Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon
Abstract
:1. Introduction
2. Materials and Methods
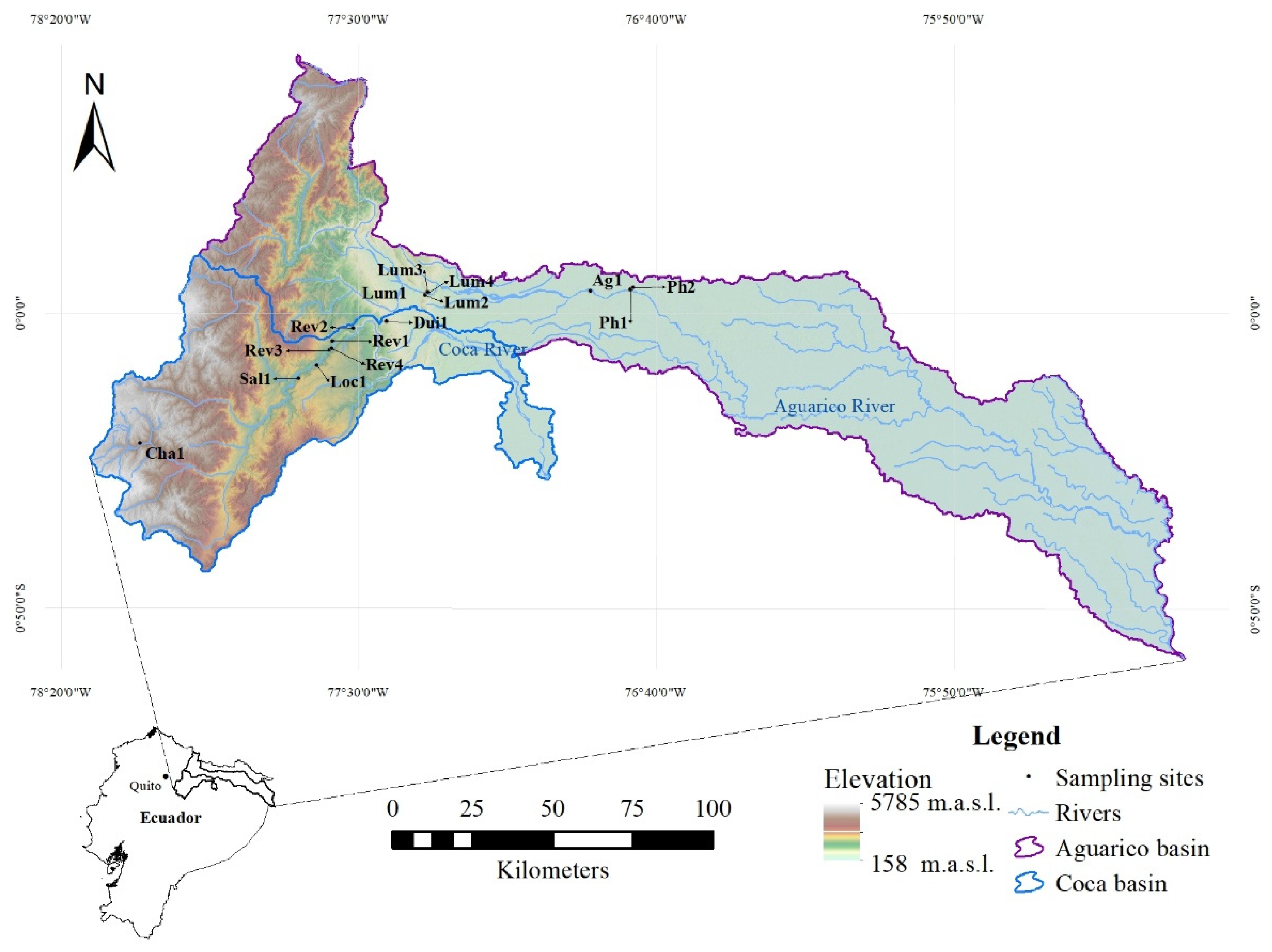
2.1. Study Area and Site Selection
2.2. Data Collection
2.2.1. Physical Chemical Analysis
2.2.2. Macroinvertebrate Data Collection
2.2.3. BMWP-Score Calculation for River Assessment
2.2.4. Trait-Allocation
2.2.5. Data Analysis
3. Results
3.1. Physical Chemical Analysis
3.2. BMWP-Col Score Calculation for River Assessment
3.3. Biological Water Quality and Functional Feeding Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Order | Family | Parasite (PA) | Collector-Filterer (CF) | Shredder (SH) | Scraper (SC) | Predator (PR) | Collector-Gatherer (CG) | Piercer (PI) |
|---|---|---|---|---|---|---|---|---|
| Acari | Acari | a | ||||||
| Amphipoda | Hyalellidae | a | ||||||
| Architaenioglossa | Ampullariidae | a | ||||||
| Coleoptera | Dryopidae | b | a,b | |||||
| Elmidae | b,c | a,b,c | b,c | |||||
| Hydrophilidae | c | a,b,c | b,c | |||||
| Lampyridae | a,b | |||||||
| Psephenidae | a,b,c | c | ||||||
| Ptilodactylidae | a,b | |||||||
| Scirtidae | b | b | a,b | b | ||||
| Decapoda | Pseudothelphusidae | d | e | |||||
| Diptera | Blephariceridae | a,b | ||||||
| Chironomidae NTP | b | c | c | b,c | a,b,c | |||
| Chironomidae TP | b | c | c | b,c | a,b,c | |||
| Dixidae | a | |||||||
| Dolichopodidae | b | |||||||
| Limoniidae | a | |||||||
| Muscidae | b | |||||||
| Psychodidae | c | c | b,c | |||||
| Simuliidae | a,b,c | b,c | b | b | ||||
| Tipulidae | b,c | c | c | b,c | c | |||
| Ephemeroptera | Baetidae | c | a,b,c | b,c | ||||
| Leptohyphidae | b | c | c | a,b,c | ||||
| Leptophlebiidae | b,c | c | b,c | a,b,c | ||||
| Oligoneuriidae | a,b | |||||||
| Haplotaxida | Tubificidae | a | ||||||
| Hemiptera | Hebridae | a,b | ||||||
| Naucoridae | a,b,c | c | ||||||
| Veliidae | a,b | |||||||
| Hirudinida | Glossiphoniidae | a | ||||||
| Lepidoptera | Pyralidae | c | c | c | ||||
| Littorinimorpha | Hydrobiidae | a | ||||||
| Megaloptera | Corydalidae | a,b,c | ||||||
| Odonata | Calopterygidae | a,b | ||||||
| Coenagrionidae | a,b,c | |||||||
| Libellulidae | a,b,c | |||||||
| Polythoridae | b | |||||||
| Opisthopora | Lumbricidae | f | ||||||
| Plecoptera | Perlidae | a,b,c | ||||||
| Sorbeoconcha | Pachychilidae | g,h | ||||||
| Trichoptera | Calamoceratidae | a,b,c | b | c | ||||
| Ecnomidae | b | |||||||
| Helicopsychidae | a,b,c | c | ||||||
| Hydrobiosidae | a,b | |||||||
| Hydropsychidae | a,b,c | c | b | b | c | |||
| Philopotamidae | a,b,c | c | ||||||
| Polycentropodidae | b | c | b,c | a | ||||
| Sericostomatidae | b | |||||||
| Xiphocentronidae | b,c | |||||||
| Tricladida | Dugesiidae | a |
| Temp | DO | DO sat | pH | Cond | NH4 | NO2 | Total N | Phosphate | Total P | Elevation | BMWP-Col | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 1.00 | |||||||||||
| DO | −0.60 * | 1.00 | ||||||||||
| DO saturation | −0.69 * | 0.68 * | 1.00 | |||||||||
| pH | −0.56 * | 0.57 * | 0.81 * | 1.00 | ||||||||
| Conductivity | −0.58 * | 0.06 | 0.47 | 0.70 * | 1.00 | |||||||
| Ammonium-N | 0.18 | 0.16 | −0.42 | −0.14 | −0.25 | 1.00 | ||||||
| Nitrite–N | 0.10 | −0.09 | −0.20 | 0.16 | 0.35 | 0.40 | 1.00 | |||||
| Total N | 0.20 | −0.35 | −0.53 * | −0.36 | −0.07 | 0.21 | 0.11 | 1.00 | ||||
| Phosphate | −0.02 | 0.57 * | 0.49 | 0.56 * | 0.16 | 0.15 | 0.17 | −0.43 | 1.00 | |||
| Total P | −0.24 | 0.27 | 0.38 | 0.69 * | 0.59 * | 0.28 | 0.59 * | −0.41 | 0.52 | 1.00 | ||
| Elevation | −0.80 * | 0.23 | 0.72 * | 0.51 | 0.65 * | −0.65 * | −0.28 | −0.23 | −0.07 | 0.06 | 1.00 | |
| BMWP-Col | −0.51 | 0.11 | 0.43 | 0.33 | 0.39 | −0.23 | −0.28 | −0.43 | 0.03 | 0.26 | 0.62 * | 1.00 |
| Order | Family | Total Abundance | Number of Sampling Sites | BMWP-Col Tolerance Score |
|---|---|---|---|---|
