Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone
Abstract
:1. Introduction
2. Materials and Methods
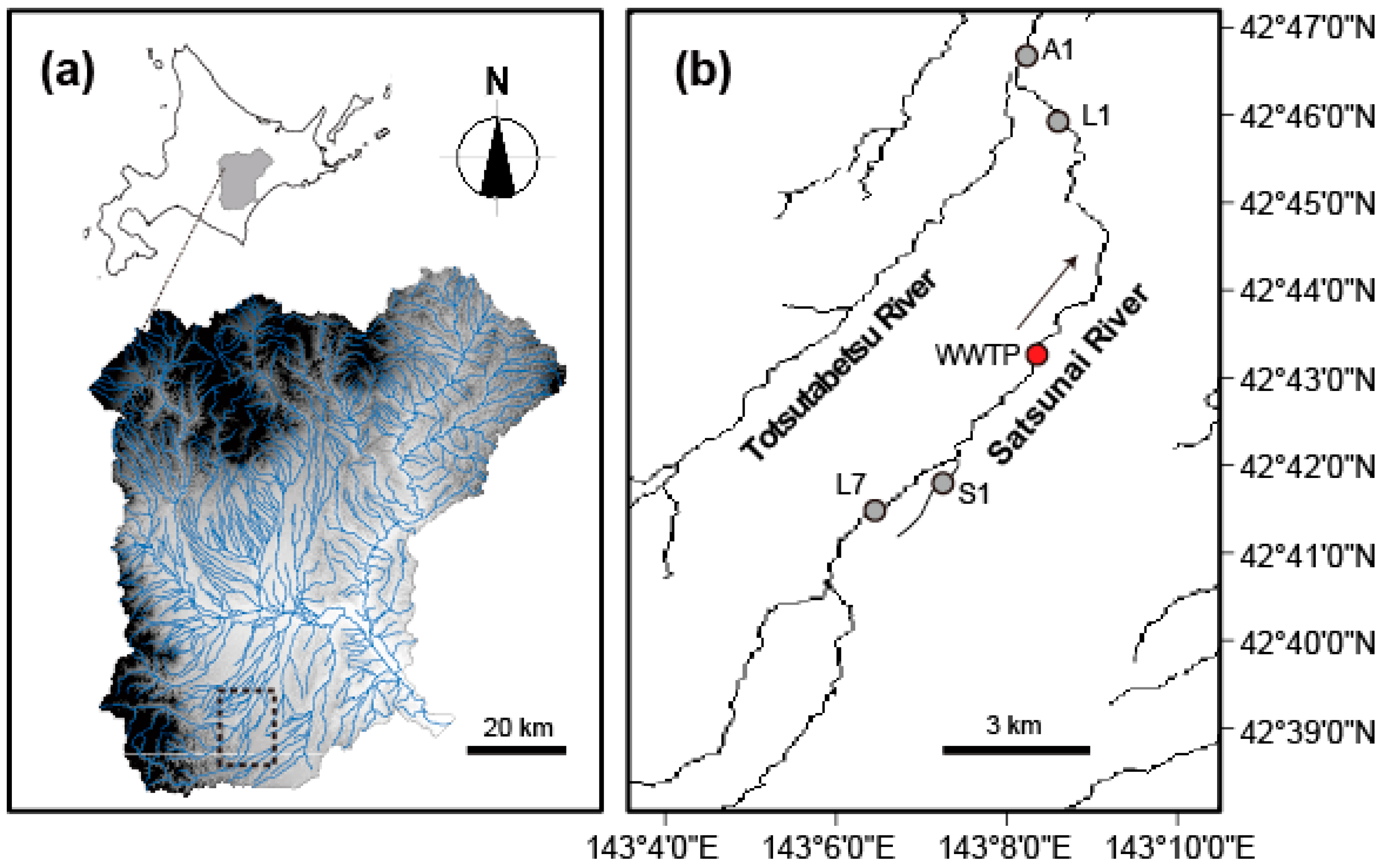
2.1. Site Description and Sampling Design
2.2. Collection of Litter Bags
2.3. Water Physicochemical Measurements
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef] [Green Version]
- Nhiwatiwa, T.; Dalu, T.; Brendonck, L. Impact of irrigation based sugarcane cultivation on the Chiredzi and Runde Rivers quality, Zimbabwe. Sci. Total Environ. 2017, 587–588, 316–325. [Google Scholar] [CrossRef]
- Collins, A.L.; Price, J.P.N.; Zhang, Y.; Gooday, R.; Naden, P.S.; Skirvin, D. Assessing the potential impacts of a revised set of on-farm nutrient and sediment ‘basic’control measures for reducing agricultural diffuse pollution across England. Sci. Total Environ. 2018, 621, 1499–1511. [Google Scholar] [CrossRef]
- Gonzales-Inca, C.; Valkama, P.; Lill, J.O.; Slotte, J.; Hietaharju, E.; Uusitalo, R. Spatial modeling of sediment transfer and identification of sediment sources during snowmelt in an agricultural watershed in boreal climate. Sci. Total Environ. 2018, 612, 303–312. [Google Scholar] [CrossRef]
- Jones, J.I.; Murphy, J.F.; Collins, A.L.; Sear, D.A.; Naden, P.S.; Armitage, P.D. The impact of fine sediment on macro-invertebrates. River Res. Appl. 2012, 28, 1055–1071. [Google Scholar] [CrossRef]
- Wagenhoff, A.; Townsend, C.R.; Phillips, N.; Matthaei, C.D. Subsidy-stress and multiple-stressor effects along gradients of deposited fine sediment and dissolved nutrients in a regional set of streams and rivers. Freshw. Biol. 2011, 56, 1916–1936. [Google Scholar] [CrossRef]
- Descloux, S.; Datry, T.; Usseglio-Polatera, P. Trait-based structure of invertebrates along a gradient of sediment colmation: Benthos versus hyporheos responses. Sci. Total Environ. 2014, 466–467, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, L.; Tolonen, K.E.; Yin, H.; Gao, J.; Zhang, Z.; Li, K.; Cai, Y. Substrate degradation and nutrient enrichment structuring macroinvertebrate assemblages in agriculturally dominated Lake Chaohu Basins, China. Sci. Total Environ. 2018, 627, 57–66. [Google Scholar] [CrossRef]
- Negishi, J.N.; Terui, A.; Nessa, B.; Miura, K.; Oiso, T.; Sumitomo, K.; Kyuka, T.; Yonemoto, M.; Nakamura, F. High resilience of aquatic community to a 100-year flood in a gravel-bed river. Landsc. Ecol. Eng. 2019, 15, 143–154. [Google Scholar] [CrossRef]
- Biggs, B.J.F. Eutrophication of streams and rivers: Dissolved nutrient-chlorophyll relationships for benthic algae. J. N. Am. Benthol. Soc. 2000, 19, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Hagen, E.M.; Webster, J.R.; Benfield, E.F. Are leaf breakdown rates a useful measure of stream integrity along an agricultural landuse gradient? J. N. Am. Benthol. Soc. 2006, 25, 330–343. [Google Scholar] [CrossRef]
