Kinetics, Mechanism, and Toxicity of Amlodipine Degradation by the UV/Chlorine Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. UV Irradiation
2.3. Kinetic Studies
2.4. Identification of Intermediates (IMs)
2.5. Toxicity Tests and Assessment
3. Results and Discussion
3.1. AML Degradation by the UV/Chlorine Process
3.2. Effect of Chlorine Dose
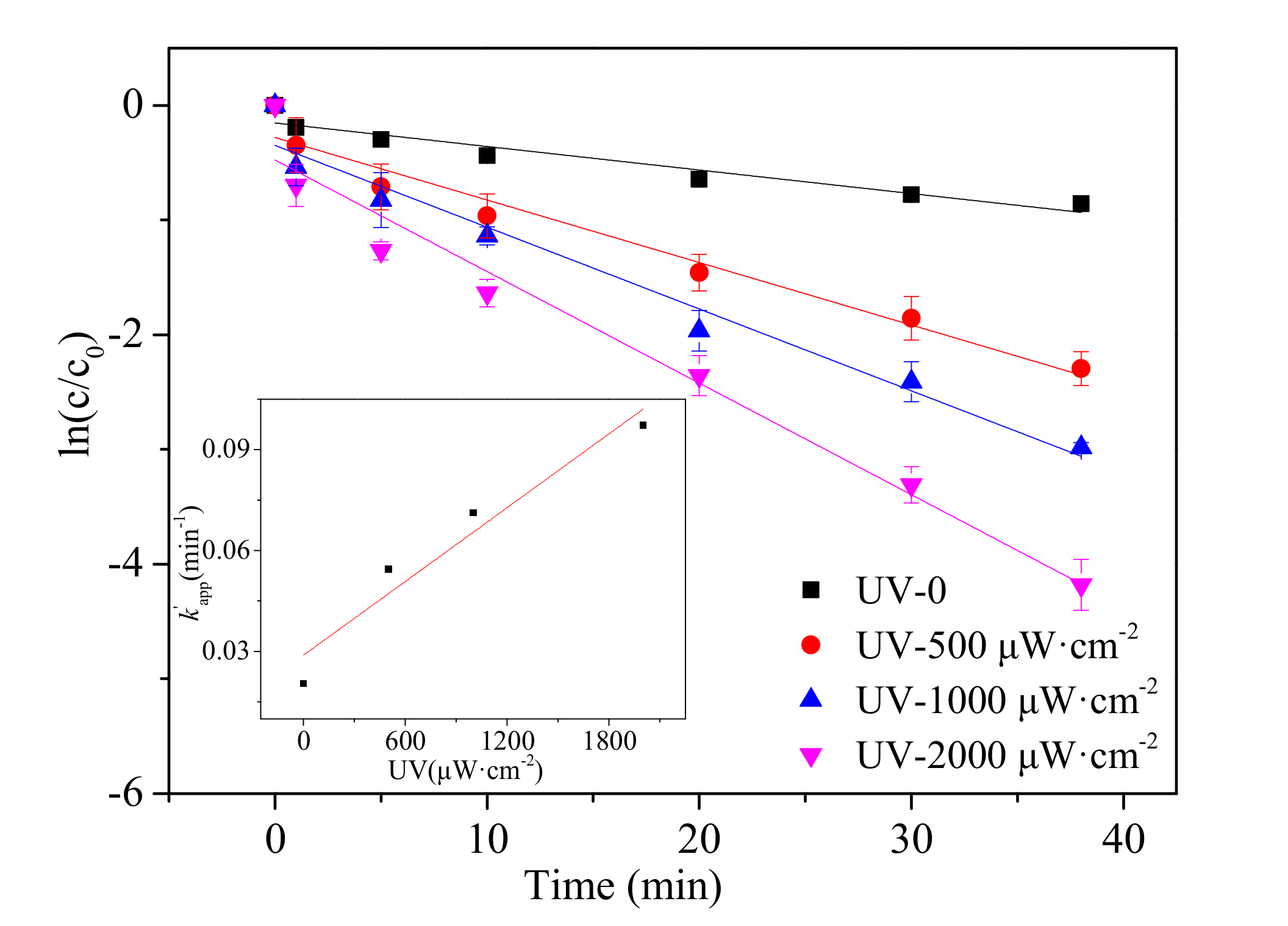
3.3. Effect of UV Intensity
3.4. Effect of pH Value
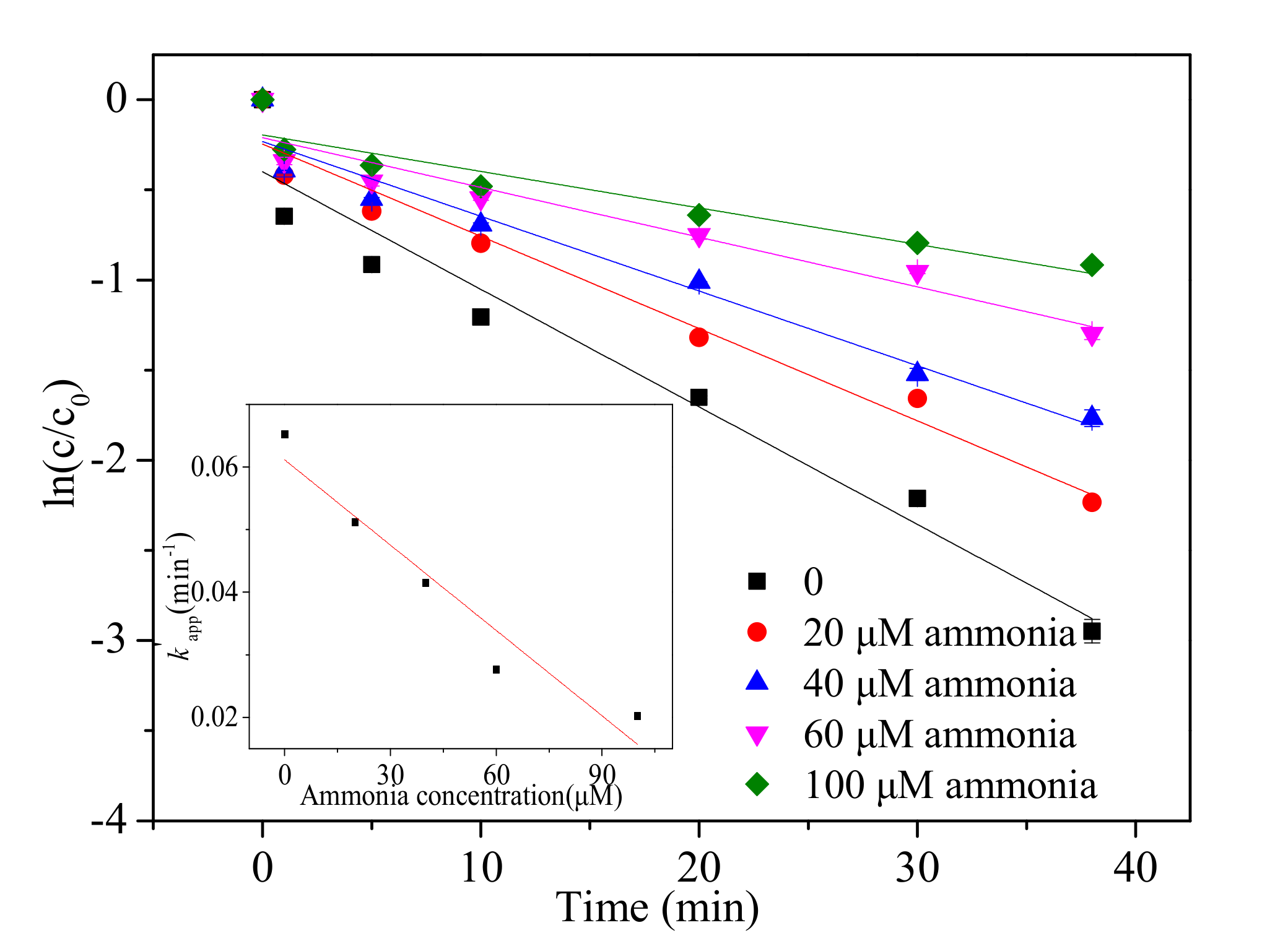
3.5. Effect of Ammonia Concentration
3.6. AML Degradation in Real Water by the UV/Chlorine Process
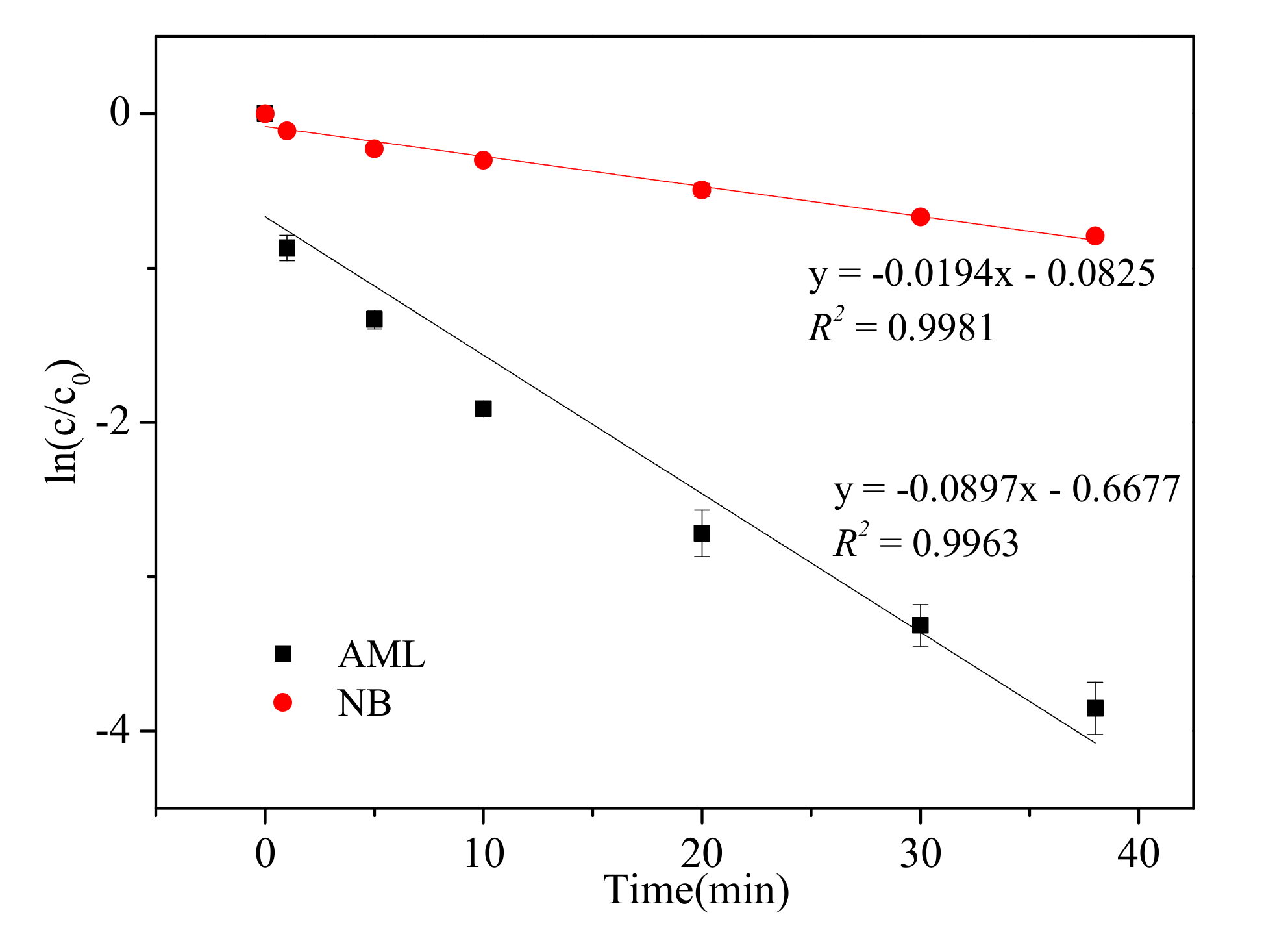
3.7. Relative Contributions of Active Ingredients
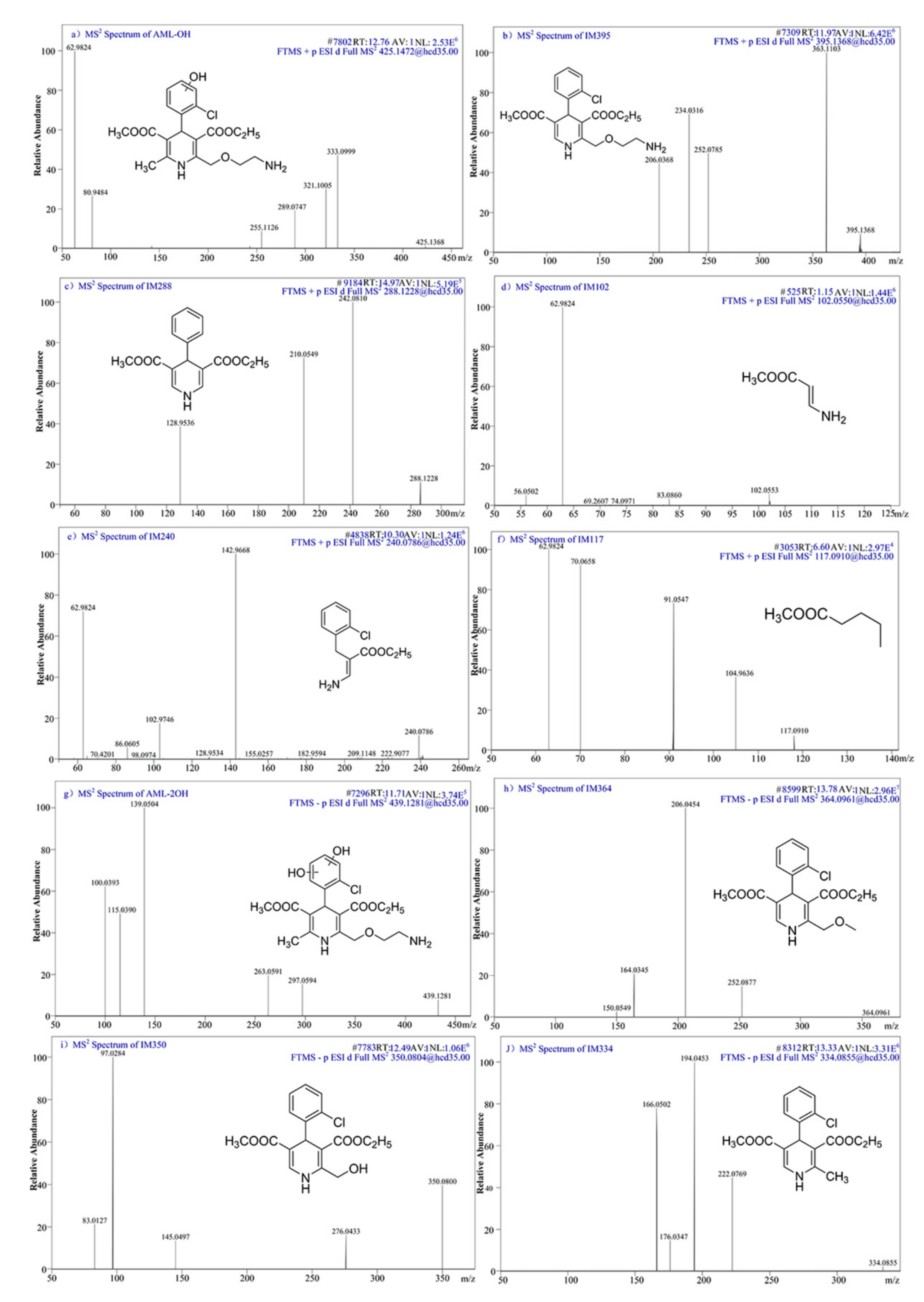
3.8. Intermediates Identification and Degradation Mechanism
3.9. Toxicity Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Total Phosphorus (mg·L−1) | COD (mg·L−1) | Ammonia Nitrogen (mg·L−1) | Dissolve Oxygen (mg·L−1) | Oxidation-Reduction Potential (mV) | PH Value |
|---|---|---|---|---|---|
| 0.39 | 8.07 | 0.62 | 5.89 | −27 | 7.2 |

References
- Enick, O.V.; Moore, M.M. Assessing the assessments: Pharmaceuticals in the environment. Environ. Impact Assess. Rev. 2007, 27, 707–729. [Google Scholar] [CrossRef]
- Delgado, L.F.; Charles, P.; Glucina, K.; Morlay, C. QSAR-like models: A potential tool for the selection of PhACs and EDCs for monitoring purposes in drinking water treatment systems—A review. Water Res. 2012, 46, 6196–6209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Bian, T.; Zhang, Y.; Zhang, F.; Yan, Y. Selective extraction of gadolinium using free-standing imprinted mesoporous carboxymethyl chitosan films with high capacity. Cellulose 2019, 26, 1209–1219. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Hu, J.; Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017, 121, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-G.; Gao, N.