| Acari | Acari | 2 | 2 | - |
| Amphipoda | Hyalellidae | 1 | 1 | 7 |
| Architaenioglossa | Ampullariidae | 1 | 1 | 6 |
| Coleoptera | Dryopidae | 1 | 1 | 6 |
| Elmidae | 46 | 9 | 7 | |
| Hydrophilidae | 10 | 5 | 4 | |
| Lampyridae | 1 | 1 | 10 | |
| Psephenidae | 12 | 2 | 10 | |
| Ptilodactylidae | 115 | 5 | 9 | |
| Scirtidae | 1 | 1 | 6 | |
| Decapoda | Pseudothelphusidae | 1 | 1 | 8 |
| Diptera | Blephariceridae | 7 | 4 | 10 |
| Chironomidae NTP | 54 | 10 | 2 | |
| Chironomidae TP | 32 | 1 | 2 | |
| Dixidae | 1 | 1 | 4 | |
| Dolichopodidae | 1 | 1 | 4 | |
| Limoniidae | 40 | 7 | - | |
| Muscidae | 1 | 1 | 5 | |
| Psychodidae | 1 | 1 | 4 | |
| Simuliidae | 11 | 2 | 6 | |
| Tipulidae | 2 | 2 | 5 | |
| Ephemeroptera | Baetidae | 336 | 9 | 5 |
| Leptohyphidae | 192 | 8 | 7 | |
| Leptophlebiidae | 193 | 10 | 8 | |
| Oligoneuriidae | 1 | 1 | 10 | |
| Haplotaxida | Tubificidae | 25 | 6 | 1 |
| Hemiptera | Hebridae | 1 | 1 | - |
| Naucoridae | 5 | 2 | 5 | |
| Veliidae | 6 | 5 | 6 | |
| Hirudinida | Glossiphoniidae | 2 | 1 | 3 |
| Lepidoptera | Pyralidae | 1 | 1 | 4 |
| Littorinimorpha | Hydrobiidae | 1 | 1 | 7 |
| Megaloptera | Corydalidae | 27 | 6 | 9 |
| Odonata | Calopterygidae | 1 | 1 | 7 |
| Coenagrionidae | 3 | 1 | 6 | |
| Libellulidae | 6 | 5 | 5 | |
| Polythoridae | 4 | 2 | 10 | |
| Opisthopora | Lumbricidae | 1 | 1 | - |
| Plecoptera | Perlidae | 206 | 6 | 10 |
| Sorbeoconcha | Pachychilidae | 2 | 2 | - |
| Trichoptera | Calamoceratidae | 2 | 2 | 10 |
| Ecnomidae | 1 | 1 | - | |
| Helicopsychidae | 9 | 4 | 9 | |
| Hydrobiosidae | 14 | 3 | 9 | |
| Hydropsychidae | 27 | 6 | 5 | |
| Philopotamidae | 9 | 3 | 9 | |
| Polycentropodidae | 2 | 1 | 7 | |
| Sericostomatidae | 6 | 3 | - | |
| Xiphocentronidae | 1 | 1 | 9 | |
| Tricladida | Dugesiidae | 1 | 1 | 6 |



References
- Ganoulis, J.; Fried, J. Towards A “Good” Transboundary Hydro-Governance: From Conflict to Shared Management, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 9783319786247. [Google Scholar]
- Giri, S. Water quality prospective in Twenty First Century: Status of water quality in major river basins, contemporary strategies and impediments: A review. Environ. Pollut. 2021, 271, 116332. [Google Scholar] [CrossRef]
- Kaushal, S.S.; McDowell, W.H.; Wollheim, W.M.; Johnson, T.A.N.; Mayer, P.M.; Belt, K.T.; Penninov, M.J. Urban evolution: The role of water. Water 2015, 7, 4063–4087. [Google Scholar] [CrossRef]
- Eriksen, T.E.; Brittain, J.E.; Søli, G.; Jacobsen, D.; Goethals, P.; Friberg, N. A global perspective on the application of riverine macroinvertebrates as biological indicators in Africa, South-Central America, Mexico and Southern Asia. Ecol. Indic. 2021, 126. [Google Scholar] [CrossRef]
- Chapman, D. Water Quality Assessments—A Guide to Use of Biota, Sediments and Water in Environmental Monitoring, 2nd ed.; CRC Press: London, UK, 1996; ISBN1 0 419 21590 5 (HB). ISBN2 0 419 21600 6 (PB). [Google Scholar]
- Helson, J.E.; Williams, D.D. Development of a macroinvertebrate multimetric index for the assessment of low-land streams in the neotropics. Ecol. Indic. 2013, 29, 167–178. [Google Scholar] [CrossRef]
- Joao, C.; Perez-Bilbao, A.; Garrido, J. Macroinvertebrates as Indicators of Water Quality in Running Waters: 10 Years of Research in Rivers with Different Degrees of Anthropogenic Impacts. Ecol. Water Qual. Water Treat. Reuse 2012. [Google Scholar] [CrossRef] [Green Version]
- Borja-Serrano, P.; Ochoa-Herrera, V.; Maurice, L.; Morales, G.; Quilumbaqui, C.; Tejera, E.; Machado, A. Determination of the microbial and chemical loads in rivers from the Quito capital province of Ecuador (Pichincha)—A preliminary analysis of microbial and chemical quality of the main rivers. Int. J. Environ. Res. Public Health 2020, 17, 5048. [Google Scholar] [CrossRef]
