Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach
Abstract
:1. Introduction
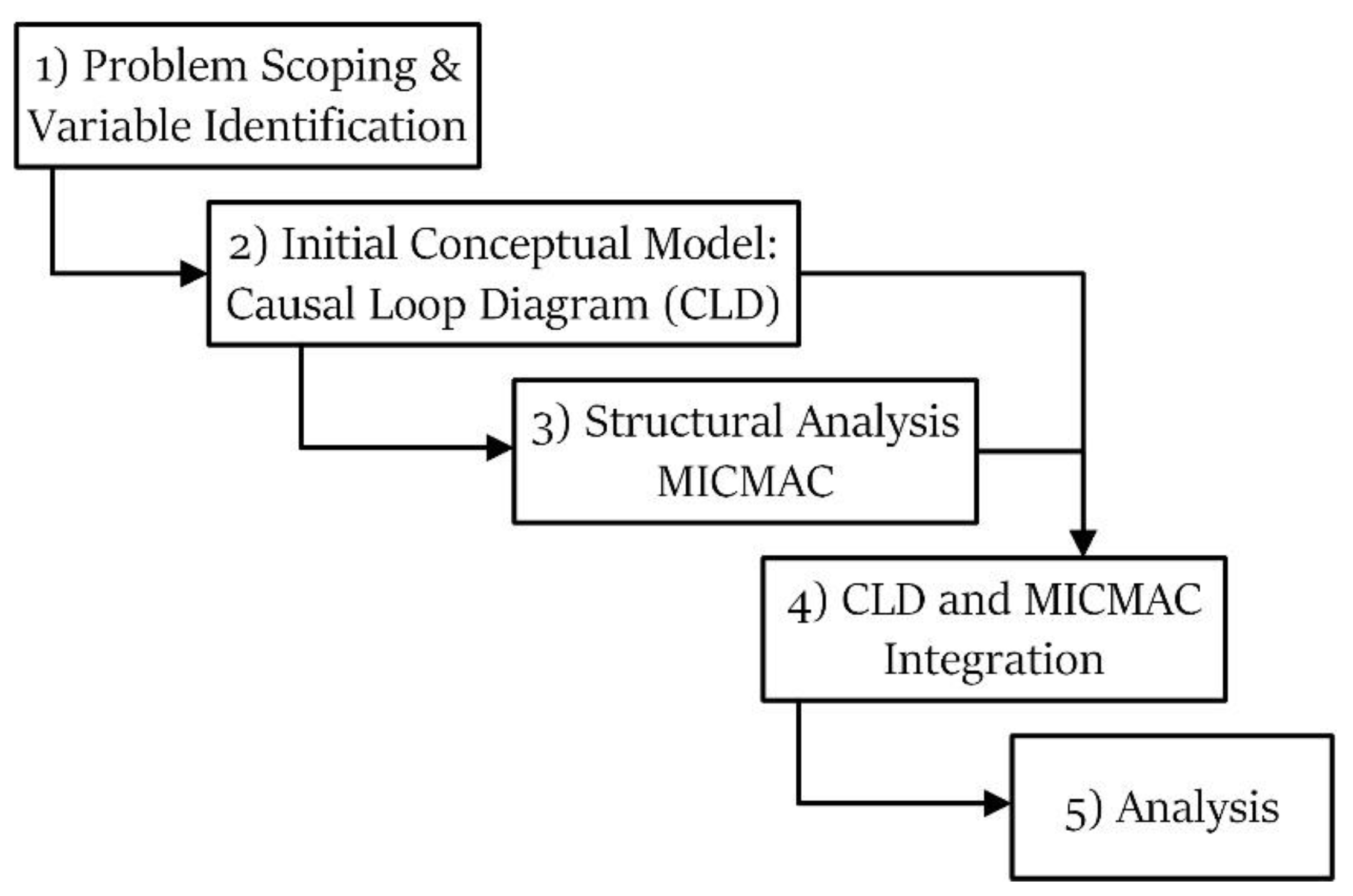
2. Method
2.1. Problem Scoping and Variable Identification
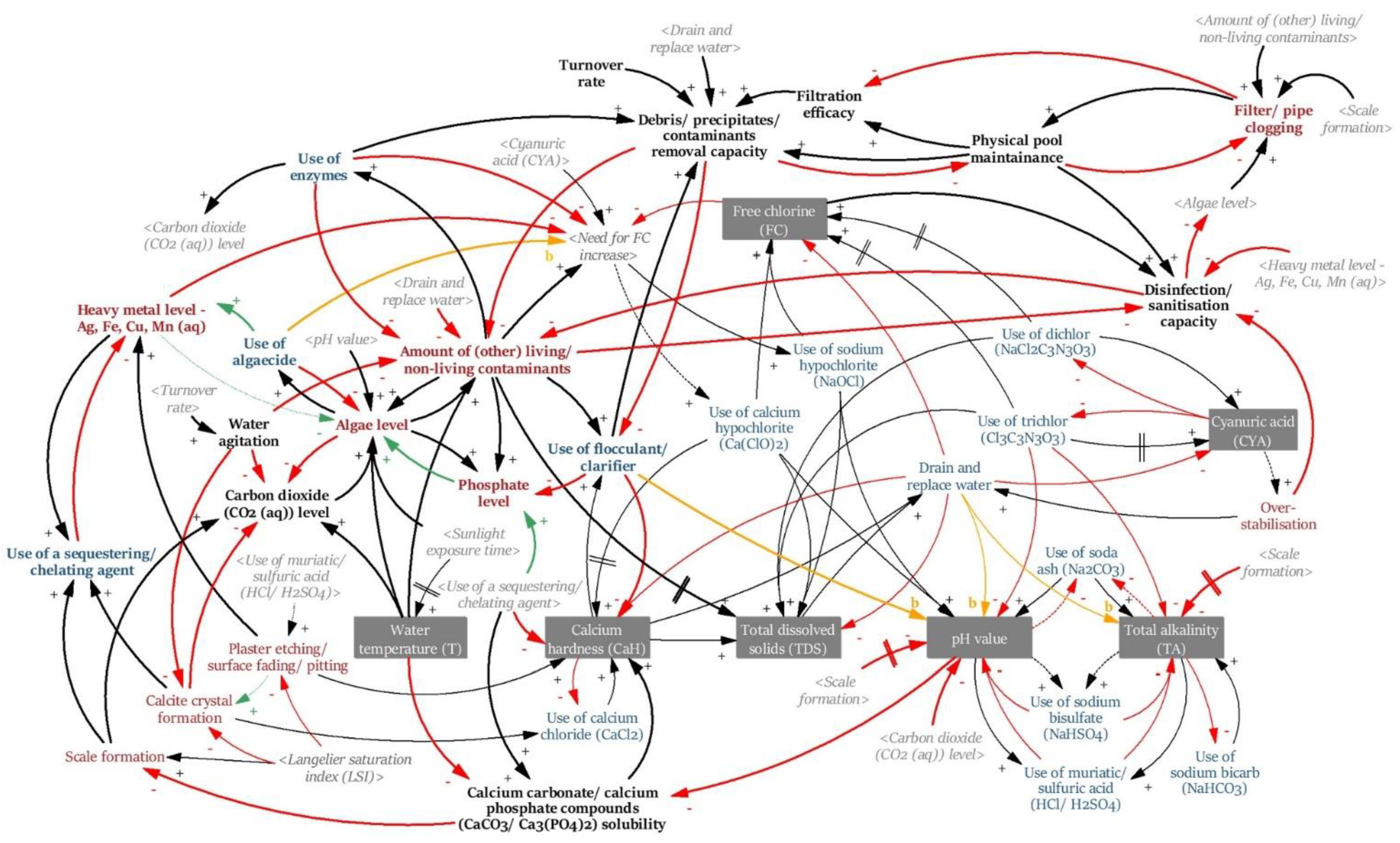
2.2. Initial Conceptual Model: Causal Loop Diagram (CLD)
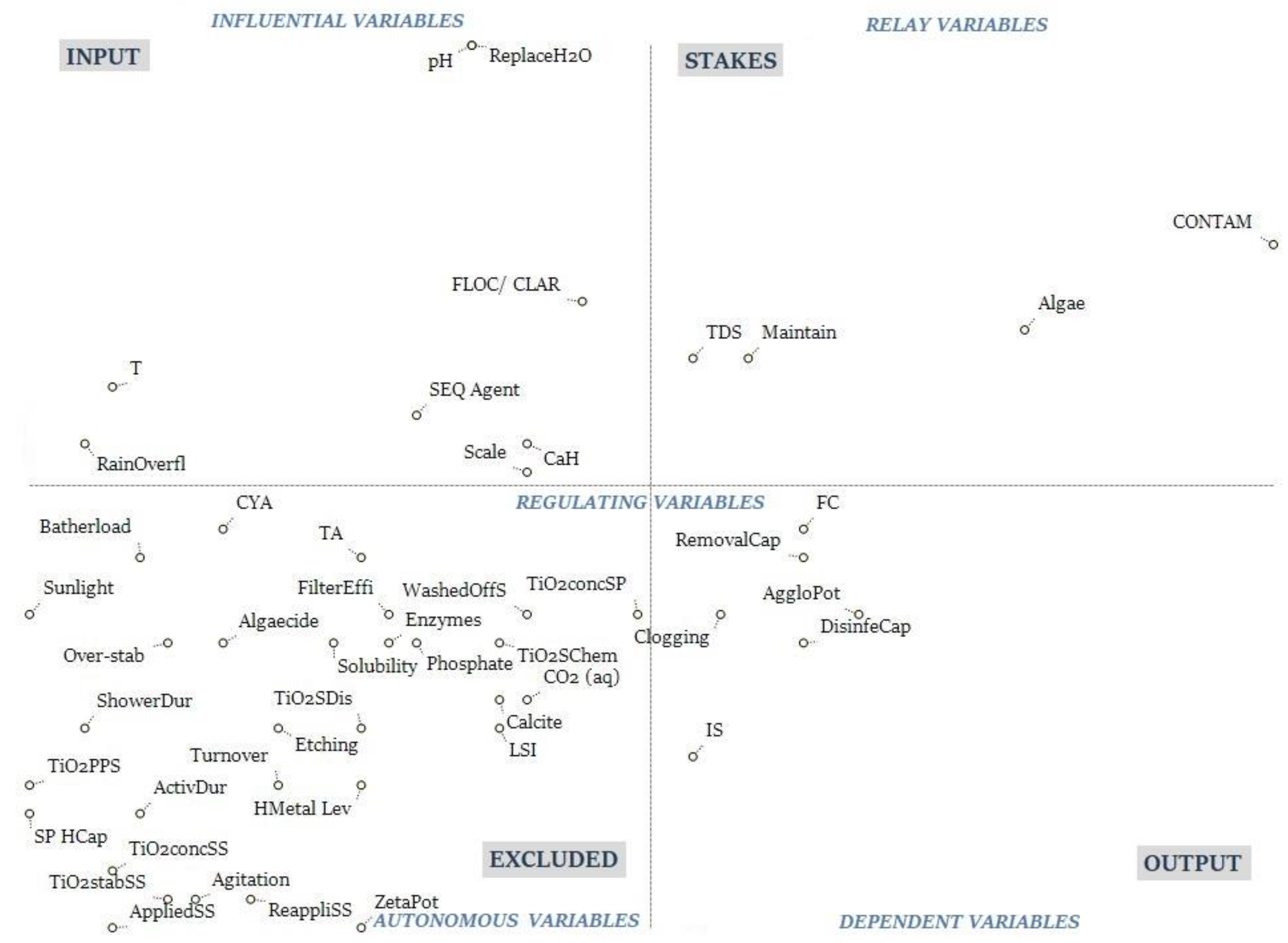
2.3. Structural Analysis (MICMAC)
2.4. CLD and MICMAC Integration
2.5. Analysis
3. Results
3.1. Problem Scoping and Variable Identification
3.2. Initial Conceptual Model: Causal Loop Diagram (CLD)

3.3. Structural Analysis (MICMAC)
3.4. CLD and MICMAC Integration
3.5. Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Variable Descriptions
| Variable (CLD) | Variable (MICMAC) | Variable Description | Reference | |
|---|---|---|---|---|
| Activity duration in swimming pool | ActivDur | The time swimming pool user (after applying sunscreen) spends in the pool for leisure activities. | ||
| Algae level | Algae | The algal density present in the SPW. Algae are microscopic plant-like organisms that contain chlorophyll and utilise photosynthesis to grow. Rain and wind introduce algae spores into the pool. Algae are typically not pathogenic but can provide an ideal substrate for bacteria. Algae control differs depending on the type of algae present in the SPW. | [9] | |
| Amount of (other) non-living/living contaminants | CONTAM | Includes environmental/human, inorganic/organic contaminants like body oils, sweat, urine, personal care products/cosmetics, dust, dirt, bacteria, and disinfection by-products. Algae and TiO2 are also SPW contaminants. However, their interactions are separately described in more detail. | [12] | |
| Amount of rain/overflow | RainOverfl | The amount of rain/excess water that overflows the sides of the pool. Assumption: rain/excess water does not contain TiO2. | ||
| Amount of sunscreen applied to skin | AppliedSS | The amount of sunscreen applied to the skin per person. Assumption: sunscreen contains TiO2. | ||
| Amount of washed-off sunscreen | WashedOffS | The amount of sunscreen residues (including other UV filters and sunscreen constituents like antioxidants, emollients) that are released into the SPW. | ||
| Bather load | Batherload | The maximum number of swimming pool users in the pool during a given amount of time. | ||
| Calcium hardness | CaH | The concentration of dissolved calcium (Ca2+) in the SPW, expressed in ppm. | ||
| Calcium carbonate/calcium phosphate compounds solubility | Solubility | The maximum amount of calcium carbonate (CaCO3)/calcium phosphate (Ca3(PO4)2) that will dissolve in a given amount of SPW at a specific temperature. The exact relationships are more complex as, for example, the carbonate/bicarbonate equilibrium (pH dependent) or interactions with other ions (e.g., Mg2+) in the pool have to be considered. | [63,88] | |