- Woodward, G.; Gessner, M.O.; Giller, P.S.; Gulis, V.; Hladyz, S.; Lecerf, A.; Malmqvist, B.; McKie, B.G.; Tiegs, S.D.; Cariss, H.; et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 2012, 336, 1438–1440. [Google Scholar] [CrossRef] [Green Version]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55, 1–4. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.G.; Vinebrooke, R.D.; Chimimba, C.T. Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hayden, B.; McWilliam-Hughes, S.M.; Cunjak, R.A. Evidence for limited trophic transfer of allochthonous energy in temperate river food webs. Freshw. Sci. 2016, 35, 544–558. [Google Scholar] [CrossRef]
- Doucett, R.R.; Marks, J.C.; Blinn, D.W.; Caron, M.; Hungate, B.A. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 2007, 88, 1587–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Gessner, M.O.; Chauvet, E.; Dobson, M. A perspective on leaf litter breakdown in streams. Oikos 1999, 85, 377–384. [Google Scholar] [CrossRef]
- Gulis, V.; Suberkropp, K. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw. Biol. 2003, 48, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Dangles, O.; Gessner, M.O.; Guérold, F.; Chauvet, E. Impacts of stream acidification on litter breakdown: Implications for assessing ecosystem functioning. J. Appl. Ecol. 2004, 41, 365–378. [Google Scholar] [CrossRef] [Green Version]
- Young, R.G.; Matthaei, C.D.; Townsend, C.R. Organic matter breakdown and ecosystem metabolism: Functional indicators for assessing river ecosystem health. J. N. Am. Benthol. Soc. 2008, 27, 605–625. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Harding, J.S.; Simon, K.S. Organic matter breakdown as a measure of stream health in New Zealand streams affected by acid mine drainage. Ecol. Indic. 2013, 24, 510–517. [Google Scholar] [CrossRef]
- Boulton, A.J.; Datry, T.; Kasahara, T.; Mutz, M.; Stanford, J.A. Ecology and management of the hyporheic zone: Stream–groundwater interactions of running waters and their floodplains. J. N. Amer. Benthol. Soc. 2010, 29, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Corson-Rikert, H.A.; Wondzell, S.M.; Haggerty, R.; Santelmann, M.V. Carbon dynamics in the hyporheic zone of a headwater mountain stream in the Cascade Mountains, Oregon. Water Resour. Res. 2016, 52, 7556–7576. [Google Scholar] [CrossRef] [Green Version]
- Burrows, R.M.; Rutlidge, H.; Bond, N.R.; Eberhard, S.M.; Auhl, A.; Andersen, M.S.; Valdez, D.G.; Kennard, M.J. High rates of organic carbon processing in the hyporheic zone of intermittent streams. Sci. Rep. 2017, 7, 13198. [Google Scholar] [CrossRef] [Green Version]
