Conserving Mekong Megafishes: Current Status and Critical Threats in Cambodia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
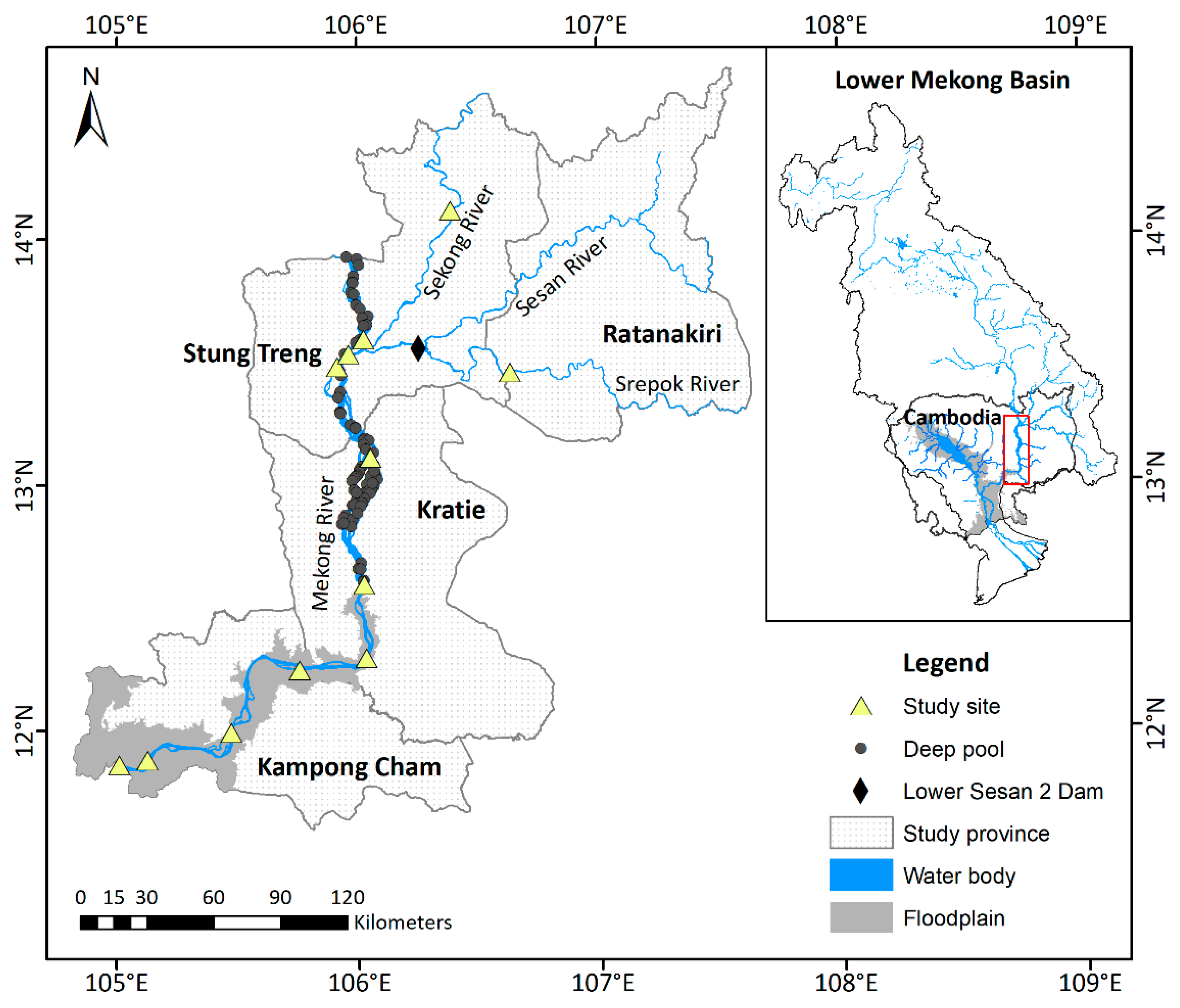
- Floodplains are known to be essential feeding and rearing habitat for many Mekong migratory fishes [48]. In our study, floodplain habitat was defined as sections of river that had close lateral connectivity with large flooded areas; these river sections also tended to have few to no deep pools in the river channel and a larger web of small streams and ox-bow lakes. The floodplain habitat type occurred in the Mekong River in Kampong Cham Province and the southernmost village sampled in Kratie Province (Figure 1, Table 2).