-Y.; Chu, W.-H.; Li, L.; Zhang, Y.-J.; Gu, J.-S.; Gu, Y.-L. Photochemical degradation of ciprofloxacin in UV and UV/H2O2 process: Kinetics, parameters, and products. Environ. Sci. Pollut. Res. 2013, 20, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Bian, T.; Wang, D.; Li, Z. One-step fabrication of imprinted mesoporous cellulose nanocrystals films for selective separation and recovery of Nd (III). Cellulose 2019, 26, 5571–5582. [Google Scholar] [CrossRef]
- Fernández, C.; González-Doncel, M.; Pro, J.; Carbonell, G.; Tarazona, J.V. Occurrence of pharmaceutically active compounds in surface waters of the Henares-Jarama-Tajo river system (Madrid, Spain) and a potential risk characterization. Sci. Total Environ. 2010, 408, 543–551. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Maulvault, A.L.; Tediosi, A.; Fernández-Tejedor, M.; Van den Heuvel, F.; Kotterman, M.; Marques, A.; Barceló, D. Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environ. Res. 2015, 143, 56–64. [Google Scholar] [CrossRef]
- Yu, C.-P.; Chu, K.-H. Occurrence of pharmaceuticals and personal care products along the West Prong Little Pigeon river in east Tennessee, USA. Chemosphere 2009, 75, 1281–1286. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Zhang, F.; Li, Z.; Yan, Y. Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J. Hazard. Mater. 2018, 353, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, T.; Wang, W.-L.; Wu, Q.-Y.; Li, A.; Hu, H.-Y. UV/chlorine as an advanced oxidation process for the degradation of benzalkonium chloride: Synergistic effect, transformation products and toxicity evaluation. Water Res. 2017, 114, 246–253. [Google Scholar] [CrossRef]