- Celi, J.E.; Villamarín, F. Freshwater ecosystems of mainland Ecuador: Diversity, issues and perspectives. Acta Limnol. Bras. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Moulatlet, G.M.; Abessa, D.M.d.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An integrative approach to identify the impacts of multiple metal contamination sources on the Eastern Andean foothills of the Ecuadorian Amazonia. Sci. Total Environ. 2020, 709, 136088. [Google Scholar] [CrossRef]
- Jaderne Houssou, N.L.; Cordero, J.D.; Bouadjio-Boulic, A.; Morin, L.; Maestripieri, N.; Ferrant, S.; Belem, M.; Peláez, J.I.; Saenz, M.; Lerigoleur, E.; et al. Synchronizing histories of exposure and demography: The construction of an agent- based model of the ecuadorian amazon colonization and exposure to oil pollution hazards. JASSS 2019, 22. [Google Scholar] [CrossRef]
- Marshall, B.G.; Veiga, M.M.; Kaplan, R.J.; Adler Miserendino, R.; Schudel, G.; Bergquist, B.A.; Guimarães, J.R.D.; Sobral, L.G.S.; Gonzalez-Mueller, C. Evidence of transboundary mercury and other pollutants in the Puyango-Tumbes River basin, Ecuador-Peru. Environ. Sci. Process. Impacts 2018, 20, 632–641. [Google Scholar] [CrossRef]
- Webb, J.; Coomes, O.T.; Mainville, N.; Mergler, D. Mercury Contamination in an Indicator Fish Species from Andean Amazonian Rivers Affected by Petroleum Extraction. Bull. Environ. Contam. Toxicol. 2015, 95, 279–285. [Google Scholar] [CrossRef]
- Antunes, A.P.; Fewster, R.M.; Venticinque, E.M.; Peres, C.A.; Levi, T.; Rohe, F.; Shepard, G.H. Empty forest or empty rivers? A century of commercial hunting in Amazonia. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, D.; Pandey, J. River ecosystem resilience risk index: A tool to quantitatively characterize resilience and critical transitions in human-impacted large rivers. Environ. Pollut. 2021, 268, 115771. [Google Scholar] [CrossRef]
- Sá-Oliveira, J.C.; Isaac, V.J.; Ferrari, S.F. Fish community structure as an indicator of the long-term effects of the damming of an Amazonian river. Environ. Biol. Fishes 2015, 98, 273–286. [Google Scholar] [CrossRef]
- Escanilla-Minchel, R.; Alcayaga, H.; Soto-Alvarez, M.; Kinnard, C.; Urrutia, R. Evaluation of the impact of climate change on runoff generation in an andean glacier watershed. Water 2020, 12, 3547. [Google Scholar] [CrossRef]
- Wongchuig, S.C.; Mello, C.R.; Chou, S.C. Projections of the impacts of climate change on the water deficit and on the precipitation erosive indexes in Mantaro River Basin, Peru. J. Mt. Sci. 2018, 15, 264–279. [Google Scholar] [CrossRef]
- Casagrande, E.; Recanati, F.; Rulli, M.C.; Bevacqua, D.; Melià, P. Water balance partitioning for ecosystem service assessment. A case study in the Amazon. Ecol. Indic. 2021, 121. [Google Scholar] [CrossRef]
- Echelpoel, W.; Van Forio, E.; Butsel, J.; Van Lock, K.; Dueñas, J.; Dominguez-granda, L.; Goethals, P.L.M. Macroinvertebrate functional feeding group structure along an impacted tropical river: The Portoviejo River (Ecuador). Limnologica 2018, 73, 12–19. [Google Scholar] [CrossRef]
- Forio, M.A.; Landuyt, D.; Bennetsen, E.; Lock, K.; Hanh, T.; Nguyen, T.; Naomi, M.; Ambarita, D.; Liz, P.; Musonge, S.; et al. Bayesian belief network models to analyse and predict ecological water quality in rivers. Ecol. Modell. 2015, 312, 222–238. [Google Scholar] [CrossRef]
- Forio, M.A.; Goethals, P.L.M. An Integrated Approach of Multi-Community Monitoring and Assessment of Aquatic Ecosystems to Support Sustainable Development. Sustainanility 2020, 12, 5603. [Google Scholar] [CrossRef]