- Deep pools are thought to be critical dry season refugia and spawning habitat for many species [37]. Pools are found throughout the Mekong Basin in Cambodia, including Kampong Cham Province and the 3S Rivers, but some are considered more important to fisheries than others [35,47]. The deep pools in the Mekong River in Kratie and Stung Treng provinces (shown in Figure 1) were identified as productive fishing grounds by local fishers; they are also larger, deeper (up to 80 m), and more numerous (95 between Sambor and the Lao border) than pools in other parts of the Cambodian Mekong [35,47]. Thus, for our study, we defined pool habitat as river sections characterized by numerous deep pools that were identified as important by local fishers. These occurred in Kratie and Stung Treng provinces (Figure 1, Table 2). It is noteworthy that less work has been done to map pools on Mekong tributaries [47].
2.2. Survey Methods
2.3. Analyses
2.3.1. Population Status
2.3.2. Body Size
2.3.3. Conservation Threats
3. Results
3.1. Sample Sizes and Assessment of Species’ Geographic Distributions
3.2. Population Status
3.3. Body Size
3.4. Conservation Threats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IUCN. The IUCN Red List of Threatened Species. Version 2019-3. 2019. Available online: http://www.iucnredlist.org (accessed on 26 February 2020).
- He, F.; Bremerich, V.; Zarfl, C.; Geldmann, J.; Langhans, S.D.; David, J.N.W.; Darwall, W.; Tockner, K.; Jähnig, S.C. Freshwater megafauna diversity: Patterns, status and threats. Divers. Distrib. 2018, 24, 1395–1440. [Google Scholar] [CrossRef] [Green Version]
- Carrizo, S.F.; Jähnig, S.C.; Bremerich, V.; Freyhof, J.; Harrison, I.; He, F.; Langhans, S.D.; Tockner, K.; Zarfl, C.; Darwall, W. Freshwater megafauna: Flagships for freshwater biodiversity under threat. Bioscience 2017, 67, 919–927. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zarfl, C.; Bremerich, V.; Henshaw, A.; Darwall, W.; Tockner, K.; Jaehnig, S.C. Disappearing giants: A review of threats to freshwater megafauna. Wiley Interdiscip. Rev. Water 2017, 4, e1208. [Google Scholar] [CrossRef]
- Fromentin, J.M.; Powers, J.E. Atlantic bluefin tuna: Population dynamics, ecology, fisheries and management. Fish Fish. 2005, 6, 281–306. [Google Scholar] [CrossRef] [Green Version]
- Artero, C.; Koenig, C.C.; Richard, P.; Berzins, R.; Guillou, G.; Bouchon, C.; Lampert, L. Ontogenetic dietary and habitat shifts in goliath grouper Epinephelus itajara from French Guiana. Endanger. Species Res. 2015, 27, 155–168. [Google Scholar] [CrossRef]
- Rainboth, W.J. Fishes of the Cambodian Mekong; Food & Agriculture Org.: Rome, Italy, 1996. [Google Scholar]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkholder, D.A.; Heithaus, M.R.; Fourqurean, J.W.; Wirsing, A.; Dill, L.M. Patterns of top-down control in a seagrass ecosystem: Could a roving apex predator induce a behaviour-mediated trophic cascade? J. Anim. Ecol. 2013, 82, 1192–1202. [Google Scholar] [CrossRef]
- Hogan, Z.; Baird, I.G.; Radtke, R.; Vander Zanden, M.J. Long distance migration and marine habitation in the tropical Asian catfish, Pangasius krempfi. J. Fish. Biol. 2007, 71, 818–832. [Google Scholar] [CrossRef]
- Auer, N.A. Importance of habitat and migration to sturgeons with emphasis on lake sturgeon. Can. J. Fish. Aquat. Sci. 1996, 53, 152–160. [Google Scholar] [CrossRef]
- Polis, G.A.; Anderson, W.B.; Holt, R.D. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Systemat. 1997, 28, 289–316. [Google Scholar] [CrossRef] [Green Version]
- Hooker, S.K.; Gerber, L.R. Marine reserves as a tool for ecosystem-based management: The potential importance of megafauna. Bioscience 2004, 54, 27–39. [Google Scholar] [CrossRef]
- Williams, P.; Reid, C. Overview of Tuna Fisheries in the Western and Central Pacific Ocean., Including Economic Conditions - 2017, Rev 1 (22 July 2018); WCPFC-TCC14-2018-IP05; Western and Central Pacific Fisheries Commission: Majuro, Republic of Marshall Islands, 2018. [Google Scholar]
- Bronzi, P.; Rosenthal, H. Present and future sturgeon and caviar production and marketing: A global market overview. J. Appl. Ichthyol. 2014, 30, 1536–1546. [Google Scholar] [CrossRef]
- Gupta, N.; Kanagavel, A.; Dandekar, P.; Dahanukar, N.; Sivakumar, K.; Mathur, V.B.; Raghavan, R. God’s fishes: Religion, culture and freshwater fish conservation in India. Oryx 2016, 50, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Hortle, K.G. Fisheries of the Mekong River Basin. Mekong Biophys. Environ. Int. River Basin 2009, 197–249. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. 2019. Available online: www.fishbase.org (accessed on 26 February 2020).