- Xiang, Y.; Fang, J.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, J.; Ma, J.; Yang, Y.; Liu, W.; Liu, Y. Degradation of atrazine by UV/chlorine: Efficiency, influencing factors, and products. Water Res. 2016, 90, 15–23. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, S.; Yang, X.; Ren, J.; Fang, J.; Shang, C.; Song, W.; Lian, L.; Zhang, X. UV/chlorine treatment of carbamazepine: Transformation products and their formation kinetics. Water Res. 2017, 116, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, R.N.; Shah, N.; Bhalani, V.; Mahajan, A.L.C. MSn and LC–MS/MS studies for the characterization of degradation products of amlodipine. J. Pharm. Anal. 2015, 5, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Elliott, W.J.; Ram, C.V.S. Calcium channel blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef]
- Hummel, D.; Löffler, D.; Fink, G.; Ternes, T.A. Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environ. Sci. Technol. 2006, 40, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Batt, A.L.; Kostich, M.S.; Lazorchak, J.M. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC−MS/MS. Anal. Chem. 2008, 80, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Spongberg, A.L.; Witter, J.D. Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. Sci. Total Environ. 2008, 397, 148–157. [Google Scholar] [CrossRef]
- Du, B.; Price, A.E.; Scott, W.C.; Kristofco, L.A.; Ramirez, A.J.; Chambliss, C.K.; Yelderman, J.C.; Brooks, B.W. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ. 2014, 466–467, 976–984. [Google Scholar] [CrossRef]
- Wang, J.; Gardinali, P.R. Uptake and depuration of pharmaceuticals in reclaimed water by mosquito fish (Gambusia holbrooki): A worst-case, multiple-exposure scenario. Environ. Toxicol. Chem. 2013, 32, 1752–1758. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Hedgespeth, M.; Wirth, E.; Fulton, M. Analysis of selected natural and synthetic hormones by LC-MS-MS using the US EPA method 1694. Anal. Methods 2011, 3, 1079–1086. [Google Scholar] [CrossRef]