- Metzler, G.M.; Smock, L.A. Storage and dynamics of subsurface detritus in a sand-bottomed stream. Can. J. Fisheries Aquat. Sci. 1990, 47, 588–594. [Google Scholar] [CrossRef]
- Rulík, M.; Zavřelová, P.; Duchoslav, M. Decomposition of two different POM types in surface water and within hyporheic sediments of a small lowland stream (Sitka, Czech Republic). Int. Rev. Hydrobiol. 2001, 86, 487–500. [Google Scholar] [CrossRef]
- Cornut, J.; Elger, A.; Lambrigot, D.; Marmonier, P.; Chauvet, E. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshw. Biol. 2010, 55, 2541–2556. [Google Scholar] [CrossRef]
- Pinay, G.; O’Keefe, T.C.; Edwards, R.T.; Naiman, R.J. Nitrate removal in the hyporheic zone of a salmon river in Alaska. River Res. Appl. 2009, 25, 367–375. [Google Scholar] [CrossRef]
- Bärlocher, F.; Seena, S.; Wilson, K.P.; Dudley Williams, D. Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw. Biol. 2008, 53, 368–379. [Google Scholar] [CrossRef]
- Stubbington, R.; Wood, P.J.; Reid, I.; Gunn, J. Benthic and hyporheic invertebrate community responses to seasonal flow recession in a groundwater-dominated stream. Ecohydrology 2011, 4, 500–511. [Google Scholar] [CrossRef]
- Nogaro, G.; Datry, T.; Mermillod-Blondin, F.; Foulquier, A.; Montuelle, B. Influence of hyporheic zone characteristics on the structure and activity of microbial assemblages. Freshw. Biol. 2013, 58, 2567–2583. [Google Scholar] [CrossRef]
- Feris, K.P.; Ramsey, P.W.; Gibbons, S.M.; Frazer, C.; Rillig, M.C.; Moore, J.N.; Gannon, J.E.; Holben, W.E. Hyporheic microbial community development is a sensitive indicator of metal contamination. Environ. Sci. Technol. 2009, 43, 6158–6163. [Google Scholar] [CrossRef]
- Sánchez-Morales, M.; Sabater, F.; Muñoz, I. Effects of urban wastewater on hyporheic habitat and invertebrates in Mediterranean streams. Sci. Total Environ. 2018, 642, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Piscart, C.; Navel, S.; Maazouzi, C.; Montuelle, B.; Cornut, J.; Mermillod-Blondin, F.; Creuze des Chatelliers, M.; Simon, L.; Marmonier, P. Leaf litter recycling in benthic and hyporheic layers in agricultural streams with different types of land use. Sci.Total Environ. 2011, 409, 4373–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, S.M.; Roline, R.A. Effects of multiple stressors on hyporheic invertebrates in a lotic system. Ecol. Indic. 2003, 3, 65–79. [Google Scholar] [CrossRef]
- Nogaro, G.; Datry, T.; Mermillod-Blondin, F.; Descloux, S.; Montuelle, B. Influence of streambed sediment clogging on microbial processes in the hyporheic zone. Freshw. Biol. 2010, 55, 1288–1302. [Google Scholar] [CrossRef]
- Harvey, J.W.; Drummond, J.D.; Martin, R.L.; McPhillips, L.E.; Packman, A.I.; Jerolmack, D.J.; Stonedahl, S.H.; Aubeneau, A.F.; Sawyer, A.H.; Larsen, L.G.; et al. Hydrogeomorphology of the hyporheic zone: Stream solute and fine particle interactions with a dynamic streambed. J. Geophys. Res. 2012, 117, G00N11. [Google Scholar] [CrossRef] [Green Version]
