Scholar] [CrossRef]
- Novakowski, G.C.; Cassemiro, F.A.; Hahn, N.S. Diet and ecomorphological relationships of four cichlid species from the Cuiabá River basin. Neotrop. Ichthyol. 2016, 14, e150151. [Google Scholar] [CrossRef]
- Corrêa, C.E.; Petry, A.C.; Hahn, N.S. Influência do ciclo hidrológico na dieta e estrutura trófica da ictiofauna do rio Cuiabá, Pantanal Mato-Grossense. Iheringia Sér. Zool. 2009, 99, 456–463. [Google Scholar] [CrossRef]
- de los Angeles Bistoni, M.; Haro, J.G.; Gutiérrez, M. Feeding of Hoplias malabaricus in the wetlands of Dulce river (Córdoba, Argentina). Hydrobiologia 1995, 316, 103–107. [Google Scholar] [CrossRef]
- Fernández, E.M.; Ferriz, R.A.; Bentos, C.A.; López, G.R. Dieta y ecomorfología de la ictiofauna del arroyo Manantiales, provincia de Buenos Aires, Argentina. Rev. Mus. Argent. Cienc. Nat. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Loureiro, V.E.; Hahn, N.S. Dieta e atividade alimentar da traíra, Hoplias malabaricus (Bloch, 1794) (Osteichthyes, Erythrinidae), nos primeiros anos de formação do reservatório de Segredo-PR. Acta Limnol. Bras. 1996, 8, 195–205. [Google Scholar]
- de Almeida, V.L.L.; Hahn, N.S.; Vazzoler, A.D.M. Feeding patterns in five predatory fishes of the high Paraná River floodplain (PR, Brazil). Ecol. Freshw. Fish 1997, 6, 123–133. [Google Scholar] [CrossRef]
- Carvalho, L.N.; Fernandes, C.H.V.; Moreira, V.S.S. Alimentação de Hoplias malabaricus (Bloch, 1794) (Osteichthyes, Erythrinidae) no rio Vermelho, Pantanal Sul Mato-Grossense. Rev. Bras. Zoociências 2002, 4, 227–236. [Google Scholar]
- Pereira, C.C.G.; Smith, W.S.; Espíndola, E.L.G. Hábitos alimenticios de nueve especies de peces del embalse de Três Irmãos, São Paulo, Brasil. Univ. Cien. 2004, 1, 33–38. [Google Scholar]
- Bortoluzzi, T.; Aschenbrenner, A.D.C.; da Silveira, C.D.R.; Roos, D.C.; Lepkoski, E.D.; Martins, J.A.; Goulart, M.G.; Querol, E.; Querol, M.V. Hábito alimentar da Sardinha Prata, Lycengraulis grossidens (Spix & Agassiz, 1829), (Pisces, Engraulidae), Rio Uruguai Médio, Sudoeste do Rio Grande do Sul, Brasil. Biodivers. Pampeana 2006, 4, 11–23. [Google Scholar]
- Mai, A.C.G.; Vieira, J.P. Review and consideration on habitat use, distribution and life history of Lycengraulis grossidens (Agassiz, 1829) (Actinopterygii, Clupeiformes, Engraulididae). Biota Neotrop. 2013, 13, 121–130. [Google Scholar] [CrossRef]
- Hartz, S.M.; Martins, A.; Barbieri, G. Dinâmica da alimentação e dieta de Oligosarcus jenynsii (Günther, 1864) na lagoa Caconde, Rio Grande do Sul, Brasil (TELEOSTEI, CHARACIDAE). Bol. Inst. Pesca 1996, 23, 21–29. [Google Scholar]
- Silva, S.H.; Meurer, S.; Zaniboni-Filho, E. Biologia alimentar e reprodutiva do peixe-cachorro (Oligosarcus jenynsii Günther, 1864) na região do alto rio Uruguai-Brasil. Acta Sci. Biol. Sci. 2004, 26, 175–179. [Google Scholar] [CrossRef]
- Nunes, D.M.; Hartz, S.M. Feeding dynamics and ecomorphology of Oligosarcus jenynsii (Gunther, 1864) and Oligosarcus robustus (Menezes, 1969) in the Lagoa Fortaleza, southern Brazil. Braz. J. Biol. 2006, 66, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.E. Alimentación de Oligosarcus jenynsii (Characiformes: Characidae) en dos embalses sobre el río Juramento, Salta, Subtrópico de Argentina. Rev. Aquatic 2016, 20, 44–50. [Google Scholar]
- Bonato, K.O.; Burres, E.D.; Fialho, C.B. Dietary differentiation in relation to mouth and tooth morphology of a neotropical characid fish community. Zool. Anz. 2017, 267, 31–40. [Google Scholar] [CrossRef]
- Zarucki, M.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Loureiro, M. Fish diversity loss in an urban stream of Uruguay throughout the last century. PANAMJAS 2011, 6, 71–75. [Google Scholar]