- Rubach, M.N.; Ashauer, R.; Buchwalter, D.B.; De Lange, H.J.; Hamer, M.; Preuss, T.G.; Töpke, K.; Maund, S.J. Framework for traits-based assessment in ecotoxicology. Integr. Environ. Assess. Manag. 2011, 7, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Koklu, R.; Sengorur, B.; Topal, B. Water quality assessment using multivariate statistical methods-a case study: Melen river system (Turkey). Water Resour. Manag. 2010, 24, 959–978. [Google Scholar] [CrossRef]
- Matoušková, M.; Dvořák, M. Assessment of physical habitat modification in the Bílina River Basin. Limnetica 2011, 30, 293–306. [Google Scholar] [CrossRef]
- Wepener, V.; van Dyk, C.; Bervoets, L.; O’Brien, G.; Covaci, A.; Cloete, Y. An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys. Chem. Earth 2011, 36, 949–962. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Lock, K.; Boets, P.; Everaert, G.; Hanh, T.; Nguyen, T.; Anne, M.; Forio, E.; Liz, P.; Musonge, S.; et al. Ecological water quality analysis of the Guayas river basin (Ecuador) based on macroinvertebrates indices. Limnologica 2016, 57, 27–59. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Boets, P.; Tien, H.; Thi, N.; Anne, M.; Forio, E.; Everaert, G.; Lock, K.; Liz, P.; Musonge, S.; et al. Impact assessment of local land use on ecological water quality of the Guayas river basin (Ecuador). Ecol. Inform. 2018, 48, 226–237. [Google Scholar] [CrossRef]
- Sotomayor, G.; Hampel, H.; Vázquez, R.F.; Goethals, P.L.M. Multivariate-statistics based selection of a benthic macroinvertebrate index for assessing water quality in the Paute River basin (Ecuador). Ecol. Indic. 2020, 111, 106037. [Google Scholar] [CrossRef] [Green Version]
- Boonsoong, B.; Sangpradub, N.; Barbour, M.T.; Simachaya, W. An implementation plan for using biological indicators to improve assessment of water quality in Thailand. Environ. Monit. Assess. 2010, 165, 205–215. [Google Scholar] [CrossRef]
- Jerves-Cobo, R.; Gonzalo, C.; Iñiguez-vela, X.; Catalina, D. Model-Based Analysis of the Potential of Macroinvertebrates as Indicators for Microbial Pathogens in Rivers. Water 2018, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- Brittain, J.E.; Eikeland, T.J. Invertebrate drift—A review Invertebrate drift—A review. Hydrobiologia 1988, 166, 77–93. [Google Scholar] [CrossRef]
- Gabriels, W.; Lock, K.; Pauw, N.; De Goethals, P.L.M. Limnologica Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnologica 2010, 40, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Forio, M.A.; Mouton, A.; Lock, K.; Boets, P.; Thi, N.; Tien, H.; Naomi, M.; Ambarita, D.; Liz, P.; Musonge, S.; et al. Fuzzy modelling to identify key drivers of ecological water quality to support decision and policy making. Environ. Sci. Policy 2017, 68, 58–68. [Google Scholar] [CrossRef]
- Dominguez-Granda, L.; Lock, K.; Goethals, P.L.M. Using multi-target clustering trees as a tool to predict biological water quality indices based on benthic macroinvertebrates and environmental parameters in the Chaguana watershed (Ecuador). Ecol. Inform. 2011, 6, 303–308. [Google Scholar] [CrossRef]
- Forio, M.; Goethals, P.L.M.; Lock, K.; Asio, V.; Bande, M.; Thas, O. Model-based analysis of the relationship between macroinvertebrate traits and environmental river conditions. Environ. Model. Softw. 2018, 106, 57–67. [Google Scholar] [CrossRef]
- Holguin-Gonzalez, J.E.; Boets, P.; Alvarado, A.; Cisneros, F.; Carrasco, M.C.; Wyseure, G.; Nopens, I.; Goethals, P.L.M. Integrating hydraulic, physicochemical and ecological models to assess the effectiveness of water quality management strategies for the River Cuenca in Ecuador. Ecol. Modell. 2013, 254, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, E.; Fernández, H.R. Macroinvertebrados Bentónicos Sudamericanos; Fundación Miguel Lillo: San Miguel de Tucumán, Argentina, 2009; ISBN 9789506680152. [Google Scholar]
- Álvarez, L.F. Metodología para la Utilización de los Macroinvertebrados Acuáticos como Indicadores de la Calidad del Agua; Alexander von Humboldt Biological Resources Research Institute: Bogotá, Colombia, 2005. [Google Scholar]