- Solagaistua, L.; Arroita, M.; Aristi, I.; Larrañaga, A.; Elosegi, A. Changes in discharge affect more surface than subsurface breakdown of organic matter in a mountain stream. Mar. Freshw. Res. 2016, 67, 1826–1834. [Google Scholar] [CrossRef]
- Mori, N.; Debeljak, B.; Škerjanec, M.; Simčič, T.; Kanduč, T.; Brancelj, A. Modelling the effects of multiple stressors on respiration and microbial biomass in the hyporheic zone using decision trees. Water Res. 2019, 149, e20. [Google Scholar] [CrossRef]
- Boulton, A.J.; Scarsbrook, M.R.; Quinn, J.M.; Burrell, G.P. Land-use effects on the hyporheic ecology of five small streams near Hamilton, New Zealand. N. Z. J. Mar. Freshwater Res. 1997, 31, 609–622. [Google Scholar] [CrossRef]
- Conroy, E.; Turner, J.N.; Rymszewicz, A.; Bruen, M.; O’Sullivan, J.J.; Lawler, D.M.; Stafford, S.; Kelly-Quinn, M. Further insights into the responses of macroinvertebrate species to burial by sediment. Hydrobiologia 2018, 805, 399–411. [Google Scholar] [CrossRef]
- Lefebvre, S.; Marmonier, P.; Pinay, G. Stream regulation and nitrogen dynamics in sediment interstices: Comparison of natural and straightened sectors of a third-order stream. River Res. Appl. 2004, 20, 499–512. [Google Scholar] [CrossRef]
- Lowell, J.L.; Gordon, N.; Engstrom, D.; Stanford, J.A.; Holben, W.E.; Gannon, J.E. Habitat heterogeneity and associated microbial community structure in a small-scale floodplain hyporheic flow path. Microb. Ecol. 2009, 58, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Soulsby, C.; Youngson, A.F.; Moir, H.; Malcolm, I.A. Fine sediment influence on salmonid spawning habitat in a lowland agricultural stream: A preliminary assessment. Sci. Total Environ. 2001, 265, 295–307. [Google Scholar] [CrossRef]
- Niyogi, D.K.; Koren, M.; Arbuckle, C.J.; Townsend, C.R. Stream communities along a catchment land-use gradient: Subsidy-stress responses to pastoral development. Environ. Manage. 2007, 39, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Aristi, I.; von Schiller, D.; Arroita, M.; Barceló, D.; Ponsatí, L.; García-Galán, M.J.; Sabater, S.; Elosegi, A.; Acuña, V. Mixed effects of effluents from a wastewater treatment plant on river ecosystem metabolism: Subsidy or stress? Freshw. Biol. 2015, 60, 1398–1410. [Google Scholar] [CrossRef]
- Menéndez, M.; Hernández, O.; Comín, F.A. Seasonal comparisons of leaf processing rates in two Mediterranean rivers with different nutrient availability. Hydrobiologia 2003, 495, 159–169. [Google Scholar] [CrossRef]