- Gonzalez, N.; Vispo, C. Aspects of the diets and feeding ecologies of fish from nine floodplain lakes of the lower Caura, Venezuelan. Scient. Guaian. 2003, 12, 329–366. [Google Scholar]
- Moreira-Hara, S.S.; Zuanon, J.A.; Amadio, S.A. Feeding of Pellona flavipinnis (Clupeiformes, Pristigasteridae) in a Central Amazonian floodplain. Iheringia Sér. Zool. 2009, 99, 153–157. [Google Scholar] [CrossRef]
- Behr, E.R.; Signor, C.A. Distribution and feeding of two sympatric species of piranhas Serrasalmus maculatus and Pygocentrus nattereri (Characidae, Serrasalminae) of the Ibicuí river, State of Rio Grande do Sul, Brazil. Iheringia Sér. Zool. 2008, 98, 501–507. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Vicentin, W.; Costa, F.E.; Súarez, Y.R. Trophic ecology of two piranha species, Pygocentrus nattereri and Serrasalmus marginatus (Characiformes, Characidae), in the floodplain of the Negro River, Pantanal. Acta Limnol. Bras. 2014, 26, 381–391. [Google Scholar] [CrossRef]
- Peterson, C.C.; McIntyre, P. Ontogenetic diet shifts in Roeboides affinis with morphological comparisons. Environ. Biol. Fishes 1998, 53, 105–110. [Google Scholar] [CrossRef]
- Novakowski, G.C.; Fugi, R.; Hahn, N.S. Diet and dental development of three species of Roeboides (Characiformes: Characidae). Neotrop. Ichthyo. 2004, 2, 157–162. [Google Scholar] [CrossRef]
- Novakowski, G.C.; Hahn, N.S.; Fugi, R. Diet seasonality and food overlap of the fish assemblage in a pantanal pond. Neotrop. Ichthyol. 2008, 6, 567–576. [Google Scholar] [CrossRef]
- Nunes, M.E. Alimentação de Juvenis de Dourado Salminus Brasiliensis e Piava Megaleporinus Obtusidens no Médio rio Uruguai. Bachelor’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2017; 30p. [Google Scholar]
- Dary, E.P.; Ferreira, E.; Zuanon, J.; Röpke, C.P. Diet and trophic structure of the fish assemblage in the mid-course of the Teles Pires River, Tapajós River basin, Brazil. Neotrop. Ichthyol. 2017, 15, e160173. [Google Scholar] [CrossRef]
- Hahn, N.S.; Fugi, R.; Peretti, D.; Russo, M.R.; Loureiro-Crippa, V.E. Estrutura trófica da ictiofauna da planície de inundação do alto rio Paraná. In A planície de inundação do alto Rio Paraná; Agostinho, A.A., Thomaz, S.M., Rodrigues, L., Gomes, L.C., Eds.; Programa PELD/CNPq: Maringá, Brazil, 2002; pp. 123–126. [Google Scholar]
- Sazima, I. Similarities in feeding behavior between some marine and freshwater fishes in two tropical communities. J. Fish Biol. 1986, 29, 53–65. [Google Scholar] [CrossRef]
- Horeau, V.; Cerdan, P.; Champeau, A.; Richard, S. Importance of aquatic invertebrates in the diet of rapids-dwelling fish in the Sinnamary River, French Guiana. J. Trop. Ecol. 1998, 14, 851–864. [Google Scholar] [CrossRef]
- Bonato, K.O.; Delariva, R.L.; da Silva, J.C. Diet and trophic guilds of fish assemblages in two streams with different anthropic impacts in the northwest of Paraná, Brazil. Zoologia 2012, 29, 27–38. [Google Scholar] [CrossRef]
- Lolis, A.A.; Andrian, I.D.F. Alimentação de Pimelodus maculatus Lacépède 1803 (Siluriformes, Pimelodidae), na planície de inundação do alto Rio Paraná, Brasil. Boletim do Instituto de Pesca 1996, 23, 23–28. [Google Scholar]
- Lima-Junior, S.E.; Goitein, R. Ontogenetic diet shifts of a Neotropical catfish, Pimelodus maculatus (Siluriformes, Pimelodidae): An ecomorphological approach. Environ. Biol. Fishes 2003, 68, 73–79. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J.; Bennemann, S. Temporal trophic shifts and feeding diversity in two sympatric, Neotropical, omnivorous fishes: Astyanax bimaculatus and Pimelodus maculatus in Río Tibagi (Paraná, Southern Brazil). Arch. Hydrobiol. 2000, 149, 285–306. [Google Scholar] [CrossRef]
