Effects of Malachite Green on the Microbiomes of Milkfish Culture Ponds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Settings of Small MCPs
2.3. Analysis of MG
2.4. Analysis of Water Quality in Small Milkfish Ponds
2.5. Next-Generation Sequencing and Data Analysis
3. Results
3.1. Effects of MG and MGDB on the Water Quality of Small MCPs
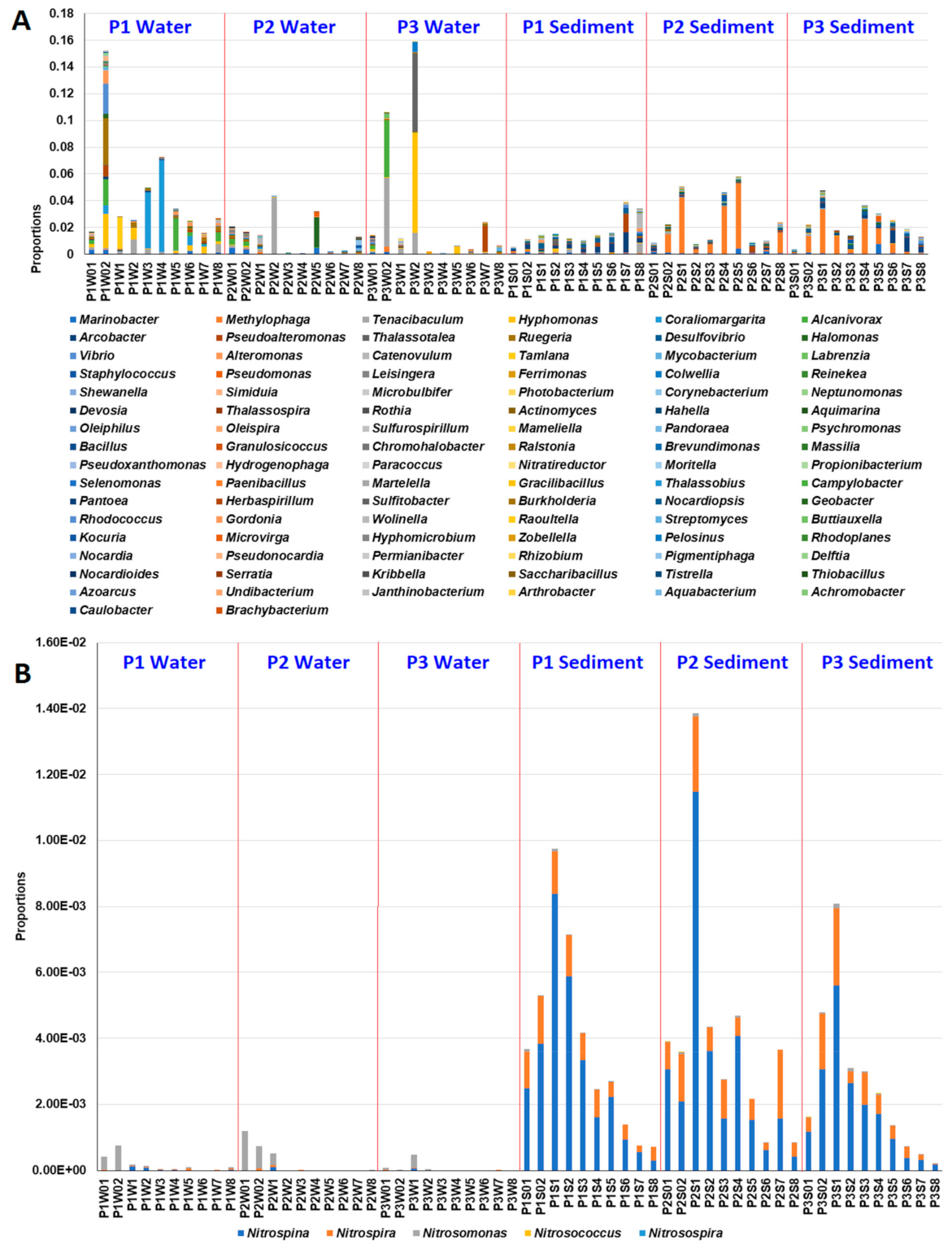
3.2. Effects of MG and MGDB on the Microbial Communities of Small MCPs
3.3. Effects of MG and MGDB on Nitrogen Cycle-Associated Microbial Communities in Small MCP
3.4. Effects of MG and MGDB on Algal, Cyanobacterial and Phototrophic Bacterial Communities in Small MCPs
3.5. Effects of MG and MGDB on Pathogenic and Xenobiotic-Degrading Microbial Communities in Small MCPs
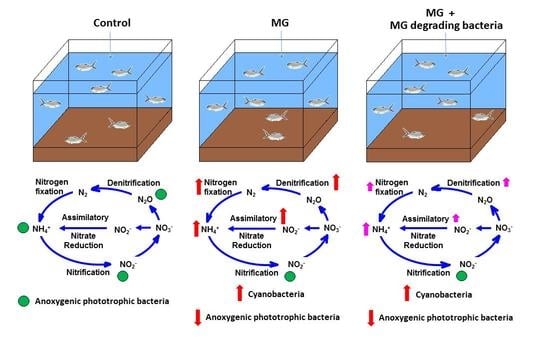
3.6. Model of MG and MGDB on Nitrogen Metabolism-Associated Microbiome in Small MCPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020; pp. 21–22. [Google Scholar]
- Bagarinao, T. Systematics, distribution, genetics and life history of Milkfish Chanos chanos. Environ. Biol. Fish. 1994, 39, 25–41. [Google Scholar] [CrossRef]
- Martinez, F.S.; Tseng, M.C.; Yeh, S.P. Milkfish (Chanos chanos) culture: Situations and trends. J. Fish. Soc. Taiwan 2006, 33, 229–244. [Google Scholar]
- Pulkkinen, K.; Suomalainen, L.R.; Read, A.F.; Ebert, D.; Rintamäki, P.; Valtonen, E.T. Intensive fish farming and the evolution of pathogen virulence: The case of columnaris disease in Finland. Proc. Biol. Sci. 2010, 77, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, W.S.; Shaapan, R.M.; Mohamed, L.A.; Gayed, S.S.R. Recent biocontrol measures for fish bacterial diseases, in particular to probiotics, bio-encapsulated vaccines, and phage therapy. Open Vet. J. 2019, 9, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Alderman, D.J. Trends in therapy and prophylaxis 1991–2001. Bul. Eur. Assoc. Fish Pathol. 2002, 22, 117–125. [Google Scholar]
- Campbell, R.E.; Lilley, J.H.; Taukhid, V.P.; Kanchanakhan, S. In vitro screening of novel treatments for Aphanomyces invadans. Aquacult. Res. 2001, 32, 223–233. [Google Scholar] [CrossRef]
- Diggles, B.K. A mycosis of juvenile spiny rock lobster, Jasus edwardsii caused by Haliphthoros sp., and possible methods of chemical control. J. Fish Dis. 2001, 24, 99–110. [Google Scholar] [CrossRef]
- Diggles, B.K. Chemotherapy of the ciliate Trichodina sp. on juvenile turbot with notes on the susceptibility of fish with abnormal pigmentation. N. Z. J. Mar. Freshw. Res. 2000, 34, 645–652. [Google Scholar] [CrossRef]