| Calcite crystal formation | Calcite | Calcite crystals (CaCO3) form when the SPW is unbalanced (LSI < 0.3) mostly due to a lack of dissolved calcium (Ca2+). Therefore, to increase the concentration of dissolved calcium (Ca2+), the SPW extracts calcium from the pool plaster. | [89] | |
| Carbon dioxide (CO2 (aq)) level | CO2 (aq) | Dissolved carbon dioxide (CO2 (aq)) is in equilibrium with carbonic acid (H2CO3). As H2CO3 is slightly acidic, the more CO2 (aq), the lower the pH. H2CO3 rapidly dissociates to hydrogen (H+), bicarbonate (HCO3−), and carbonate (CO32−) ions. This combination of alkaline (HCO3−, CO32−) and acidic (H+) substances helps to control the pH in the SPW (see TA). In addition, due to the equilibrium of dissolved CO2 (aq) and atmospheric CO2 (g), CO2 will naturally outgas, and hence, cause the pH to rise to approximately 8.2.CO2 will leave the SPW until the equilibrium of dissolved CO2 (aq) and atmospheric CO2 (g) is reached. This causes the pH to rise naturally to approximately 8.2. Similarly, the pH will not rise above 8.2 as the atmospheric pressure will force CO2 back into solution (Henry’s Law). | [90] | |
| Cyanuric acid (Cyanurate correction factor) | CYA |  | The cyanuric acid (CYA) concentration in the SPW, expressed in ppm. CYA reduces the photochemical degradation of free chlorine by UV rays. However, using CYA impairs the oxidation capacity of free chlorine by reducing the oxidation-reduction potential (ORP). Also, as CYA forms alkaline ionisation products, the cyanurate alkalinity contributes to the total alkalinity, thus increasing the buffering capacity of SPW. | [63,70,91,92] |
| Debris/precipitates/contaminants removal capacity | RemovalCap | The ability to remove debris/precipitates/contaminants from the SPW. Their nature can be of organic or inorganic particulate matter. | ||
| Disinfection/sanitisation capacity | DisinfeCap | The ability of a disinfectant to disinfect/sanitise the SPW. Primarily, disinfectants reduce the number of harmful microorganisms, but they can also oxidise, for example, bather waste. | ||
| Drain and replace water | ReplaceH2O | Drain and replace a specified volume of SPW with fresh tap/well/rainwater. | ||
| Filter efficacy | FilterEffi | The ability of the chosen filtration system (cartridge/sand filters) to remove suspended particulate matter from the SPW. The filter medium, the water flow rate per unit area, regular filter cleaning (i.e., hosing down, soaking in a clean fluid for cartridge filters/backwashing for sand filters), the pore size (cartridge filter)/grade of filter sand size, single or multi-grade beds (sand filter) are key determinants. | [10,12,13] | |