- Bonato, K.O.; Fialho, C.B. Evidence of niche partitioning under ontogenetic influences among three morphologically similar Siluriformes in small subtropical streams. PLoS ONE 2014, 9, e110999. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschek, O.; Yamamoto, K.C.; Carvalho-Neto, F.G.M. Diet composition of fish assemblage of Lake Tupe, Amazonas, Brazil. Ver. Colombiana Cienc. Anim. 2013, 5, 313–326. [Google Scholar] [CrossRef]
- Rodrigues, G.G.; Hartz, S.M. Food dynamics of fish and the interaction with macroinvertebrates from a shallow lake in Southern Brazil. Verh. Int. Verein. Limnol. 2001, 27, 3309–3314. [Google Scholar] [CrossRef]
- Corrêa, F. Estrutura Trófica da Assembléia de Peixes Numa Área de Banhado do Parque Nacional da Lagoa do Peixe (RS). Ph.D. Thesis, Universidade Federal do Rio Grande, Rio Grande, Brazil, 2011. [Google Scholar]
- Saccol-Pereira, A. Variação Sazonal e Estrutura Trófica da Assembléia de Peixes do Delta do Rio Jacuí, RS, Brasil. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2008. [Google Scholar]
- González Sagrario, M.; Ferrero, L. The trophic role of Cyphocharax voga (Hensel, 1869) according to foraging area and diet analysis in turbid shallow lakes. Fundam. Appl. Limnol. 2013, 183, 75–88. [Google Scholar] [CrossRef]
- Corrêa, F.; Piedras, S.R.N. Alimentação de Cyphocharax voga (Hensel, 1869) (Characiformes, Curimatidae) no arroio Corrientes, Pelotas, Rio Grande Do Sul, Brasil. Biotemas 2008, 21, 117–122. [Google Scholar] [CrossRef]
- Angelescu, V.; Gneri, F.S. Adaptaciones del aparato digestivo al régimen alimenticio de algunos peces del rio Uruguay y rio de La Plata. Inst. Nac. Investig. Cienc. Nat. 1949, 1, 161–281. [Google Scholar]
- Dourado, E.C.d.S.; Benedito-Cecilio, E.; Latini, J.D. O grau de trofia do ambiente influencia a quantidade de energia dos peixes. In Biocenoses em Reservatórios: Padrões Espaciais e Temporais; Rodrigues, L., Thomaz, S.M., Agostinho, A.A., Gomes, L.C., Eds.; Rima Editora: São Carlos, Brazil, 2005; pp. 211–222. [Google Scholar]
- Delariva, R.L.; Agostinho, A.A. Relationship between morphology and diets of six Neotropical Loricariids. J. Fish Biol. 2001, 58, 832–847. [Google Scholar] [CrossRef]
- Hahn, N.S.; Fugi, R.; Loureiro-Crippa, V.E.; Peretti, A.C.; Russo, M.R. Trophic structure of the fish fauna. In Structure and Functioning of the Paraná River and its Floodplains; Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., Miranda, L.E., Eds.; EDUEM: Maringá, Brazil, 2004; pp. 139–143. [Google Scholar]
- Zardo, É.L.; Behr, E.R. Trophic ecology of Loricariichthys melanocheilus Reis & Pereira, 2000 (Siluriformes: Loricariidae) in Ibicuí river, southern Brazil. Acta Sci. Biol. Sci. 2016, 38, 10.4025. [Google Scholar]
- Fugi, R.; Hahn, N.S.; Agostinho, A.A. Feeding styles of five species of bottom-feeding fishes of the high Paraná River. Environ. Biol. Fish. 1996, 46, 297–307. [Google Scholar] [CrossRef]
- Fugi, R.; Agostinho, A.A.; Hahn, N.S. Trophic morphology of five benthic-feeding fish species of a tropical floodplain. Rev. Brasil. Biol. 2001, 61, 27–33. [Google Scholar] [CrossRef]
- Khallaf, E.A.; Alne-na-ei, A.A. Feeding ecology of Oreochromis niloticus (Linnaeus) & Tilapia zillii (Gervias) in a Nile canal. Hydrobiologia 1987, 146, 57–62. [Google Scholar]
- Monaco, I.d.A.; Resende, E.K. Alimentação de Liposarcus anisitsi e Potamorhina squamoralevis, peixes abundantes na Baía Tuiuiú, Pantanal, Mato Grosso do Sul, Brasil. In Proceedings of the 5° Simpósio sobre Recursos Naturais e Socioeconômicos do Pantanal, Corumba, MS, Brazil, 9–12 November 2010. [Google Scholar]
- Pereira, R.A.C.; Resende, E.K. Peixes detritívoros da planície inundável do Rio Miranda, Pantanal, Mato Grosso Do Sul, Brasil. Embrapa Bol. Pesq. 1998, 12, 1–50. [Google Scholar]