- Engebrecht, J.; Nealson, K.; Silverman, M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell 1983, 32, 773–781. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Giddings, J.M.; Salvito, D.; Putt, A.E. Acute toxicity of 4-amino musk xylene to Daphnia magna in laboratory water and natural water. Water Res. 2000, 34, 3686–3689. [Google Scholar] [CrossRef]
- Xiang, H.; Shao, Y.; Gao, N.; Lu, X.; An, N.; Tan, C.; Zheng, Z. Degradation of diuron by chlorination and UV/chlorine process: Degradation kinetics and the formation of disinfection by-products. Sep. Purif. Technol. 2018, 202, 365–372. [Google Scholar] [CrossRef]
- Deng, L.; Huang, C.H.; Wang, Y.L. Effects of combined UV and chlorine treatment on the formation of trichloronitromethane from amine precursors. Environ. Sci. Technol. 2014, 48, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.; Barreto Crespo, M.T.; Pereira, V.J. Drinking water treatment of priority pesticides using low pressure UV photolysis and advanced oxidation processes. Water Res. 2010, 44, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Zhang, Y.; Xiang, H.; Guo, Y. Degradation of diclofenac by UV-activated persulfate process: Kinetic studies, degradation pathways and toxicity assessments. Ecotoxicol. Environ. Saf. 2017, 141, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Wang, T.; Hu, H.Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]