- Nuwer, R. Critically Endangered Giant Fish on Menu at Luxury Restaurants; National Geographic: Washington, DC, USA, 2018. [Google Scholar]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The global decline of freshwater megafauna. Glob. Chang. Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef]
- Ngor, P.B.; McCann, K.S.; Grenouillet, G.; So, N.; McMeans, B.C.; Fraser, E.; Lek, S. Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci. Rep. 2018, 8, 8947. [Google Scholar] [CrossRef] [Green Version]
- MRC. Transboundary Fisheries Management Issues in the Mekong and Sekong Rivers; Mekong River Commission: Vientiane, Lao PDR, 2017. [Google Scholar]
- Ziv, G.; Baran, E.; Nam, S.; Rodriguez-Iturbe, I.; Levin, S.A. Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin. Proc. Natl. Acad. Sci. USA 2012, 109, 5609–5614. [Google Scholar] [CrossRef] [Green Version]
- Dugan, P.J.; Barlow, C.; Agostinho, A.A.; Baran, E.; Cada, G.F.; Chen, D.Q.; Cowx, I.G.; Ferguson, J.W.; Jutagate, T.; Mallen-Cooper, M.; et al. Fish migration, dams, and loss of ecosystem services in the Mekong Basin. Ambio 2010, 39, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.W.; Healey, M.; Dugan, P.; Barlow, C. Potential effects of dams on migratory fish in the Mekong River: Lessons from salmon in the Fraser and Columbia Rivers. Environ. Manag. 2011, 47, 141–159. [Google Scholar] [CrossRef]
- Baran, E.; Myschowoda, C. Dams and fisheries in the Mekong Basin. Aquat. Ecosyst. Health Manag. 2009, 12, 227–234. [Google Scholar] [CrossRef]
- Olden, J.D.; Hogan, Z.S.; Vander Zanden, M.J. Small fish, big fish, red fish, blue fish: Size-biased extinction risk of the world’s freshwater and marine fishes. Glob. Ecol. Biogeogr. 2007, 16, 694–701. [Google Scholar] [CrossRef]
- Jennings, S.; Reynolds, J.D.; Mills, S.C. Life history correlates of responses to fisheries exploitation. Proc. R. Soc. B Biol. Sci. 1998, 265, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Gray, T.N.E.; Phommachak, A.; Vannachomchan, K.; Guegan, F. Using local ecological knowledge to monitor threatened Mekong megafauna in Lao PDR. PLos ONE 2017, 12, e0183247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUCN. Strategic Planning for Species Conservation: A handbook, Version 1.0; IUCN Species Survival Commission, Species Conservation Planning Task Force; 2008. Available online: https://www.iucn.org/downloads/scshandbook_2_12_08_compressed.pdf (accessed on 6 June 2020).
- IUCN. Strategic Planning for Species Conservation: An Overview, Version 1.0; IUCN Species Survival Commission, Species Conservation Planning Task Force; 2008. Available online: https://www.iucn.org/downloads/scsoverview_1_12_2008.pdf (accessed on 6 June 2020).