- Giora, J.; Fialho, C.B. Feeding biology of Steindachnerina brevipinna (Characiformes, Curimatidae) in the Ibicuí-mirim river, Rio Grande do Sul, Brazil. Iheringia Sér. Zool. 2003, 93, 277–281. [Google Scholar] [CrossRef]
- Flores, S.; Hirt, L.; Araya, P. Estructura y dinámica de la comunidad íctica del arroyo Yabotí, Reserva de Biosfera Yabotí, Misiones, Argentina. Rev. Mex. Biodiv. 2015, 86, 386–395. [Google Scholar] [CrossRef]
- Casatti, L.; Mendes, H.F.; Ferreira, K.M. Aquatic macrophytes as feeding site for small fishes in the Rosana Reservoir, Paranapanema River, Southeastern Brazil. Braz. J. Biol. 2003, 63, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Bialetzki, A.; Nakatani, K. Mudanças ontogênicas no trato digestório e dieta de Apareiodon affinis (Steindachner, 1879) (Osteichthyes, Parodontidae). Acta Sci. Biol. Sci. 2004, 26, 291–298. [Google Scholar]
- Abes, S.D.S.; Agostinho, A.A.; Okada, E.K.; Gomes, L.C. Diet of Iheringichthys labrosus (Pimelodidae, Siluriformes) in the Itaipu Reservoir, Paraná River, Brazil-Paraguay. Braz. Archiv. Biol. Technol. 2001, 44, 101–105. [Google Scholar] [CrossRef]
- Makrakis, M.C.; Nakatani, K.; Bialetzki, A.; Sanches, P.V.; Baumgartner, G.; Gomes, L.C. Ontogenetic shifts in digestive tract morphology and diet of fish larvae of the Itaipu Reservoir, Brazil. Environ. Biol. Fish. 2005, 72, 99–107. [Google Scholar] [CrossRef]
- Teixeira, I.; Bennemann, S.T. Ecomorfologia refletindo a dieta dos peixes em um reservatório no sul do Brasil. Biota Neotrop. 2007, 7, 67–76. [Google Scholar] [CrossRef]
- Fagundes, C.K.; Behr, E.R.; Kotzian, C.B. Diet of Iheringichthys labrosus (Siluriformes, Pimelodidae) in the Ibicuí river, southern Brazil. Iheringia Sér. Zool. 2008, 98, 60–65. [Google Scholar] [CrossRef]
- García, M.L.; Protogino, L.C. Invasive freshwater molluscs are consumed by native fishes in South America. J. Appl. Ichthyol. 2005, 21, 34–38. [Google Scholar] [CrossRef]
- López Cazorla, A.C.; Durán, W.; Tejera, L. Alimentación de la ictiofauna del río Sauce Grande, provincia de Buenos Aires, Argentina. Biol. Acuat. 2003, 20, 73–79. [Google Scholar]
- Loureiro-Crippa, V.E.; Hahn, N.S. Use of food resources by the fish fauna of a small reservoir (rio Jordão, Brazil) before and shortly after its filling. Neotrop. Ichthyol. 2006, 4, 357–362. [Google Scholar] [CrossRef]
- Bastos, R.F.; Miranda, S.F.; Garcia, A.M. Diet and feeding strategy of Characidium rachovii (Characiformes, Crenuchidae) in coastal plain streams of southern Brazil. Iheringia Sér. Zool. 2013, 103, 335–341. [Google Scholar] [CrossRef]
- González-Bergonzoni, I. Dieta de Peces de Agua Dulce: Efectos de Factores Climáticos y Complejidad del Hábitat. Master’s Thesis, Universidad de la República, Pedeciba, Uruguay, 2011; 67p. [Google Scholar]
- de Mérona, B.; Vigouroux, R.; Horeau, V. Changes in food resources and their utilization by fish assemblages in a large tropical reservoir in South America (Petit-Saut Dam, French Guiana). Acta Oecol. 2003, 24, 147–156. [Google Scholar] [CrossRef]
- de Mérona, B.; Vigouroux, R.; Tejerina-Garro, F.L. Alteration of fish diversity downstream from Petit-Saut Dam in French Guiana. Implication of ecological strategies of fish species. Hydrobiologia 2005, 551, 33–47. [Google Scholar] [CrossRef]
- Sánchez, R.M.; Galvis, G.; Victoriano, P.F. Relationship between digestive tract characteristics and diets of fishes from Yucao River, Meta River system (Colombia). Gayana 2003, 67, 75–86. [Google Scholar] [CrossRef]
- Longoni, L.S. Biologia alimentar e reprodutiva do cará Gymnogeophagus gymnogenys (Perciformes: Cichlidae) na Região do Delta do Jacuí, Rio Grande do Sul. Bachelors Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2009; 42p. [Google Scholar]