- Graeber, D.; Jensen, T.M.; Rasmussen, J.J.; Riis, T.; Wiberg-Larsen, P.; Baattrup-Pedersen, A. Multiple stress response of lowland stream benthic macroinvertebrates depends on habitat type. Sci. Total Environ. 2017, 599–600, 1517–1523. [Google Scholar] [CrossRef]
- Cook, S.C.; Housley, L.; Back, J.A.; King, R.S. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 2018, 99, 47–56. [Google Scholar] [CrossRef]
- Atashgahi, S.; Aydin, R.; Dimitrov, M.R.; Sipkema, D.; Hamonts, K.; Lahti, L.; Maphosa, F.; Kruse, T.; Saccenti, E.; Springael, D.; et al. Impact of a wastewater treatment plant on microbial community composition and function in a hyporheic zone of a eutrophic river. Sci. Rep. 2015, 5, 17284. [Google Scholar] [CrossRef] [PubMed]
- Negishi, J.N.; Hibino, A.; Miura, K.; Kawanishi, R.; Watanabe, N.; Toyoda, K. Coupled benthic–hyporheic responses of macroinvertebrates to surface water pollution in a gravel-bed river. Freshw. Sci. 2019, 38, 591–604. [Google Scholar] [CrossRef]
- Folt, C.L.; Chen, C.Y.; Moore, M.V.; Burnaford, J. Synergism and antagonism among multiple stressors. Limnol. Oceanography 1999, 44, 864–877. [Google Scholar] [CrossRef] [Green Version]
- Matthaei, C.D.; Piggott, J.J.; Townsend, C.R. Multiple stressors in agricultural streams: Interactions among sediment addition, nutrient enrichment and water abstraction. J. Appl. Ecol. 2010, 47, 639–649. [Google Scholar] [CrossRef]
- Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Climate warming and agricultural stressors interact to determine stream macroinvertebrate community dynamics. Glob. Chang. Biol. 2015, 21, 1887–1906. [Google Scholar] [CrossRef] [PubMed]
- Beermann, A.J.; Elbrecht, V.; Karnatz, S.; Ma, L.; Matthaei, C.D.; Piggott, J.J.; Leese, F. Multiple-stressor effects on stream macroinvertebrate communities: A mesocosm experiment manipulating salinity, fine sediment and flow velocity. Sci. Total Environ. 2018, 610–611, 961–971. [Google Scholar] [CrossRef]
- Kanda, F. Survival curves and longevity of the leaves of Alnus japonica vat. arguta in Kushiro Marsh. Vegetatio 1996, 124, 61–66. [Google Scholar] [CrossRef]
- Xu, X.; Shibata, H. Landscape patterns of overstory litterfall and related nutrient fluxes in a cool-temperate forest watershed in northern Hokkaido, Japan. J. For. Res. 2007, 18, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Muller, O.; Tayanagi, S.; Nakaji, T.; Hiuraa, T. Experimental branch warming alters tall tree leaf phenology and acorn production. Agric. For. Meteorol. 2010, 150, 1026–1029. [Google Scholar] [CrossRef]
- Alam, M.K.; Negishi, J.N.; Rahman, M.A.T.; Tolod, J.R. Stable isotope ratios of emergent adult aquatic insects can be used as indicators of water pollution in the hyporheic food web. Ecol. Indic. 2020, 118, 106738. [Google Scholar] [CrossRef]