| Filter/pipe clogging | Clogging | The degree to which the filter media/pipe is clogged through excessive use of flocculants/coagulants, scale/debris build-up. This impairs the water flow rate and filter efficacy. | ||
| Free (active) chlorine | FC |  | Free (active) chlorine (FC) is a form of chlorine that can act as a sanitiser (and oxidiser) and can be added in various forms to the SPW, for example, as sodium or calcium hypochlorite—see equation (1a) and (1b), respectively. All forms produce HOCl (hypochlorous acid) in water, which is the most desirable, active form of FC. The pH predominantly determines the degree of dissociation of HOCl to H+ (hydrogen ion) and OCl¯ (hypochlorite ion, a less effective form of FC) in the SPW, see equation (2). | [10,73] |
| Heavy metal level | HMetal Lev | The heavy metal level corresponds to the concentration of dissolved heavy metals such as silver (Ag+), iron (Fe2+), copper (Cu2+), and manganese (Mn2+) in the SPW. In high, non-chelated concentrations, these metals can be easily oxidised, for example, by disinfectant products, which can lead to metal stains or turn the colour of the water. | [10] | |
| Heteroagglomeration potential | AggloPot | The heteroagglomeration potential is the likelihood of individual, separated suspended particulate matter to form assemblages. This mechanism can often be induced by adding salts or other chemicals such as coagulants/flocculants. | ||
| Ionic strength | IS | The ionic strength represents the concentration of all ions present in the SPW and is equal to half of the sum of each ion’s molar concentration multiplied by their valence squared. | ||
| Langelier saturation index | LSI | The Langelier Saturation Index (LSI) is a measure to indicate the SPW balance. If perfectly balanced, the LSI is zero. Undersaturated SPW (low LSI) will seek to dissolve calcium from, for example, the pool plaster surface, whereas oversaturated SPW (high LSI) will deposit any form of calcium carbonate (CaCO3) into the SPW to reduce the amount of dissolved calcium (Ca2+). The LSI can be calculated as LSI = pH + T + CaH + [TA − (CYA correction factor @ pH)] − TDS. | [62] | |
| pH value | pH | Abbreviation for “potential or power of hydrogen”. The pH is defined as the negative decadic logarithm of the hydrogen ion activity. The pH value indicates the basicity or acidity of water on a scale from zero (the most acidic) to 14 (the most basic). | [93,94] | |