- Chan, S.; Putrea, S.; Sean, K.; Hortle, K.G. Using local knowledge to inventory deep pools, important fish habitats in Cambodia. In Proceedings of the 6th Technical Symposium on Mekong Fisheries, Mekong River Commission, Vientiane, Laos, 26–28 November 2003; pp. 57–76. [Google Scholar]
- Halls, A.S.; Conlan, I.; Wisesjindawat, W.; Phouthavongs, K.; Viravong, S.; Chan, S.; Vu, V.A. Atlas of Deep Pools in the Lower Mekong River and Some of Its Tributaries; MRC Technical Paper No. 31; Mekong River Commission: Phnom Penh, Cambodia, 2013; p. 69. [Google Scholar]
- Máiz-Tomé, L. Freshwater Key Biodiversity Areas in the Lower Mekong River Basin; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2019. [Google Scholar]
- Poulsen, A.; Poeu, O.; Viravong, S.; Suntornratana, U.; Thanh Tung, N. Deep Pools as Dry Season Fish Habitats in the Mekong Basin; MRC Technical Paper No. 4; Mekong River Commission: Phnom Penh, Cambodia, 2002; p. 22. [Google Scholar]
- Hogan, Z.S. Threatened fishes of the world: Pangasianodon gigas Chevey, 1931 (Pangasiidae). Environ. Biol. Fishes 2004, 70, 210. [Google Scholar] [CrossRef]
- Ramsar Sites Information Service (RSIS) Middle Stretches of Mekong River North of Stoeng Treng. Available online: https://rsis.ramsar.org/ris/999 (accessed on 7 September 2018).
- Baran, E.; Saray, S.; Teoh, S.J.; Tran, T.C. Fish. and Fisheries in the Sekong, Sesan and Srepok Basins (3S Rivers, Mekong Watershed), with Special Reference to the Sesan River; Project Report: Challenge Program. on Water & Food Mekong Project MK3 “Optimizing the Management of a Cascade of Reservoirs at the Catchment level”; International Centre for Environmental Management (ICEM): Hanoi, Vietnam, 2013. [Google Scholar]
- Poizat, G.; Baran, E. Fishermen‘s knowledge as background information in tropical fish ecology: A quantitative comparison with fish sampling results. Environ. Biol. Fishes 1997, 50, 435–449. [Google Scholar] [CrossRef]
- Drew, J.A. Use of traditional ecological knowledge in marine conservation. Conserv. Biol. 2005, 19, 1286–1293. [Google Scholar] [CrossRef]
- Turvey, S.T.; Trung, C.T.; Quyet, V.D.; Nhu, H.V.; Thoai, D.V.; Tuan, V.C.A.; Hoa, D.T.; Kacha, K.; Sysomphone, T.; Wallate, S.; et al. Interview-based sighting histories can inform regional conservation prioritization for highly threatened cryptic species. J. Appl. Ecol. 2015, 52, 422–433. [Google Scholar] [CrossRef]
- Nash, H.C.; Wong, M.H.G.; Turvey, S.T. Using local ecological knowledge to determine status and threats of the Critically Endangered Chinese pangolin (Manis pentadactyla) in Hainan, China. Biol. Conserv. 2016, 196, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, A.F.; Hortle, K.G.; Valbo-Jorgensen, J.; Chan, S.; Chhuon, C.K.; Viravong, S.; Bouakhamvongsa, K.; Suntornratana, U.; Yoorong, N.; Nguyen, T.T.; et al. Distribution and Ecology of Some Important Riverine Fish Species of the Mekong River Basin; MRC Technical Paper No. 10; Mekong River Commission: Phnom Penh, Cambodia, 2004. [Google Scholar]
- Koemsoeun, S. Lower Sesan II dam opens. Phnom Penh Post, 18 December 2018. [Google Scholar]
- MRC. Planning Atlas of the Lower Mekong River Basin; Mekong River Commission: Phnom Penh, Cambodia, 2011. [Google Scholar]
- Poulsen, A.F.; Poeu, O.; Viravong, S.; Suntornratana, U.; Thanh Tung, N. Fish Migrations of the Lower Mekong River Basin: Implications for Development, Planning and Environmental Management; MRC Technical Paper No. 8; Mekong River Commission: Phnom Penh, Cambodia, 2002; p. 62. [Google Scholar]
- Berkes, F.; Colding, J.; Folke, C. Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 2000, 10, 1251–1262. [Google Scholar] [CrossRef]
- Davis, A.; Wagner, J.R. Who knows? On the importance of identifying “experts” when researching local ecological knowledge. Hum. Ecol. 2003, 31, 463–489. [Google Scholar] [CrossRef]
- Birkeland, C.; Dayton, P.K. The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 2005, 20, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Kolm, N. Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 2229–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.A. Size-dependent territory defense by a damselfish. Oecologia 1985, 67, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.R.; McPhail, J.D. Food abundance, intruder pressure, and body size as determinants of territory size in juvenile steelhead trout (Oncorhynchus mykiss). Behaviour 1998, 135, 65–82. [Google Scholar] [CrossRef]
- Trippel, E.A.; Kjesbu, O.S.; Solemdal, P. Effects of adult age and size structure on reproductive output in marine fishes. In Early Life History and Recruitment in Fish Populations; Chambers, R.C., Trippel, E.A., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 31–62. [Google Scholar]
- Marteinsdottir, G.; Begg, G.A. Essential relationships incorporating the influence of age, size and condition on variables required for estimation of reproductive potential in Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser. 2002, 235, 235–256. [Google Scholar] [CrossRef]
- FiA. Law on Fisheries; Fisheries Administration, Ministry of Agriculture, Forestry and Fisheries: Phnom Penh, Cambodia, 2006.