- Boulton, A.J.; Boon, P.I. A review of methodology used to measure leaf litter decomposition in lotic environments: Time to turn over an old leaf? Aust. J. Mar. Freshw. Res. 1991, 42, 1–43. [Google Scholar] [CrossRef]
- Cornut, J.; Clivot, H.; Chauvet, E.; Elger, A.; Pagnout, C.; Gue´rold, F. Effect of acidification on leaf litter decomposition in benthic and hyporheic zones of woodland streams. Water Res. 2012, 46, 6430–6444. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Tanida, K. Aquatic Insects of Japan: Manual with Keys and Illustrations, 2nd ed.; Tokai University Press: Kanagawa, Japan, 2018; p. 1730, (In Japanese). ISBN 978-448-601-774-5. [Google Scholar]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America, 5th ed.; Kendal Hunt Publishing Company: Dubuque, IA, USA, 2019; p. 1498. ISBN 978-152-496-854-0. [Google Scholar]
- Webster, J.R.; Benfield, E.F. Vascular plant breakdown in freshwater ecosystems. Ann. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Navel, S.; Mermillod-Blondin, F.; Montuelle, B.; Chauvet, E.; Simon, L.; Piscart, C.; Marmonier, P. Interactions between fauna and sediment control the breakdown of plant matter in river sediments. Freshw. Biol. 2010, 55, 753–766. [Google Scholar] [CrossRef]
- Vander Vorste, R.; Mermillod-Blondin, F.; Hervant, F.; Mons, R.; Datry, T. Gammarus pulex (Crustacea: Amphipoda) avoids increasing water temperature and intraspecific competition through vertical migration into the hyporheic zone: A mesocosm experiment. Aquat. Sci. 2017, 79, 45–55. [Google Scholar] [CrossRef]
- Akatsuka, K.; Komai, T. Pseudocrangonyx, a new genus of subterranean amphipods from Japan. Annot. Zool. Jpn. 1922, 10, 119–126. [Google Scholar]
- Stubbington, R.; Wood, P.J.; Reid, I. Spatial variability in the hyporheic zone refugium of temporary streams. Aquat. Sci. 2011, 73, 499–511. [Google Scholar] [CrossRef]
- Xu, M.Z.; Wang, Z.Y.; Pan, B.Z.; Zhao, N. Distribution and species composition of macroinvertebrates in the hyporheic zone of bed sediment. Int. J. Sediment Res. 2012, 27, 129–140. [Google Scholar] [CrossRef]
- Grattan, R.M.; Suberkropp, K. Effects of nutrient enrichment on yellow poplar leaf decomposition and fungal activity in streams. J. N. Am. Benthol. Soc. 2001, 20, 33–43. [Google Scholar] [CrossRef]
- Menéndez, M.; Descals, E.; Riera, T.; Moya, O. Leaf litter breakdown in Mediterranean streams: Effect of dissolved inorganic nutrients. Hydrobiologia 2011, 669, 143–155. [Google Scholar] [CrossRef]