- Yin, K.; Deng, L.; Luo, J.; Crittenden, J.; Liu, C.; Wei, Y.; Wang, L. Destruction of phenicol antibiotics using the UV/H2O2 process: Kinetics, byproducts, toxicity evaluation and trichloromethane formation potential. Chem. Eng. J. 2018, 351, 867–877. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; Von Gunten, U. Degradation kinetics of atrazine and its degradation products with ozone and OH radicals: A predictive tool for drinking water treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; He, Q.; Deng, Y.; Liu, H. The role of reactive oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef]
- Wang, D.; Bolton, J.R.; Hofmann, R. Medium pressure UV combined with chlorine advanced oxidation for trichloroethylene destruction in a model water. Water Res. 2012, 46, 4677–4686. [Google Scholar] [CrossRef]
- Watts, M.J.; Hofmann, R.; Rcdsenfeldt, E.J. Low-pressure UV/Cl2 for advanced oxidation of taste and odor. J. Am. Water Work. Assoc. 2012, 104, E58–E65. [Google Scholar] [CrossRef] [Green Version]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef]
- Yin, K.; Deng, Y.; Liu, C.; He, Q.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Kinetics, pathways and toxicity evaluation of neonicotinoid insecticides degradation via UV/chlorine process. Chem. Eng. J. 2018, 346, 298–306. [Google Scholar] [CrossRef]
- Nowell, L.H.; Hoigné, J. Photolysis of aqueous chlorine at sunlight and ultraviolet wavelengths—II. Hydroxyl radical production. Water Res. 1992, 26, 599–605. [Google Scholar] [CrossRef]
- Nam, S.W.; Yoon, Y.; Choi, D.J.; Zoh, K.D. Degradation characteristics of metoprolol during UV/chlorination reaction and a factorial design optimization. J. Hazard. Mater. 2015, 285, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Adams, C.D. Determination of monochloramine formation rate constants with stopped-flow spectrophotometry. Environ. Sci. Technol. 2004, 38, 1435–1444. [Google Scholar] [CrossRef]