- Ngor, P.B.; Legendre, P.; Oberdorff, T.; Lek, S. Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-3S river system. Ecol. Indic. 2018, 88, 103–114. [Google Scholar] [CrossRef]
- Jennings, S.; Greenstreet, S.P.R.; Reynolds, J.D. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J. Anim. Ecol. 1999, 68, 617–627. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet. Res. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Ratner, B.D.; So, S.; Mam, K.; Oeur, I.; Kim, S. Conflict and collective action in Tonle Sap fisheries: Adapting governance to support community livelihoods. Nat. Resour. Forum 2017, 41, 71–82. [Google Scholar] [CrossRef]
- Kurien, J. Shocking Reality: An ‘illegal’ fisher in a Cambodian village reforms himself after realizing the ill effects of ‘electro-fishing’. Samudra Rep. Triannu. J. Int. Collect. Support Fishworks 2007, 48, 3536. [Google Scholar]
- Dolan, C.R.; Miranda, L.E. Immobilization thresholds of electrofishing relative to fish size. Trans. Am. Fish. Soc. 2003, 132, 969–976. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, M.E.; Holtgrieve, G.W.; Ngor, P.B.; Dang, T.D.; Piman, T. Maintaining perspective of ongoing environmental change in the Mekong floodplains. Curr. Opin. Environ. Sustain. 2019, 37, 1–7. [Google Scholar] [CrossRef]
- Hortle, K.G.; Bamrungrach, P. Fisheries Habitat and Yield in the Lower Mekong Basin; MRC Technical Paper No. 47; Mekong River Commission: Phnom Penh, Cambodia, 2015; p. 80. [Google Scholar]
- Open Development Cambodia. Fisheries Conservation Areas. Available online: https://opendevelopmentcambodia.net/profiles/fisheries-conservation-areas/ (accessed on 24 February 2020).