- Roldán-Pérez, G. Bioindicación de la Calidad del Agua en Colombia. Uso del método BMWP/Col; Universidad de Antioquia: Medellín, Colombia, 2003; 170p. [Google Scholar]
- Verberk, W.C.E.P.; Noordwijk, C.G.E.; Van Hildrew, A.G. Delivering on a promise: Integrating species traits to transform descriptive community ecology into a predictive science. Freshw. Sci. 2013, 32, 531–547. [Google Scholar] [CrossRef] [Green Version]
- Menezes, S.; Baird, D.J.; Soares, A.M.V.M. Beyond taxonomy: A review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- Tall, L.; Méthot, G.; Armellin, A.; Pinel-alloul, B. Bioassessment of Benthic Macroinvertebrates in Wetland Habitats of Lake Saint-Pierre (St. Lawrence River). J. Great Lakes Res. 2008, 34, 599–614. [Google Scholar] [CrossRef]
- Calapez, A.R.; Serra, S.R.Q.; Santos, J.M.; Branco, P.; Ferreira, T.; Hein, T.; Brito, A.G.; Feio, M.J. The effect of hypoxia and flow decrease in macroinvertebrate functional responses: A trait-based approach to multiple-stressors in mesocosms. Sci. Total Environ. 2018, 637–638, 647–656. [Google Scholar] [CrossRef] [PubMed]
- James, A.B.W.; Dewson, Z.S.; Death, R.G. Do stream macroinvertebrates use instream refugia in response to severe short-term flow reduction in New Zealand streams ? Freshw. Biol. 2008, 53, 1316–1334. [Google Scholar] [CrossRef]
- Pardo, I.; García, L. Abstraction in small lowland streams: Unforeseen hypoxia and anoxia effects. Sci. Total Environ. 2016, 568, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.B.; Kefford, B.J.; Metzeling, L.; Liess, M.; Burgert, S.; Marchant, R.; Pettigrove, V.; Goonan, P.; Nugegoda, D. A trait database of stream invertebrates for the ecological risk assessment of single and combined effects of salinity and pesticides in South-East Australia. Sci. Total Environ. 2011, 409, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Foden, W.B.; Butchart, S.H.M.; Stuart, S.N.; Vié, J.C.; Akçakaya, H.R.; Angulo, A.; DeVantier, L.M.; Gutsche, A.; Turak, E.; Cao, L.; et al. Identifying the World’s Most Climate Change Vulnerable Species: A Systematic Trait-Based Assessment of all Birds, Amphibians and Corals. PLoS ONE 2013, 8, 5427. [Google Scholar] [CrossRef] [Green Version]
- Dolédec, S.; Statzner, B.; Bournard, M. Species traits for fututre biomonitoring across ecoregions. Freshw. Biol. 1999, 42, 737–758. [Google Scholar] [CrossRef]
- Torres, M.C.; Fierro, V.; Páez, S.; Plúa, F.; Carrera, M.I.; Moscoso, N.; Cazco, R.; Tamayo, G.; Ruiz, M.; Narváez, M.; et al. La Economía de los ecosistemas y la biodiversidad. Informe final: Resultados de las modelaciones biofísicas, valoración económica y propuesta de políticas. Cuenca del Río Coca, Ecuador; Quito, Ecuador. 2018. Available online: https://www.epn.edu.ec/wp-content/uploads/2018/03/Informe-Final-TEEB-Cuenca-Rio-Coca.pdf (accessed on 22 March 2021).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Alexiades, A.V.; Encalada, A.C.; Lessmann, J.; Guayasamin, J.M. Spatial prediction of stream physicochemical parameters for the Napo River Basin, Ecuador. J. Freshw. Ecol. 2019, 34, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, S.; Terneus, F. Nexo agua—energía: Análisis del flujo hídrico del Proyecto Hidroeléctrico Coca Codo Sinclair The water-energy nexus: Analysis of the water flow of the Coca Codo Sinclair Hydroelectric Project. Ingenius 2019, 21, 53–62. [Google Scholar]
- Organization of American States (OAS). Plan de Ordenamiento y Manejo de las Cuencas de los Ríos San Miguel y Putumayo Indice Prefacio Instituciones citadas; Washington, DC, USA. 1987. Available online: https://www.oas.org/dsd/publications/Unit/oea49s/oea49s.pdf (accessed on 25 March 2021).