- Madsen, H.C.K.; Buchmann, K.; Mellergaard, S. Treatment of trichodiniasis in eel (Anguilla anguilla) reared in recirculation systems in Denmark: Alternatives to formaldehyde. Aquaculture 2000, 186, 221–231. [Google Scholar] [CrossRef]
- Rodriguez, J.L.T.; Fernandez, M.T.S. Attempts at oral pharmacological treatment of Ichthyophthirius multifiliis in rainbow trout, Oncorhynchus mykiss. J. Fish Dis. 2001, 24, 249–252. [Google Scholar] [CrossRef]
- Tieman, D.M.; Goodwin, A.E. Treatments for itch infestations in channel catfish evaluated under static and flow-through water conditions. N. Am. J. Aquacult. 2001, 63, 293–299. [Google Scholar] [CrossRef]
- Flores, C.J.; Flores, C.R.; Ibarra, V.F.; Vera-Montenegro, Y.; Vasquez, P.C. Evaluation of chemotherapeutic drugs against cichlidogyriasis in tilapia fish (Oreochromis hornorum) in Mexico. Rev. Latinoam. Microbiol. 1995, 37, 179–187. [Google Scholar]
- Gouvello, R.; Pobel, T.; Richards, R.H.; Gould, C. Field efficacy of a ten days treatment of fumagillin against proliferative kidney disease in rainbow trout, Oncorhynchus mykiss. Aquaculture 1999, 171, 27–40. [Google Scholar] [CrossRef]
- Molnar, K. Effect of exposure to malachite green solution on common carp fry with Dactylogyrus vastator (monogenea) infection. Acta. Vet. Hung. 1995, 43, 277–286. [Google Scholar] [PubMed]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Mantovani, A. Health risk assessment of toxicologically relevant residues in emerging countries: A pilot study on Malachite Green residues in farmed freshwater fish of Armenia. Food Chem. Toxicol. 2020, 143, 111526. [Google Scholar] [CrossRef]
- Dubreil, E.; Mompelat, S.; Kromer, V.; Guitton, Y.; Danion, M.; Morin, T.; Hurtaud-Pessel, D.; Verdon, E. Dye residues in aquaculture products: Targeted and metabolomics mass spectrometric approaches to track their abuse. Food Chem. 2019, 294, 355–367. [Google Scholar] [CrossRef]
- Chu, Y.L.; Chimeddulam, D.; Sheen, L.Y.; Wu, K.Y. Probabilistic risk assessment of exposure to leucomalachite green residues from fish products. Food Chem. Toxicol. 2013, 62, 770–776. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, Z.; Li, D. Review of methods for the detection and determination of malachite green and leuco-malachite green in aquaculture. Crit. Rev. Anal. Chem. 2019, 49, 1–20. [Google Scholar] [CrossRef]