| Over-stabilisation | Over-stab | Over-stabilisation is the build-up of cyanuric acid (CYA) in SPW that results from the use of chlorinated isocyanurates. As a result, the effectiveness of chlorine in killing pathogens is significantly impaired. | [95] | |
| Phosphate level | Phosphate | The phosphate level comprises various types of organic and inorganic phosphorus compounds. However, most phosphorous compounds will eventually break down and convert to orthophosphates (PO43−), which serve as nutrient for algae. Test kits usually only test for PO43− as most abundant phosphate type in the SPW. | [96,97,98] | |
| Physical pool maintenance | Maintain | The physical pool maintenance includes activities such as cleaning (skimming, scrubbing, vacuuming) pool surfaces/filter/pipes as per the manufacturer’s guidelines. | ||
| Plaster etching/surface fading/pitting | Etching | Plaster etching/surface fading/pitting is generally caused by unbalanced SPW or wrong usage of acid products, which results in irreversible damage to the pool. | ||
| Reapplication frequency/day | ReappliSS | The number of sunscreen reapplications per day per person. | ||
| Scale formation | Scale | Scale formation refers to three types of calcium scale deposition resulting from chemically unbalanced SPW, namely calcium carbonate (CaCO3)—and sometimes calcium phosphate (Ca3(PO4)2) or calcium sulfate (CaSO4). | ||
| Shower duration before pool use | ShowerDur | The duration of the shower taken by the pool user before using the pool. Assumption: sunscreen applied by the pool users is partially washed off during the shower. | ||
| Sunlight exposure time | Sunlight | This variable describes how long the SPW is exposed to full sunlight. | ||
| Swimming pool holding capacity | SP HCap | The maximum water holding capacity in a SP. | ||
| TiO2 concentration in applied sunscreen | TiO2concSS | The TiO2 concentration in the sunscreen that is applied to the user’s skin. | ||
| TiO2 particle surface chemistry | TiO2SChem | The TiO2 particle surface can either be hydrophilic or hydrophobic depending on the sunscreen type (oil-in-water or water-in-oil). | ||
| TiO2 particle surface (coating) dissolution | TiO2SDis | The percentage of the depletion/dissolution of the TiO2 particle surface (coating); e.g., aluminium oxide (hydroxide) Al2O3/Al2(OH)3 dissolution caused by chlorine. | [83,84] | |
| TiO2 primary particle size | TiO2PPS | The primary particle size of TiO2 particles used in the sunscreen. | ||
| TiO2 stability (chemical and physical) in sunscreen formulation | TiO2stabSS | The TiO2 particle stability to maintain dispersion in the sunscreen formulation. | ||
| Total alkalinity | TA | The total alkalinity (aka buffering capacity) is the concentration of dissolved alkaline substances, e.g., hydroxide (OH−), carbonate (CO32−), bicarbonate (HCO3−) ions in the SPW. | ||