- Selmo, A.T. Estudo comparado da dieta de duas espécies simpátricas de Gymnogeophagus (Perciformes, Cichlidae) em um riacho no sul do Brasil. Bachelors Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2010; 29p. [Google Scholar]
- Ibañez, C.; Tedesco, P.A.; Bigorne, R.; Hugueny, B.; Pouilly, M.; Zepita, C.; Zubieta, J.; Oberdorff, T. Dietary-morphological relationship in fish assemblages of small forested streams in the Bolivian Amazon. Aquat. Living Resour. 2007, 20, 131–142. [Google Scholar] [CrossRef]
- Rodrigues, F.L.; Bemvenuti, M.A. Hábito alimentar e osteologia da boca do peixe-rei, Odontesthes humensis de Buen (Atheriniformes, Atherinopsidae) na Lagoa Mirim, Rio Grande do Sul, Brasil. Rev. Bras. Zool. 2001, 18, 793–802. [Google Scholar] [CrossRef]
- Fugi, R.; Hahn, N.S.; Novakowski, G.C.; Balassa, G.C. Feeding ecology of Pachyurus bonariensis (Perciformes, Sciaenidae) in two bays of the Pantanal, Mato Grosso State, Brazil. Iheringia Sér. Zool. 2007, 97, 343–347. [Google Scholar] [CrossRef]
- Lima, D.O.; Behr, E.R. Feeding ecology of Pachyurus bonariensis Steindachner, 1879 (Sciaenidae: Perciformes) in the Ibicuí River, Southern Brazil: Ontogenetic, seasonal and spatial variations. Braz. J. Biol. 2010, 70, 503–509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Mérona, B.; Dos Santos, G.M.; De Almeida, R.G. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environ. Biol. Fishes 2001, 60, 375–392. [Google Scholar] [CrossRef]
- Barili, E.; Fugi, R.; Novakowski, G.C.; Agostinho, A.A. Impoundment effects in the population of Auchenipterus osteomystax (Siluriformes: Auchenipteridae): A Neotropical reservoir case. Rev. Biol. Trop. 2012, 60, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Demétrio, J.A.; Gomes, L.C.; Latini, J.D.; Agostinho, A.A. Influence of net cage farming on the diet of associated wild fish in a Neotropical reservoir. Aquaculture 2012, 330, 172–178. [Google Scholar] [CrossRef]
- Strictar-Pereira, L.; Agostinho, A.A.; Gomes, L.C. Cage culture with tilapia induces alteration in the diet of natural fish populations: The case of Auchenipterus osteomystax. Braz. J. Biol. 2010, 70, 1021–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suiberto, M.R.; Galuch, A.V.; Bialetzki, A.; Nakatani, K. Ontogenetic shifts in the digestive tube and diet of Bryconamericus stramineus Eigenmann, 1908 (Osteichthyes, Characidae). Acta Limnol. Bras. 2009, 21, 465–472. [Google Scholar]
- Brandão-Gonçalves, L.; Lima-Junior, E.; Suarez, Y.R. Feeding habits of Bryconamericus stramineus Eigenmann, 1908 (Characidae), in different streams of Guiraí River Sub-Basin, Mato Grosso do Sul, Brazil. Biota Neotrop. 2009, 9, 135–143. [Google Scholar] [CrossRef]
- Artioli, L.G.S.; Prates-Júnior, P.H.S.; Diefenthaeler, F.; Fontoura, N.F. Período reprodutivo e alimentação de Astyanax alburnus no canal cornélios, capão da canoa, Rio Grande do Sul (Teleostei, Characiformes, Characidae). Biociências 2003, 11, 115–122. [Google Scholar]
- Delariva, R.L.; Hahn, N.S.; Kashiwaqui, E.A.L. Diet and trophic structure of the fish fauna in a subtropical ecosystem: Impoundment effects. Neotrop. Ichthyol. 2013, 11, 891–904. [Google Scholar] [CrossRef]
- Vilella, F.S.; Becker, F.G.; Hartz, S.M. Diet of Astyanax species (Teleostei, Characidae) in an Atlantic forest river in Southern Brazil. Braz. Arch. Biol. Technol. 2002, 45, 223–232. [Google Scholar] [CrossRef]
- Bemvenuti, M.A. Silversides in South Brazil: Morphological and ecological aspects. Biocell 2006, 30, 111–118. [Google Scholar] [PubMed]
- Brancolini, F.; Maroñas, M.E.; Sendra, E.D. Dieta de Pseudocorynopoma doriae (Characiformes: Characidae) en el arroyo de La Choza, Buenos Aires, Argentina. Biol. Acuática 2015, 30, 259–265. [Google Scholar]