- Rainboth, W.J. Aaptosyax grypus, a new genus and species of large piscivorous cyprinids from the middle Mekong River. Jpn. J. Ichthyol. 1991, 38, 231–237. [Google Scholar] [CrossRef]
- Kc, K.B.; Bond, N.; Fraser, E.D.G.; Elliott, V.; Farrell, T.; McCann, K.; Rooney, N.; Bieg, C. Exploring tropical fisheries through fishers’ perceptions: Fishing down the food web in the Tonlé Sap, Cambodia. Fish. Manag. Ecol. 2017, 24, 452–459. [Google Scholar] [CrossRef]
- Ratcliffe, R. Cambodia scraps plans for Mekong hydropower dams. The Guardian, 19 March 2020. [Google Scholar]





| Scientific Name/English Name | Local Names | Max Length/Weight | IUCN Status | Species Abbr |
|---|---|---|---|---|
| Aaptosyax grypus Giant salmon carp | Trey pasanak (Kh), Pa sanak, Pa sanak gnai (Lao) | 130 cm SL/30 kg | Critically Endangered | agry |
| Catlocarpio siamensis Giant barb | Trey kolriang (Kh), Pa kaho (Lao) | 300 cm TL/300 kg | Critically Endangered | csia |
| Luciocyprinus striolatus | Trey sroum dao (Kh) | 200 cm SL | Endangered | lstr |
| Pangasianodon gigas Mekong giant catfish | Trey reach (Kh), Pa boeuk (Lao) | 300 cm TL/350 kg | Critically Endangered | pgig |
| Pangasius sanitwongsei Giant pangasius or dog-eating catfish | Trey po pruy (Kh), Pa leum (Lao) | 300 cm SL/300 kg | Critically Endangered | psan |
| Probarbus jullieni Isok barb | Trey trasak (Kh), Pa eun, Pa eun ta deng (Lao) | 150 cm SL/70 kg | Critically Endangered | pjul |
| Urogymnus polylepis Giant freshwater whipray | Trey bor bel yeak, Trey bawbel (Kh), Pa fa hang, Pa fa lai (Lao) | 240 cm WD/600 kg | Endangered | upol |
| Wallago micropogon | Trey stourk (Kh), Pa khoun (Lao) | 154 cm SL/96 kg | Data deficient | wmic |
| Village | Habitat | No. Fishers |
|---|---|---|
| Mekong/Kampong Cham (34) | ||
| Svay Leu | Floodplain | 7 |
| Prek Koy | Floodplain | 10 |
| Prek Toch | Floodplain | 10 |
| Roka Knol 3 | Floodplain | 7 |
| Mekong/Kratie (21) | ||
| Chheu Teal Plous | Floodplain | 8 |
| Sambok | Pool | 5 |
| Koh Khnhe | Pool | 8 |
| Mekong/Stung Treng (21) | ||
| Sma Koh | Pool | 5 |
| Ba Chung | Pool | 7 |
| Koh Khon Den | Pool | 9 |
| Sekong/Stung Treng (10) | ||
| Pha Bang | Tributary | 10 |
| Srepok/Ratanakiri (10) | ||
| Sre Angkrong | Tributary | 10 |
| Grand Total | 96 | |
| River | Mekong | Sekong | Srepok | TOTAL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Province | Kampong Cham | Kratie | Stung Treng | Stung Treng | Ratanakiri | |||||||
| Total Fishers | 34 | 21 | 21 | 10 | 10 | 96 | ||||||
| Summary of High-Quality Interviews by Species | ||||||||||||
| Species | Ct | % | Ct | % | Ct | % | Ct | % | Ct | % | Ct | % |
| agry | 0 | 0 | 1 | 5 | 1 | 5 | 1 | 10 | 0 | 0 | 3 | 3 |
| csia | 21 | 62 | 15 | 71 | 6 | 29 | 1 | 10 | 1 | 10 | 44 | 46 |
| lstr | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 1 | 10 | 2 | 2 |
| pgig | 3 | 9 | 6 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 9 |
| psan | 3 | 9 | 7 | 33 | 4 | 19 | 0 | 0 | 5 | 50 | 19 | 20 |
| pjul | 20 | 59 | 15 | 71 | 12 | 57 | 6 | 60 | 9 | 90 | 62 | 65 |
| upol | 0 | 0 | 10 | 48 | 7 | 33 | 0 | 0 | 1 | 10 | 18 | 19 |
| wmic | 10 | 29 | 17 | 81 | 8 | 38 | 9 | 90 | 7 | 70 | 51 | 53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, T.; Pin, K.; Ngor, P.B.; Hogan, Z. Conserving Mekong Megafishes: Current Status and Critical Threats in Cambodia. Water 2020, 12, 1820. https://doi.org/10.3390/w12061820
Campbell T, Pin K, Ngor PB, Hogan Z. Conserving Mekong Megafishes: Current Status and Critical Threats in Cambodia. Water. 2020; 12(6):1820. https://doi.org/10.3390/w12061820
Chicago/Turabian StyleCampbell, Teresa, Kakada Pin, Peng Bun Ngor, and Zeb Hogan. 2020. "Conserving Mekong Megafishes: Current Status and Critical Threats in Cambodia" Water 12, no. 6: 1820. https://doi.org/10.3390/w12061820
APA StyleCampbell, T., Pin, K., Ngor, P. B., & Hogan, Z. (2020). Conserving Mekong Megafishes: Current Status and Critical Threats in Cambodia. Water, 12(6), 1820. https://doi.org/10.3390/w12061820