- Adel, M.; Dadar, M.; Oliveri, C.G. Antibiotics and malachite green residues in farmed rainbow trout (Oncorhynchus mykiss) from the Iranian markets: A risk assessment. Int. J. Food Prop. 2017, 20, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xia, S.; Han, X.; Fu, Z. Simultaneous Determination of Malachite Green, Chloramphenicols, Sulfonamides, and Fluoroquinolones Residues in Fish by Liquid Chromatography-Mass Spectrometry. J. Anal. Methods Chem. 2020, 2020, 3725618. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Fan, S.; Lien, C.-W.; Unnikrishnan, B.; Wang, Y.-S.; Chu, H.-W.; Huang, C.-C.; Hsu, P.-H.; Chang, H.-T. Graphene oxide membrane as an efficient extraction and ionization substrate for spray-mass spectrometric analysis of malachite green and its metabolite in fish samples. Anal. Chim. Acta. 2018, 1003, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J.C.; Rizzato, J.A.; Ferraz de Queiroz, J.; Reyes, F.G. Considerations on the use of malachite green in aquaculture and analytical aspects of determining the residues in fish: A review. J. Aquat. Food Prod. Technol. 2011, 20, 273–294. [Google Scholar] [CrossRef]
- Barani, A.; Tajik, H. Malachite green residue in farmed fish in north-west part of Iran. Int. J. Food Prop. 2017, 20, S580–S585. [Google Scholar] [CrossRef] [Green Version]
- Donya, S.M.; A Farghaly, A.; A Abo-Zeid, M.; Aly, H.F.; A Ali, S.; A Hamed, M.; El-Rigal, N.S. Malachite green induces genotoxic effect and biochemical disturbances in mice. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 469–482. [Google Scholar]
- Culp, S.J.; Mellick, P.W.; Trotter, R.W.; Greenlees, K.J.; Kodell, R.L.; Beland, F.A. Carcinogenicity of malachite green chloride and leucomalachite green in B6C3F1 mice and F344 rats. Food Chem. Toxicol. 2006, 44, 1204–1212. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Malachite Green Chloride and Leucomalachite Green. (CAS NOS. 569-64-2 and 129-73-7) in F344/N Rats and B6C3F1 Mice (Feed Studies); Technical Report Series (National Toxicology Program (U.S.)), no. 527; U.S. Dept. of Health and Human Services, National Toxicology Program: Washington, DC, USA, 2005; pp. 1–312. [Google Scholar]
- Yao, X.; Zhang, Y.; Zhang, L.; Zhou, Y. A bibliometric review of nitrogen research in eutrophic lakes and reservoirs. J. Environ. Sci. 2018, 66, 274–285. [Google Scholar] [CrossRef]
- Liang, Z.; Soranno, P.A.; Wagner, T. The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.H. Bacteria as biological control agents of freshwater cyanobacteria: Is it feasible beyond the laboratory? Appl. Microbiol. Biotechnol. 2018, 102, 9911–9923. [Google Scholar] [CrossRef]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.B.; Olinda, R.A.; Souza, B.A.; Cyrino, J.E.; Dias, C.T.; Queiroz, J.F.; Tavares, L.H. Efficiency of bioaugmentation in the removal of organic matter in aquaculture systems. Braz. J. Biol. 2011, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Maria Gabriela, P.F.; Fabiana, P.M.; João Paulo, V.L.; Humber, A.A.; William, S.; Eudes, S.C. Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res. 2017, 45, 167–176. [Google Scholar]