| Total dissolved solids | TDS | Total dissolved solids are the total amount of dissolved matter, including salts, minerals, metals, and contaminants in the SPW. | [99] | |
| Total TiO2 concentration in SPW | TiO2concSP | The total particulate TiO2 concentration in SPW. | ||
| Turnover rate | Turnover | The turnover rate refers to the time during which a net volume of SPW passes through the filtration system. | [10] | |
| Use of algaecide | Algaecide | The use of an algaecide refers to the use of natural/synthetic substances used for killing and controlling algae. | ||
| Use of enzymes | Enzymes | The use of enzymes refers to proteins that accelerate the chemical reactions of other substances (e.g., the breakdown of oil or other non-living contaminants) without being used up or altered. | ||
| Use of flocculant/clarifier | FLOC/CLAR | The use of a flocculant or clarifier enhances the agglomeration of suspended particulate matter in the SPW. | ||
| Use of sequestering/chelating agent | SEQ Agent | The use of a sequestering or chelating agent refers to the use of chemicals that control the formation of scale or stains by preventing the precipitations of metal ions. | ||
| Water agitation | Agitation | Water agitation refers to promoting gas exchange, which increases the rate at which oxygen (O2) dissolves in but also carbon dioxide (CO2) releases from SPW. This can be done purposefully through fountains and springs etc. or caused by the movement of pool users. | ||
| Water temperature | T | The temperature of the SPW. | ||
| Zeta potential | ZetaPot | The zeta potential refers to the electrical potential at the slipping plane, which separates the mobile fluid from the fluid attached to the particle surface. This is an important factor to indicate the stability of a colloidal dispersion. | [100] | |
Appendix B. MICMAC
| 0 = No influence 1 = Weak influence 2 = Moderate influence 3 = Strong influence | 1: ActivDur | 2: AppliedSS | 3: Batherload | 4: ReappliSS | 5: ShowerDur | 6: CaH | 7: CO2 (aq) | 8: CYA | 9: FC | 10: LSI | 11: pH | 12: TA | 13: TDS | 14: T | 15: Solubility | 16: AggloPot | 17: IS | 18: ZetaPot | 19: RainOverfl | 20: Sunlight | 21: RemovalCap | 22: DisinfeCap | 23: FilterEffi | 24: Maintain | 25: SP HCap | 26: Turnover | 27: Agitation | 28: TiO2concSS | 29: TiO2SDis | 30: TiO2SChem | 31: TiO2PPS | 32: TiO2stabSS | 33: ReplaceH2O | 34: SEQ Agent | 35: Algaecide | 36: Enzymes | 37: FLOC/CLAR | 38: Algae | 39: CONTAM | 40: WashedOffS | 41: Calcite | 42: Clogging | 43: HMetal Lev | 44: Over-stab | 45: Phosphate | 46: Etching | 47: Scale | 48: TiO2concSP |