- Tian, F.; Liu, W.; Guo, G.; Qiang, Z.; Zhang, C. Kinetics and mechanism of dimethoate chlorination during drinking water treatment. Chemosphere 2014, 103, 181–187. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.; Zhang, D.; Yin, K.; Wang, D.; Zhang, W.; Liu, C.; Yang, C.; Wei, Y.; Wang, L. The individual and Co-exposure degradation of benzophenone derivatives by UV/H2O2 and UV/PDS in different water matrices. Water Res. 2019, 159, 102–110. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, M.; Gao, N.; Chu, W.; Li, K.; Chen, S. Degradation of phenacetin by the UV/chlorine advanced oxidation process: Kinetics, pathways, and toxicity evaluation. Chem. Eng. J. 2018, 335, 520–529. [Google Scholar] [CrossRef]
- Yin, K.; He, Q.; Liu, C.; Deng, Y.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism. Chemosphere 2018, 212, 56–66. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Nojavan, S.; Haghgoo, S.; Mohammadi, A. Development of a stability-indicating CE assay for the determination of amlodipine enantiomers in commercial tablets. Electrophoresis 2008, 29, 4583–4592. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.-Y.; Xu, L. Transformation of benzophenone-type UV filters by chlorine: Kinetics, products identification and toxicity assessments. J. Hazard. Mater. 2016, 311, 263–272. [Google Scholar] [CrossRef]
- Zhu, B.; Zonja, B.; Gonzalez, O.; Sans, C.; Pérez, S.; Barceló, D.; Esplugas, S.; Xu, K.; Qiang, Z. Degradation kinetics and pathways of three calcium channel blockers under UV irradiation. Water Res. 2015, 86, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qu, R.; Pan, X.; Wang, Z. Oxidative degradation of triclosan by potassium permanganate: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Res. 2016, 103, 215–223. [Google Scholar] [CrossRef] [PubMed]




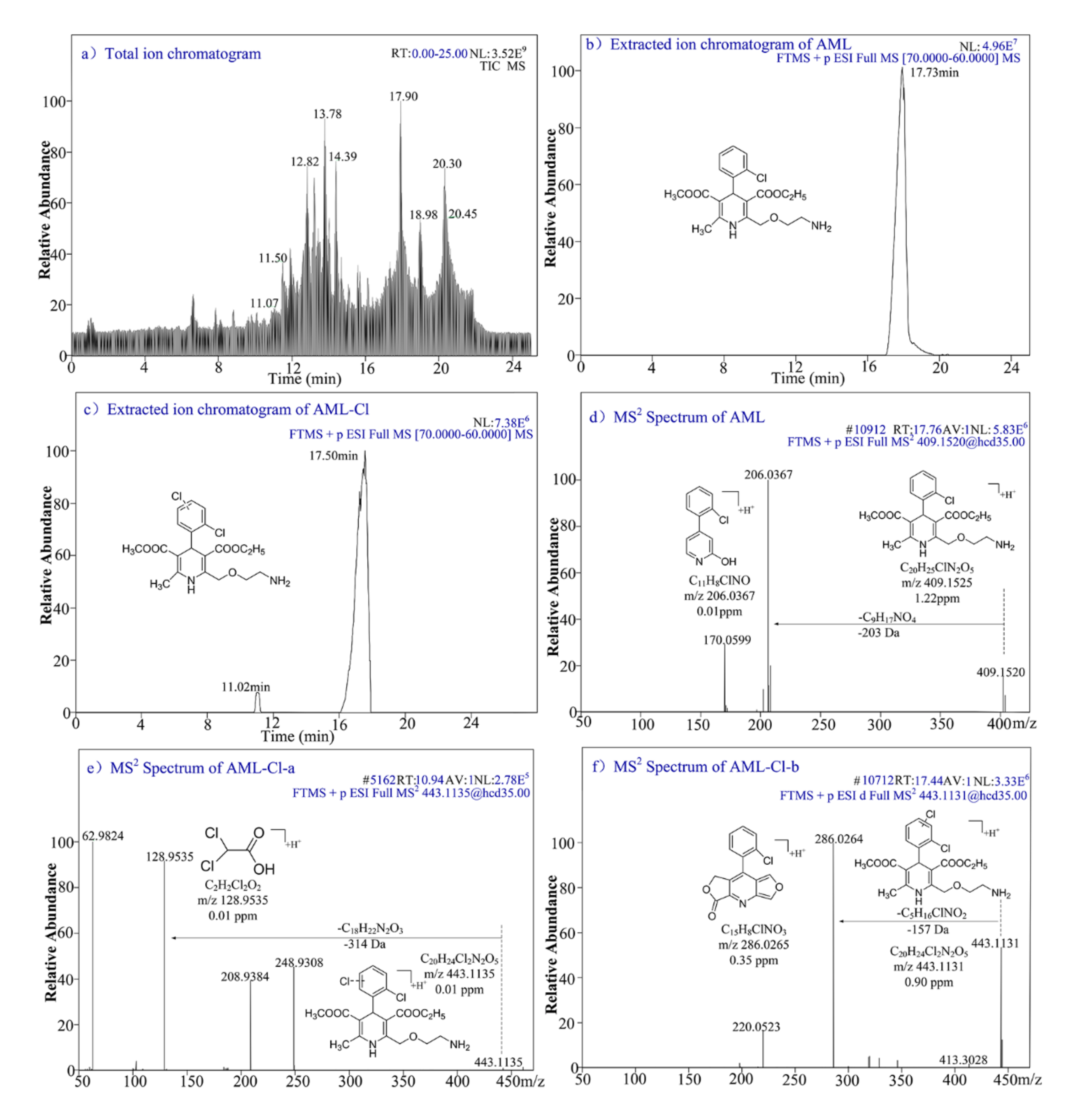