- Zhou, Q.; Li, K.; Jun, X.; Bo, L. Role and functions of beneficial microorganisms in sustainable aquaculture. Bioresour. Technol. 2009, 100, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.V.; Chang, Y.T.; Chao, W.L.; Yeh, S.L.; Kuo, D.L.; Yang, C.W. Effects of sulfamethoxazole and sulfamethoxazole-degrading bacteria on water quality and microbial communities in milkfish ponds. Environ. Pollut. 2019, 252, 305–316. [Google Scholar] [CrossRef]
- Song, J.; Han, G.; Wang, Y.; Jiang, X.; Zhao, D.; Li, M.; Yang, Z.; Ma, Q.; Parales, R.E.; Ruan, Z.; et al. Pathway and kinetics of malachite green biodegradation by Pseudomonas veronii. Sci. Rep. 2020, 10, 4502. [Google Scholar] [CrossRef]
- Tao, Y.; Meng, L.; Guo, Y.; Han, M.; Sun, C.; Wang, F.; Li, J. Biological Decolorization and Degradation of Malachite Green by Pseudomonas sp. YB2: Process Optimization and Biodegradation Pathway. Curr. Microbiol. 2017, 74, 1210–1215. [Google Scholar] [CrossRef]
- Vignesh, A.; Manigundan, K.; Santhoshkumar, J.; Shanmugasundaram, T.; Gopikrishnan, V.; Radhakrishnan, M.; Joseph, J.; Ayyasamy, P.M.; Kumar, G.D.; Meganathan, R.; et al. Microbial degradation, spectral analysis and toxicological assessment of malachite green by Streptomyces chrestomyceticus S20. Bioprocess Biosyst. Eng. 2020, 43, 1457–1468. [Google Scholar] [CrossRef]
- Wang, J.; Gao, F.; Liu, Z.; Qiao, M.; Niu, X.; Zhang, K.-Q.; Huang, X. Pathway and molecular mechanisms for malachite green biodegradation in Exiguobacterium sp. MG2. PLoS ONE 2012, 7, e51808. [Google Scholar] [CrossRef]
- Yang, C.W.; Chao, W.L.; Hsieh, C.Y.; Chang, B.V. Biodegradation of Malachite Green in Milkfish Pond Sediments. Sustainability 2019, 11, 4179. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.N.; Briones, A.; Diana, J.; Raskin, L. Ammonia-oxidizing archaea and nitrite-oxidizing nitrospiras in the biofilter of a shrimp recirculating aquaculture system. FEMS Microbiol. Ecol. 2013, 83, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van De Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Newell, S.E.; Davis, T.W.; Johengen, T.H.; Gossiaux, D.; Burtner, A.; Palladino, D.; McCarthy, M.J. Reduced forms of nitrogen are a driver of non-nitrogen-fixing harmful cyanobacterial blooms and toxicity in Lake Erie. Harmful Algae 2019, 81, 86–93. [Google Scholar] [CrossRef]