- Galacatos, K.; Barriga-Salazar, R.; Stewart, D. Seasonal and Habitat Influences on Fish Communities within the Lower Yasuni River Basin of the Ecuadorian Amazon. Environ. Biol. Fishes 2004, 71, 33–51. [Google Scholar] [CrossRef]
- Bass, M.S.; Finer, M.; Jenkins, C.N.; Kreft, H.; Cisneros-Heredia, D.F.; McCracken, S.F.; Pitman, N.C.A.; English, P.H.; Swing, K.; Villa, G.; et al. Global conservation significance of Ecuador’s Yasuní National Park. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, J.; Guayasamin, J.M.; Casner, K.L.; Flecker, A.S.; Funk, W.C.; Ghalambor, C.K.; Gill, B.A.; Jácome-Negrete, I.; Kondratieff, B.C.; Poff, L.R.N.; et al. Freshwater vertebrate and invertebrate diversity patterns in an Andean-Amazon basin: Implications for conservation efforts. Neotrop. Biodivers. 2016, 2, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Mena, C.F.; Bilsborrow, R.E.; McClain, M.E. Socioeconomic drivers of deforestation in the Northern Ecuadorian Amazon. Environ. Manag. 2006, 37, 802–815. [Google Scholar] [CrossRef]
- Rivera-Parra, J.L.; Vizcarra, C.; Mora, K.; Mayorga, H.; Dueñas, J.C. Spatial distribution of oil spills in the north eastern Ecuadorian Amazon: A comprehensive review of possible threats. Biol. Conserv. 2020, 252. [Google Scholar] [CrossRef]
- PetroAmazonas, E. Nuestra Producción. Available online: https://www.petroamazonas.gob.ec/ (accessed on 18 January 2021).
- López, V. El proyecto hidroeléctrico Coca Codo Sinclair y la gobernanza energética en la Amazonía ecuatoriana. Let. Verdes Rev. Latinoam. Estud. Socioambientales 2011. [Google Scholar] [CrossRef] [Green Version]
- van Teijlingen, K. Minería a gran escala, pluralismo territorial y contención: Un mapeo de encuentros y desencuentros en la amazonía ecuatoriana. Estud. Atacameños. Arqueol. Antropol. Surandinas 2019, 275–299. [Google Scholar] [CrossRef]
- Lu, F.; Gray, C.; Bilsborrow, R.E.; Mena, C.F.; Erlien, C.M.; Bremner, J.; Barbieri, A.; Walsh, S.J. Contrasting Colonist and Indigenous Impacts on Amazonian Forests. Conserv. Biol. 2010, 24, 881–885. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, G. On indigeneity, change, and representation in the northeastern Ecuadorian Amazon. Environ. Plan. A 2005, 37, 285–303. [Google Scholar] [CrossRef]
- Everaert, G.; Neve, J.; De Boets, P.; Dominguez-granda, L.; Mereta, S.T.; Ambelu, A.; Hoang, T.H.; Goethals, P.L.M.; Thas, O. Comparison of the Abiotic Preferences of Macroinvertebrates in Tropical River Basins. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Mereta, S.T.; Boets, P.; De Meester, L.; Goethals, P.L.M. Development of a multimetric index based on benthic macroinvertebrates for the assessment of natural wetlands in Southwest Ethiopia. Ecol. Indic. 2013, 29, 510–521. [Google Scholar] [CrossRef]
- Dudgeon, D.; Gao, B.W. Biodiversity and ecosystem functioning in a species-poor guild: A test using tropical stream detritivores. Hydrobiologia 2010, 652, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.Q.; Dudgeon, D. The influence of flow and season upon leaf-litter breakdown in monsoonal Hong Kong streams. Hydrobiologia 2011, 663, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.; Gutiérrez-Fonseca, P.E. Functional feeding groups of aquatic insect families in Latin America: A critical analysis and review of existing literature. Rev. Biol. Trop. 2014, 62, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tomanova, A.; Moya, N.; Oberdorff, T. Using macroinvertebrate biological traits for assessing biotic integrity of Neotropical streams. River Res. Appl. 2008, 24, 1230–1239. [Google Scholar] [CrossRef]
- Wehrtmann, I.S.; Hernández-Díaz, D.; Cumberlidge, N. Freshwater crabs as predators and prey: The case of Ptychophallus uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America. Lat. Am. J. Aquat. Res. 2019, 47, 18–26. [Google Scholar] [CrossRef]
- Yang, C.; Wehrtmann, I.S.; Wenger, S.J.; Rugenski, A.T. Neotropical freshwater crabs (Decapoda: Pseudothelphusidae) shred leaves. Nauplius 2020, 28. [Google Scholar] [CrossRef]
- Nessimian, J.L.; Dorvillé, L.F.M.; Sanseverino, A.M.; Baptista, D.F. Relation between flood pulse and functional composition of the macroinvertebrate benthic fauna in the lower Rio Negro, Amazonas, Brazil. Amazoniana 1998, 15, 35–50. [Google Scholar]
- Pearson, T.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. An Annu. Rev. 1978, 16, 229–331. [Google Scholar]
- Schmera, D.; Heino, J.; Podani, J.; Erős, T.; Dolédec, S. Functional diversity: A review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 2017, 787, 27–44. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing, 4.0.3 ed. Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 28 December 2020).