| No. | Compounds | LC Retention Time (min) | Molecular Formula | [M+H]+ | Molecular Structures | ||
|---|---|---|---|---|---|---|---|
| Theoretical m/z | Measured m/z | ∆ (ppm) | |||||
| 1 | AML | 17.73 | C20H25ClN2O5 | 409.1525 | 409.1522 | 0.73 | 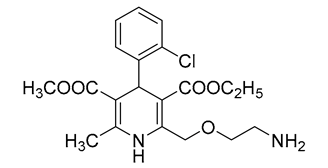 |
| 2 | AML-Cl-a | 11.02 | C20H24Cl2N2O5 | 443.1135 | 443.1135 | 0.01 |  |
| 3 | AML-Cl-b | 17.50 | C20H24Cl2N2O5 | 443.1135 | 443.1131 | 0.90 |  |
| 4 | AML-OH | 12.67 | C20H25ClN2O6 | 425.1474 | 425.1472 | 0.47 | 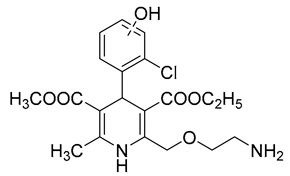 |
| 5 | IM395 | 11.97 | C19H23ClN2O5 | 395.1368 | 395.1368 | 0.01 |  |
| 6 | IM288 | 14.97 | C16H17NO4 | 288.1230 | 288.1228 | 0.69 |  |
| 7 | IM102 | 1.15 | C4H7NO2 | 102.0550 | 102.0550 | 0.01 | 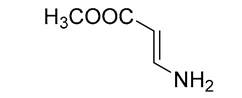 |
| 8 | IM240 | 10.30 | C12H14ClNO2 | 240.0786 | 240.0786 | 0.01 |  |
| 9 | IM117 | 6.60 | C6H12O2 | 117.0910 | 117.0910 | −0.01 | 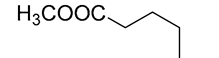 |
| No. | Compounds | LC Retention Time (min) | Molecular Formula | [M-H]− | Molecular Structures | ||
|---|---|---|---|---|---|---|---|
| Theoretical m/z | Measured m/z | ∆ (ppm) | |||||
| 1 | AML | 17.73 | C20H25ClN2O5 | 407.1379 | 407.1380 | −0.25 | 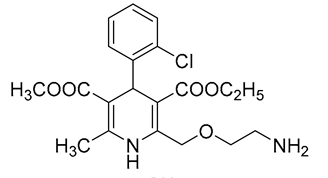 |
| 10 | AML−2OH | 11.71 | C20H25ClN2O7 | 439.1278 | 439.1281 | −0.68 |  |
| 11 | IM364 | 13.78 | C18H20ClNO5 | 364.0957 | 364.0961 | −1.09 |  |
| 12 | IM350 | 12.49 | C17H18ClNO5 | 350.0801 | 350.0804 | −0.86 |  |
| 13 | IM334 | 13.33 | C17H18ClNO4 | 334.0852 | 334.0855 | −0.89 |  |
| No. | Products | Molecular Weight | CAS Number | GC Retention Time (min) | Molecular Structures |
|---|---|---|---|---|---|
| 14 | Acetic acid | 60.0211 | 64-19-7 | 8.2587 |  |
| 15 | Oxalic acid | 89.9953 | 144-62-7 | 17.6322 | 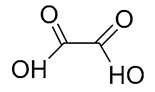 |