- Killberg-Thoreson, L.; Baer, S.E.; Sipler, R.E.; Reay, W.G.; Roberts, Q.N.; Bronk, D.A. Seasonal Nitrogen Uptake Dynamics and Harmful Algal Blooms in the York River, Virginia. Estuar. Coast. 2020. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Wu, X.; Yang, Y.; Xu, X.; Zheng, B.; Deng, W.; Shao, Z.; Lu, L.; Wang, L.; et al. A study on the relationship between metabolism of Cyanobacteria and chemical oxygen demand in Dianchi Lake, China. Water Environ. Res. 2019, 91, 1650–1660. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Xiao, X.; Liu, J.; Wu, C.; Chen, H.; Kerr, P.; Shurin, J. Seasonal changes in phosphorus competition and allelopathy of a benthic microbial assembly facilitate prevention of cyanobacterial blooms. Environ. Microbiol. 2017, 19, 2483–2494. [Google Scholar] [CrossRef]
- Koblížek, M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol. Rev. 2015, 39, 854–870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shu, M.; Wang, Y.; Fu, L.; Li, W.; Deng, B.; Liang, Q.; Shen, W. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J. Microbiol. Biotechnol. 2014, 30, 2523–2531. [Google Scholar] [CrossRef]
- Chang, B.V.; Liao, C.S.; Chang, Y.T.; Chao, W.L.; Yeh, S.L.; Kuo, D.L.; Yang, C.W. Investigation of a farm-scale multitrophic recirculating aquaculture system with the addition of Rhodovulum sulfidophilum for milkfish (Chanos chanos) coastal aquaculture. Sustainability 2019, 11, 1880. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Zhang, X.H.; Boon, N.; Bossier, P. Probiotics in aquaculture of China—Current state, problems and prospect. Aquaculture 2009, 290, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Kowaltowski, A.J.; Turin, J.; Indig, G.L.; Vercesi, A.E. Mitochondrial effects of triarylmethane dyes. J. Bioenerg. Biomembr. 1999, 31, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Glanville, S.D.; Clark, A.G. Inhibition of human glutathione S-transferases by basic triphenylmethane dyes. Life Sci. 1997, 60, 1535–1544. [Google Scholar] [CrossRef]
- Debnam, P.; Glanville, S.; Clark, A.G. Inhibition of glutathione S-transferases from rat liver by basic triphenylmethane dyes. Biochem. Pharmacol. 1993, 45, 1227–1233. [Google Scholar] [CrossRef]
- Panandiker, A.; Fernandes, C.; Rao, K.V. The cytotoxic properties of malachite green are associated with the increased demethylase, aryl hydrocarbon hydroxylase and lipid peroxidation in primary cultures of Syrian hamster embryo cells. Cancer Lett. 1992, 67, 93–101. [Google Scholar] [CrossRef]
- Panandiker, A.; Fernandes, C.; Rao, T.K.; Rao, K.V. Morphological transformation of Syrian hamster embryo cells in primary culture by malachite green correlates well with the evidence for formation of reactive free radicals. Cancer Lett. 1993, 74, 31–36. [Google Scholar] [CrossRef]