- RStudio Team RStudio: Integrated Development for R. Available online: https://www.rstudio.com/ (accessed on 28 December 2020).
- Grande, J.A.; Jiménez, A.; Borrego, J.; de la Torre, M.L.; Gómez, T. Relationships Between Conductivity and pH in Channels Exposed to Acid Mine Drainage Processes: Study of a Large Mass of Data Using Classical Statistics. Water Resour. Manag. 2010, 24, 4579–4587. [Google Scholar] [CrossRef]
- Nguyen, T.; Boets, P.; Lock, K.; Anne, M.; Forio, E.; Echelpoel, W.; Van Butsel, J.; Van Dueñas, J.A.; Everaert, G.; Elvin, L.; et al. Water quality related macroinvertebrate community responses to environmental gradients in the Portoviejo River (Ecuador). Ann. Limnol. J. Limnol. 2017, 53, 203–219. [Google Scholar] [CrossRef]
- de Brouwer, J.H.F.; Verdonschot, P.F.M.; Eekhout, J.P.C.; Verdonschot, R.C.M. Macroinvertebrate taxonomic and trait-based responses to large-wood reintroduction in lowland streams. Freshw. Sci. 2020, 39, 693–703. [Google Scholar] [CrossRef]
- Ding, N.; Yang, W.; Zhou, Y.; González-Bergonzoni, I.; Zhang, J.; Chen, K.; Vidal, N.; Jeppesen, E.; Liu, Z.; Wang, B. Different responses of functional traits and diversity of stream macroinvertebrates to environmental and spatial factors in the Xishuangbanna watershed of the upper Mekong River Basin, China. Sci. Total Environ. 2017, 574, 288–299. [Google Scholar] [CrossRef]
- Statzner, B.; Bêche, L.A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- Friberg, N.; Bonada, N.; Bradley, D.C.; Dunbar, M.J.; Edwards, F.K.; Grey, J.; Hayes, R.B.; Hildrew, A.G.; Lamouroux, N.; Trimmer, M.; et al. Biomonitoring of Human Impacts in Freshwater Ecosystems. The Good, the Bad and the Ugly. 2011, 44, 1–68. [Google Scholar]
- Armitage, P.D.; Moss, D.; Wright, J.F.; Furse, M.T. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res. 1983, 17, 333–347. [Google Scholar] [CrossRef]
- Buss, D.; Baptista, D.; Nessimian, J.; Egler, M. Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 2004, 518, 179–188. [Google Scholar] [CrossRef]
- Chukwu, L.; Nwachukwu, S. Impact of refined petroleum spills on water quality, macro-invertebrate and microbial communities of a tropical aquatic environment. Journal of environmental biology. J. Environ. Biol. 2005, 26, 449–458. [Google Scholar]
- Couceiro, S.R.M.; Hamada, N.; Forsberg, B.R.; Padovesi-Fonseca, C. Effects of anthropogenic silt on aquatic macroinvertebrates and abiotic variables in streams in the Brazilian Amazon. J. Soils Sediments 2010, 10, 89–103. [Google Scholar] [CrossRef]
- Marques, M.M.; Barbosa, F. Biological quality of waters from an impacted tropical watershed (middle Rio Doce basin, southeast Brazil), using benthic macroinvertebrate communities as an indicator. Hydrobiologia 2001, 457, 69–76. [Google Scholar] [CrossRef]
- Vinson, M.R.; Dinger, E.C.; Kotynek, J.; Dethier, M. Effects of oil pollution on aquatic macroinvertebrate assemblages in Gabon wetlands. Afr. J. Aquat. Sci. 2008, 33, 261–268. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Mercado, M.; Peña-Cortés, F.; Tapia, J.; Hauenstein, E.; Caputo, L.; Vargas-Chacoff, L. Landscape composition as a determinant of diversity and functional feeding groups of aquatic macroinvertebrates in southern rivers of the Araucanía, Chile. Lat. Am. J. Aquat. Res. 2015, 43, 186–200. [Google Scholar] [CrossRef]
- Brewin, P.A.; Buckton, S.T.; Ormerod, S.J. The seasonal dynamics and persistence of stream macroinvertebrates in Nepal: Do monsoon floods represent disturbance? Freshw. Biol. 2000, 44, 581–594. [Google Scholar] [CrossRef]
- Mesa, L.M. Influence of riparian quality on macroinvertebrate assemblages in subtropical mountain streams. J. Nat. Hist. 2014, 48, 1153–1167. [Google Scholar] [CrossRef]
- Buss, D.F.; Carlisle, D.M.; Chon, T.S.; Culp, J.; Harding, J.S.; Keizer-Vlek, H.E.; Robinson, W.A.; Strachan, S.; Thirion, C.; Hughes, R.M. Stream biomonitoring using macroinvertebrates around the globe: A comparison of large-scale programs. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Wagner, R.; Suetfeld, R.; Junk, W.J. How do plant-herbivore interactions of trees influence coarse detritus processing by shredders in aquatic ecosystems of different latitudes? SIL Proc. 1922–2010 2002, 28, 815–821. [Google Scholar] [CrossRef]