- Ferriz, R.A.; Fernandez, E.M.; López, G.R.; Bentos, C.A. Alimentación de Pseudocorynopoma doriai (Pisces: Characidae) en el arroyo El Portugués, provincia de Buenos Aires, Argentina. Rev. Mus. Arg. Cienc. Nat. 2012, 14, 243–251. [Google Scholar] [CrossRef]
- Andrade-López, J.; Machado-Allison, A. Morphological and ecological aspects of the Heptapteridae and Auchenipteridae species in the Morichal Nicolasito (Aguaro River, Guárico State, Venezuela). Bol. Acad. C Fís Mat. Nat. 2009, 69, 35–52. [Google Scholar]
- Santin, M.T.M.; Baggio, M.M.; Agostinho, A.A.; Bialetzki, A. Mudanças ontogênicas no trato digestório e na dieta de Trachelyopterus galeatus. Bol. Inst. Pesca São Paulo 2015, 41, 57–68. [Google Scholar]
- Mol, J.H. Ontogenetic diet shifts and diet overlap among three closely related Neotropical armoured catfishes. J. Fish Biol. 1995, 47, 788–807. [Google Scholar] [CrossRef]
- Winemiller, K.O. Feeding and reproductive biology of the currito, Hoplosternum littorale, in the Venezuelan llanos with comments on the possible function of the enlarged male pectoral spines. Environ. Biol. Fishes 1987, 20, 219–227. [Google Scholar] [CrossRef]
- Dias, T.S. Estudo da Dieta de Oito Espécies da Subfamília Cheirodontinae (Characiformes: Characidae) em Diferentes Sistemas Lacustres nos Estados do Rio Grande do Norte e Rio Grande do Sul. Unpublished M.Sc. Dissertation, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2007; 89p. [Google Scholar]
- Scanferla, A.F.L.; Súarez, Y.R. Flood pulse are the main determinant of feeding dynamics and composition of Odontostilbe pequira (Characiformes: Characidae) in southern Pantanal, Brazil. Acta Limnol. Bras. 2016, 28, e19. [Google Scholar] [CrossRef]
- Fiori, L.F.; Alves, G.H.Z.; Hahn, N.S.; Benedito, E. Influence of feeding plasticity on the fitness of small Neotropical characids. Iheringia. Sér. Zool. 2016, 106, 1–6. [Google Scholar] [CrossRef][Green Version]
- Santos, C.L.; Dos Santos, I.A.; Da Silva, C.J. Ecologia trófica de peixes ocorrentes em bancos de macrófitas aquáticas na baia Caiçara, Pantanal Mato-Grossense. Braz. J. Biosci. 2009, 7, 473–476. [Google Scholar]
- Aguiaro, T.; Caramaschi, É.P. Trophic guilds in fish assemblages in three coastal lagoons of Rio de Janeiro State (Brazil). Verh. Int. Verein. Limnol. 1998, 26, 2166–2169. [Google Scholar] [CrossRef]
- Aguiaro, T.; Castelo Branco, C.W.; Verani, J.R.; Caramaschi, É.P. Diet of the clupeid fish Platanichthys platana (Regan, 1917) in two different Brazilian coastal lagoons. Braz. Arch. Biol. Technol. 2003, 46, 215–222. [Google Scholar] [CrossRef]
- Pereira, L.S.; Agostinho, A.A.; Delariva, R.L. Effects of river damming in Neotropical piscivores and omnivorous fish: Feeding, body condition and abundances. Neotrop. Ichthyol. 2016, 14, e150044. [Google Scholar] [CrossRef]
- Esteves, K.E. Feeding ecology of three Astyanax species (Characidae, Tetragonopterinae) from a floodplain lake of Mogi-Guaçú River, Paraná River basin, Brazil. Environ. Biol. Fishes 1996, 46, 83–101. [Google Scholar] [CrossRef]
- Wolff, L.L.; Abilhoa, V.; Rios, F.S.A.; Donatti, L. Spatial, seasonal and ontogenetic variation in the diet of Astyanax aff. fasciatus (Ostariophysi: Characidae) in an Atlantic Forest river, Southern Brazil. Neotrop. Ichthyol. 2009, 7, 257–266. [Google Scholar] [CrossRef]
- Arcifa, M.S.; Northcote, T.G.; Froehlich, O. Interactive ecology of two cohabiting characin fishes (Astyanax fasciatus and Astyanax bimaculatus) in an eutrophic Brazilian reservoir. J. Trop. Ecol. 1991, 7, 257–268. [Google Scholar] [CrossRef]
- Barbosa, P.M.M.; Matsumura-Tundisi, T. Consumption of zooplanktonic organisms by Astyanax fasciatus Cuvier, 1819 (Osteichthyes, Characidae) in Lobo (Broa) Reservoir, São Carlos, SP, Brazil. Hydrobiologia 1984, 113, 171–181. [Google Scholar] [CrossRef]