- Panandiker, A.; Maru, G.B.; Rao, K.V. Dose-response effects of malachite green on free radical formation, lipid peroxidation and DNA damage in Syrian hamster embryo cells and their modulation by antioxidants. Carcinogenesis 1994, 15, 2445–2448. [Google Scholar] [CrossRef]
- Culp, S.J.; Beland, F.A.; Heflich, R.H.; Benson, R.W.; Blankenship, L.R.; Webb, P.J.; Mellick, P.W.; Trotter, R.W.; Shelton, S.D.; Greenlees, K.J.; et al. Mutagenicity and carcinogenicity in relation to DNA adduct formation in rats fed leucomalachite green. Mutat. Res. 2002, 506–507, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.R.; Higson, S.L.; Scott, J.E. Methyl green and its analogues bind selectively to AT-rich regions of native DNA. Eur. J. Histochem. 1992, 36, 263–270. [Google Scholar]
- Müller, W.; Gautier, F. Interactions of heteroaromatic compounds with nucleic acids. A- T-specific non-intercalating DNA ligands. Eur. J. Biochem. 1975, 54, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.D. Influence of cationic triphenylmethane dyes upon DNA polymerization and product hydrolysis by Escherichia coli polymerase I. Biochemistry 1977, 16, 30–33. [Google Scholar] [CrossRef] [PubMed]







| Water (mg/L) | Sediment (mg/Kg) | |||||
|---|---|---|---|---|---|---|
| Day | Pond 1 | Pond 2 | Pond 3 | Pond 1 | Pond 2 | Pond 3 |
| 0 | ND | 1.95 ± 0.22 | 1.92 ± 0.31 | ND | 1.98 ± 0.42 | 1.90 ± 0.29 |
| 7 | ND | 1.58 ± 0.18 | 1.45 ± 0.29 | ND | 1.73 ± 0.26 | 1.52 ± 0.22 |
| 14 | ND | ND | ND | ND | 1.29 ± 0.32 | 1.08 ± 0.19 |
| 21 | ND | ND | ND | ND | 1.02 ± 0.12 | 0.91 ± 0.12 |
| 28 | ND | ND | ND | ND | 0.82 ± 0.10 | 0.75 ± 0.09 |
| 35 | ND | ND | ND | ND | 0.68 ± 0.09 | 0.42 ± 0.08 |
| 42 | ND | ND | ND | ND | 0.35 ± 0.06 | 0.12 ± 0.05 |
| 49 | ND | ND | ND | ND | 0.21 ± 0.08 | 0.07 ± 0.01 |
| 56 | ND | ND | ND | ND | 0.10 ± 0.05 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-W.; Chang, Y.-T.; Hsieh, C.-Y.; Chang, B.-V. Effects of Malachite Green on the Microbiomes of Milkfish Culture Ponds. Water 2021, 13, 411. https://doi.org/10.3390/w13040411
Yang C-W, Chang Y-T, Hsieh C-Y, Chang B-V. Effects of Malachite Green on the Microbiomes of Milkfish Culture Ponds. Water. 2021; 13(4):411. https://doi.org/10.3390/w13040411
Chicago/Turabian StyleYang, Chu-Wen, Yi-Tang Chang, Chi-Yen Hsieh, and Bea-Ven Chang. 2021. "Effects of Malachite Green on the Microbiomes of Milkfish Culture Ponds" Water 13, no. 4: 411. https://doi.org/10.3390/w13040411
APA StyleYang, C.-W., Chang, Y.-T., Hsieh, C.-Y., & Chang, B.-V. (2021). Effects of Malachite Green on the Microbiomes of Milkfish Culture Ponds. Water, 13(4), 411. https://doi.org/10.3390/w13040411