| 16 | Glycolic acid | 76.0160 | 79-14-1 | 17.9978 |  |
| 17 | Lactic acid | 90.0316 | 50-21-5 | 20.5232 | 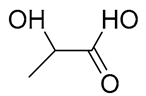 |
| Compounds | Fish 96 h-LC50 (mg·L−1) | Daphnid 48 h-LC50 (mg·L−1) | Green Algae 96 h-EC50 (mg·L−1) |
|---|---|---|---|
| AML | 290.0 | 155.5 | 144.3 |
| AML-Cl | 82.9 | 50.3 | 46.8 |
| AML-OH | 813.5 | 398.3 | 368.5 |
| AML-2OH | 2278.7 | 1018.4 | 939.7 |
| IM395 | 868.6 | 419.8 | 388.3 |
| IM288 | 201.2 | 107.9 | 100.2 |
| IM102 | 28,420.7 | 8802.8 | 8032.8 |
| IM240 | 60.7 | 35.8 | 33.3 |
| IM117 | 131.1 | 67.4 | 62.4 |
| IM364 | 106.1 | 61.7 | 57.4 |
| IM350 | 433.2 | 220.8 | 204.6 |
| IM334 | 19.9 | 13.5 | 12.6 |
| Acetic acid | 25,785.7 | 12,302.4 | 8704.6 |
| Oxalic acid | 168,000.0 | 67,876.8 | 39,658.6 |
| Glycolic acid | 355,000.0 | 152,000.0 | 95,559.1 |
| Lactic acid | 177,000.0 | 79065.5 | 51,783.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, S.; Du, E.; Sha, Y.; Zheng, L.; Peng, M.; Ling, L. Kinetics, Mechanism, and Toxicity of Amlodipine Degradation by the UV/Chlorine Process. Water 2020, 12, 3150. https://doi.org/10.3390/w12113150
Xu J, Zhou S, Du E, Sha Y, Zheng L, Peng M, Ling L. Kinetics, Mechanism, and Toxicity of Amlodipine Degradation by the UV/Chlorine Process. Water. 2020; 12(11):3150. https://doi.org/10.3390/w12113150
Chicago/Turabian StyleXu, Jianye, Siqi Zhou, Erdeng Du, Yongjun Sha, Lu Zheng, Mingguo Peng, and Ling Ling. 2020. "Kinetics, Mechanism, and Toxicity of Amlodipine Degradation by the UV/Chlorine Process" Water 12, no. 11: 3150. https://doi.org/10.3390/w12113150
APA StyleXu, J., Zhou, S., Du, E., Sha, Y., Zheng, L., Peng, M., & Ling, L. (2020). Kinetics, Mechanism, and Toxicity of Amlodipine Degradation by the UV/Chlorine Process. Water, 12(11), 3150. https://doi.org/10.3390/w12113150