- Abelha, M.C.F.; Goulart, E. Oportunismo trófico de Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes, Cichlidae) no reservatório de Capivari, Estado do Paraná, Brasil. Acta Sci. Biol. Sci. 2004, 26, 37–45. [Google Scholar] [CrossRef]
- Bastos, R.F.; Condini, M.V.; Varela Junior, A.S.; Garcia, A.M. Diet and food consumption of the pearl cichlid Geophagus brasiliensis (Teleostei: Cichlidae): Relationships with gender and sexual maturity. Neotrop. Ichthyol. 2011, 9, 825–830. [Google Scholar] [CrossRef]
- Moraes, M.F.P.; de Freitas Barbola, I.; Duboc, L.F. Feeding habits and morphometry of digestive tracts of Geophagus brasiliensis (Osteichthyes, Cichlidae), in a lagoon of high Tibagi River, Paraná State, Brazil. Publ. UEPG Biol. Health Sci. 2004, 10, 37–45. [Google Scholar] [CrossRef]
- Cantanhêde, G.; Hahn, N.S.; Gubiani, É.A.; Fugi, R. Invasive molluscs in the diet of Pterodoras granulosus (Valenciennes, 1821) (Pisces, Doradidae) in the Upper Paraná River floodplain, Brazil. Ecol. Freshw. Fish 2008, 17, 47–53. [Google Scholar] [CrossRef]
- de Souza-Stevaux, M.C.; Negrelle, R.R.; Citadini-Zanette, V. Seed dispersal by the fish Pterodoras granulosus in the Paraná River Basin, Brazil. J. Trop. Ecol. 1994, 10, 621–626. [Google Scholar] [CrossRef]
- Arcifa, M.S.; Froehlich, O.; Northcote, T.G. Distribution and feeding ecology of fishes in a tropical Brazilian reservoir. Soc. Cienc. Nat. La Salle 1988, 48, 301–326. [Google Scholar]
- Villares Junior, G.A.; Gomiero, L.M.; Goitein, R. Biological aspects of Schizodon nasutus Kner, 1858 (Characiformes, Anostomidae) in the low Sorocaba river basin, São Paulo state, Brazil. Braz. J. Biol. 2011, 71, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Zardo, É.L.; da Rosa, V.M.; Behr, E.R. Distribution and feeding of fish from Anostomidae family in Ibicuí River, Río Grande do Sul, Brazil. Bol. Inst. Pesca São Paulo 2016, 42, 156–166. [Google Scholar] [CrossRef]
- Sverlij, S.B.; López, H.L.; Schenke, R.L.D.; Ros, A.E. Peces del Río Uruguay: Guía ilustrado de las especies más comunes del rio Uruguay inferior y el embalse de Salto Grande; CARU: Montevideo, Uruguay, 1998; 89p. [Google Scholar]
- de Resende, E.K. Trophic structure of fish assemblages in the lower Miranda river, Pantanal, Mato Grosso do Sul State, Brazil. Rev. Bras. Biol. 2000, 60, 389–403. [Google Scholar] [CrossRef]
- Winemiller, K.O. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 1989, 8, 225–241. [Google Scholar] [CrossRef]
- Zaniboni-Filho, E.; Meurer, S.; Schibatta, O.A.; Nuñer, A.P.O. Catálogo ilustrado de peixes do alto rio Uruguai; Editora da UFSC: Florianópolis, Brazil, 2000. [Google Scholar]
- Teixeira-de Mello, F.; Gonzalez-Bergonzoni, I.; Viana, F.; Saizar, C. Length–weight relationships of 26 fish species from the middle section of the Negro River (Tacuarembó-Durazno, Uruguay). J. Appl. Ichthyol. 2011, 27, 1413–1415. [Google Scholar] [CrossRef]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Vidal, N.; DÁnatro, A.; Stebniki, S.; González-Bergonzoni, I.; Teixeira-de Mello, F. Monitoreo de la pesca artesanal en el Rïo Uruguay a través de información generada por pescadores de la zona de Nuevo Berlín, Fray Bentos y Las Cañas (Período 2007–2017); UPM: Montevideo, Uruguay, 2018. [Google Scholar]
- Iwaszkiw, J.; Lacoste, F. La pesca artesanal en la Cuenca del Plata (Argentina) y sus implicancias en la conservación de la biodiversidad. Rev. Mus. Arg. Cienc. Nat. 2011, 13, 21–25. [Google Scholar] [CrossRef]
- Vidal, N.; DÁnatro, A.; Stebniki, S.; González-Bergonzoni, I.; Teixeira-de Mello, F. Monitoreo de la pesca artesanal en el Rïo Uruguay a través de información generada por pescadores de la zona de Nuevo Berlín, Fray Bentos y Las Cañas (Período 2007–2016); UPM: Montevideo, Uruguay, 2017. [Google Scholar]