- Gulis, V.; Ferreira, V.; Graça, M.A.S. Stimulation of leaf litter decomposition and associated fungi and invertebrates by moderate eutrophication: Implications for stream assessment. Freshw. Biol. 2006, 51, 1655–1669. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E.; Schindlbacher, A.; Siciliano, S.; Breulmann, M.; Yannarell, A.; Beman, J.M.; Abell, G.; Philippot, L.; Prosser, J.; et al. Microbes as engines of ecosystem function: When does community structure enhance predictions of ecosystem processes? Front. Microbiol. 2016, 7, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| (a) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Site (S), Type (T), S × T | −66.65 | 157.31 | 0.74 | |
| 1st Reduced model | ||||
| S, T | −67.99 | 149.99 | <0.001S, 0.93 T | |
| 2nd Reduced model | ||||
| S | −68.06 | 146.11 | ||
| T | −85.06 | 178.13 | ||
| (b) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Site (S), Type (T), S × T | 109.68 | −189.4 | 0.98 | |
| 1st Reduced model | ||||
| S, T | 109.06 | −200.1 | <0.05S, 0.13 T | |
| 2nd Reduced model | ||||
| S | 107.07 | −200.1 | ||
| T | 105.04 | −198.1 | ||
| (c) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Site (S), Type (T), S × T | −97.47 | 224.9 | <0.05 | |
| 1st Reduced model | ||||
| S, T | −105.81 | 229.6 |
| (a) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Type | −4.25 | 18.5 | <0.05 | |
| Null model | ||||
| N.A. | −8.20 | 22.4 | ||
| (b) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Detritivore | −18.99 | 46.0 | <0.01 | |
| Null model | ||||
| N.A. | −23.63 | 53.3 |
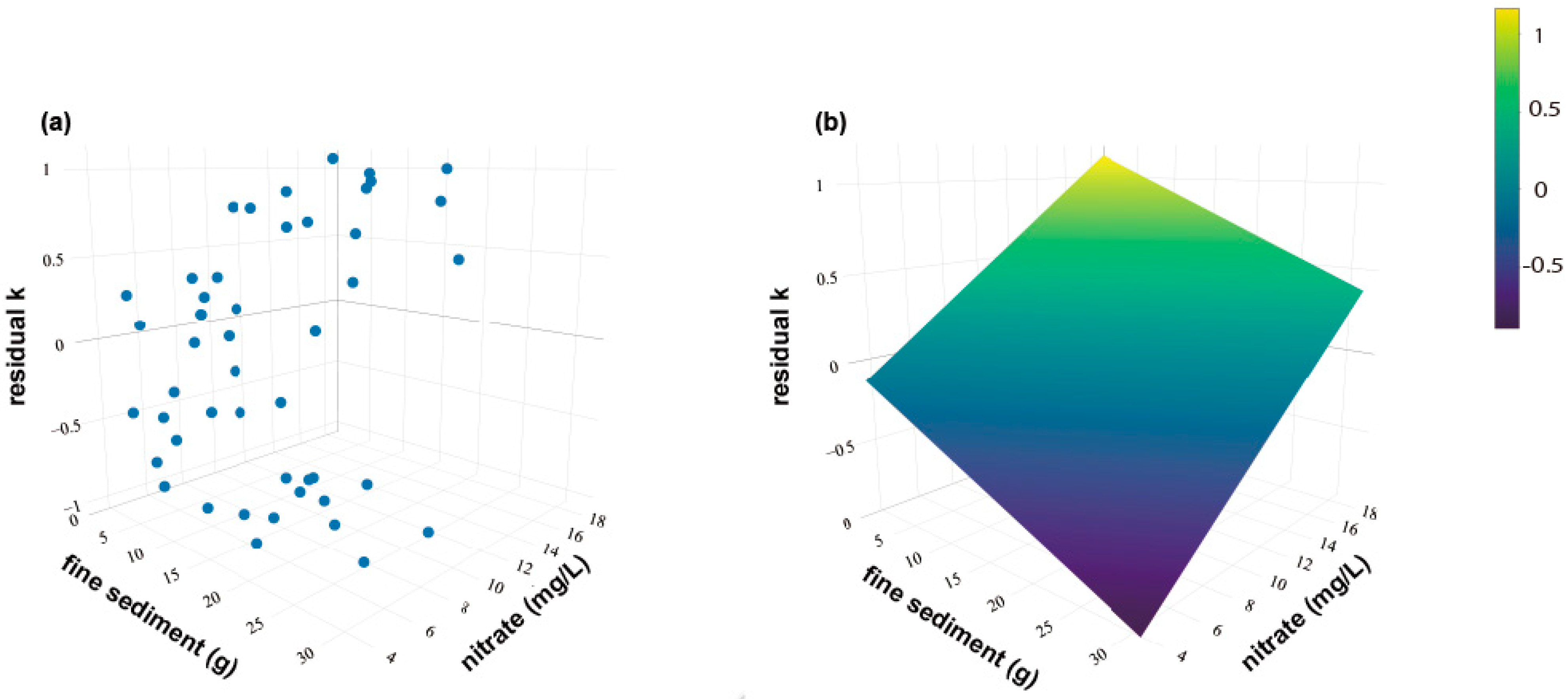
| (a) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Nitrate (N), sediment (S), N × S | −11.43 | 38.9 | 0.44 | |
| 1st Reduced model | ||||
| N, S | −11.73 | 37.5 | <0.001N, <0.01S | |
| 2nd Reduced model | ||||
| N | −16.99 | 46 | ||
| S | −19.28 | 50.6 | ||
| (b) | Explanatory Variables | logLik | AIC | p-Value |
| Full model | ||||
| Nitrate (N), sediment (S), detritivores (D) | 9.0 | −2.1 | <0.01N, 0.09 S, <0.001D | |
| 1st Reduced model | ||||
| N, S | −5.61 | 25.2 | ||
| S, D | 4.35 | 5.3 | ||
| D, N | 7.58 | −1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.K.; Negishi, J.N.; Pongsivapai, P.; Yamashita, S.; Nakagawa, T. Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone. Water 2021, 13, 1340. https://doi.org/10.3390/w13101340
Alam MK, Negishi JN, Pongsivapai P, Yamashita S, Nakagawa T. Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone. Water. 2021; 13(10):1340. https://doi.org/10.3390/w13101340
Chicago/Turabian StyleAlam, Md. Khorshed, Junjiro N. Negishi, Pongpet Pongsivapai, Shohei Yamashita, and Tomohiro Nakagawa. 2021. "Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone" Water 13, no. 10: 1340. https://doi.org/10.3390/w13101340
APA StyleAlam, M. K., Negishi, J. N., Pongsivapai, P., Yamashita, S., & Nakagawa, T. (2021). Additive Effects of Sediment and Nutrient on Leaf Litter Decomposition and Macroinvertebrates in Hyporheic Zone. Water, 13(10), 1340. https://doi.org/10.3390/w13101340