- Jacobsen, D.; Encalada, A. The macroinvertebrate fauna of Ecuadorian highland streams in the wet and dry season. Arch. fur Hydrobiol. 1998, 142, 53–70. [Google Scholar] [CrossRef]
- Junk, W.; Bayley, P.B.; Sparks, R.E. The Flood Pulse Concept in River-Floodplain Systems. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Buss, D.; Salles, F. Using Baetidae species as biological indicators of environmental degradation in a Brazilian river basin. Environ. Monit. Assess. 2007, 130, 365–372. [Google Scholar] [CrossRef]
- Damanik-Ambarita, M.N.; Everaert, G.; Anne, M.; Forio, E.; Hanh, T.; Nguyen, T.; Lock, K.; Liz, P.; Musonge, S.; Suhareva, N.; et al. Generalized Linear Models to Identify Key Hydromorphological and Chemical Variables Determining the Occurrence of Macroinvertebrates in the Guayas River Basin (Ecuador). Water 2016, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Thodsen, H.; Andersen, H.E.; Tornbjerg, H.; Baattrup-Pedersen, A.; Riis, T. Flow regimes filter species traits of benthic diatom communities and modify the functional features of lowland streams—A nationwide scale study. Sci. Total Environ. 2019, 651, 357–366. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B.; Novak, J.A.; Higgins, M.J.; Wessell, K.J.; Lessard, J.L. Development and application of a macroinvertebrate functional-group approach in the bioassessment of remnant river oxbows in southwest Florida. J. N. Am. Benthol. Soc. 2002, 21, 290–310. [Google Scholar] [CrossRef] [Green Version]
- Buss, D.F.; Vitorino, A.S. Rapid Bioassessment Protocols using benthic macroinvertebrates in Brazil: Evaluation of taxonomic sufficiency. J. N. Am. Benthol. Soc. 2010, 29, 562–571. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2019. Available online: https://www.ipbes.net/sites/default/files/downloads/spm_unedited_advance_for_posting_htn.pdf (accessed on 2 April 2021).






| Variable | Unit | Mean | Min | Max | Std |
|---|---|---|---|---|---|
| Temperature (Temp) | °C | 20.1 | 9.4 | 24.2 | 3.8 |
| Dissolved Oxygen (DO) | mg·L−1 | 8.0 | 6.4 | 9.0 | 0.6 |
| DO saturation (DOsat) | % | 97 | 62 | 105 | 10 |
| pH | 7.6 | 6.5 | 8.4 | 0.6 | |
| Conductivity (Cond) | µS·cm−1 | 126 | 15 | 902 | 221 |
| Ammonium-N (NH4–N) | mg·L−1 | 0.02 | 0.00 | 0.12 | 0.03 |
| Nitrite-N (NO2–N) a | mg·L−1 | 0.04 | 0.00 | 0.17 | 0.04 |
| Total N (TN) a | mg·L−1 | 0.4 | 0.1 | 0.9 | 0.3 |
| Phosphate (PO4–P) | mg·L−1 | 0.27 | 0.01 | 0.90 | 0.26 |
| Total P (TP) | mg·L−1 | 0.4 | 0.1 | 2.1 | 0.5 |
| Flow velocity b | m·s−1 | 0.53 | 0.14 | 1.25 | 0.37 |
| Elevation | M (asl) | 994 | 263 | 2872 | 723 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, S.; Eurie Forio, M.A.; Lock, K.; Vandenbroucke, M.; Oña, T.; Gualoto, M.; Goethals, P.L.M.; Van der heyden, C. Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon. Water 2021, 13, 1692. https://doi.org/10.3390/w13121692
Cabrera S, Eurie Forio MA, Lock K, Vandenbroucke M, Oña T, Gualoto M, Goethals PLM, Van der heyden C. Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon. Water. 2021; 13(12):1692. https://doi.org/10.3390/w13121692
Chicago/Turabian StyleCabrera, Santiago, Marie Anne Eurie Forio, Koen Lock, Marte Vandenbroucke, Tania Oña, Miguel Gualoto, Peter L. M. Goethals, and Christine Van der heyden. 2021. "Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon" Water 13, no. 12: 1692. https://doi.org/10.3390/w13121692
APA StyleCabrera, S., Eurie Forio, M. A., Lock, K., Vandenbroucke, M., Oña, T., Gualoto, M., Goethals, P. L. M., & Van der heyden, C. (2021). Variations in Benthic Macroinvertebrate Communities and Biological Quality in the Aguarico and Coca River Basins in the Ecuadorian Amazon. Water, 13(12), 1692. https://doi.org/10.3390/w13121692