- Luz, R.K.; Portella, M.C. Freqüencia alimentar na larvicultura do trairão (Hoplias lacerdae). Rev. Bras. Zootec. 2005, 34, 1442–1448. [Google Scholar] [CrossRef]
- Penchaszadeh, P.E.; Darrigran, G.; Angulo, C.; Averbuj, A.; Brogger, M.; Dogliotti, A.; Pirez, N. Predation of the invasive freshwater mussel Limnoperna fortunei (Dunker 1857) (Mytilidae) by the fish Leporinus obtusidens Valenciennes, 1846 (Anostomidae) in the Rio de la Plata, Argentina. J. Shelfish Res. 2000, 19, 229–231. [Google Scholar]
- Luz-Agostinho, K.D.G.; Agostinho, A.A.; Gomes, L.C.; Júlio, H.F., Jr. Influence of flood pulses on diet composition and trophic relationship among piscivorous fish in the upper Paraná River floodplain. Hydrobiologia 2008, 607, 187–198. [Google Scholar] [CrossRef]
- Freitas, T.M.S.; Almeida, V.H.D.; Valente, R.D.; Montag, I.F.D. Feeding ecology of Auchenipterichthys longimanus (Siluriformes: Auchenipteridae) in a riparian flooded forest of Eastern Amazonia, Brazil. Neotrop. Ichtiol. 2011, 9, 629–636. [Google Scholar] [CrossRef]
- Brejão, G.C.; Gerhard, P.; Zuanon, J. Functional trophic composition of the ichthyofauna of forest streams in eastern Brazilian Amazon. Neotrop. Ichthyol. 2013, 11, 361–373. [Google Scholar] [CrossRef]
- D’Anatro, A.; Vidal, N.; Gonzalez-Bergonzoni, I.; Teixeira de Mello, F.; Tana, J.; Naya, D. Geographic and seasonal variation analysis of digestive morphology in the catfish Iheringichthys labrosus along lower Río Uruguay. Animal Physiol. 2013, 5, 9–13. [Google Scholar]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse in river-floodplain systems. In Proceedings of International Large River Symposium; Dodge, D.P., Ed.; Canadian Special Publication of Fisheries and Aquatic Sciences, 0706-6481; Department of Fisheries and Oceans: Ottawa, ON, Canada, 1989; Volume 106, pp. 110–127. [Google Scholar]
- Winemiller, K.O.; Jepsen, D.B. Effects of seasonality and fish movement on tropical river food webs. J. fish Biol. 1998, 53, 267–296. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Carl Saunders, W. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Boil. 2005, 50, 201–220. [Google Scholar] [CrossRef]
- Malmqvist, B.; Rundle, S.D.; Covich, A.P.; Hildrew, A.G.; Robinson, C.T.; Townsend, C.R. Prospects for streams and rivers: An ecological perspective. In Aquatic Ecosystems: Trends and Global Prospects; Polunin, N.V.C., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 19–29. ISBN 978-0-521-83327-1. [Google Scholar]
- Agostinho, A.A.; Pelicice, F.M.; Gomes, L.C. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz. J. Biol. 2008, 68, 1119–1132. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Léveque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 2005, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Winemiller, K.O.; McIntyre, P.B.; Castello, L.; Fluet-Chouinard, E.; Giarrizzo, T.; Nam, S.; Baird, I.G.; Darwan, W.; Lujan, N.K.; Harrison, I.; et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 2016, 351, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Zaniboni-Filho, E. Mosaic environments shape the distribution of Neotropical freshwater ichthyoplankton. Ecol. Freshw. Fish 2019. [Google Scholar] [CrossRef]
- Dudgeon, D. Large-Scale hydrological changes in tropical Asia: Prospects for riverine biodiversity. The construction of large dams will have an impact on the biodiversity of tropical Asian rivers and their associated wetlands. BioScience. 2000, 50, 793–806. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A global boom in hydropower dam construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).