| 1: Activity duration in swimming pool | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2: Amount of sunscreen applied to skin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3: Bather load | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 4: Reapplication frequency/day | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5: Shower duration before pool use | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6: Calcium hardness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | |
| 7: Carbon dioxide (CO2) level in water | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8: Cyanuric acid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | |
| 9: Free (active) chlorine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10: Langelier saturation index | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | |
| 11: pH value | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 3 | 2 | 0 | 0 | 3 | 1 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | 2 | 0 | |
| 12: Total alkalinity | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | |
| 13: Total dissolved solids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | |
| 14: Water temperature | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 15: Calcium carbonate/calcium phosphate compounds solubility | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | |
| 16: Heteroagglomeration potential | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 17: Ionic strength | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 18: Zeta Potential | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 19: Amount of rain/overflow | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | |
| 20: Sunlight exposure time | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 21: Debris/precipitates/contaminants removal capacity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | |
| 22: Disinfection/sanitisation capacity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 23: Filtration efficacy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 24: Physical pool maintenance | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | |
| 25: Swimming pool holding capacity | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 26: Turnover rate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 27: Water agitation | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 28: TiO2 concentration in applied sunscreen | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| 29: TiO2 particle surface (coating) dissolution | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 30: TiO2 particle surface chemistry | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 31: TiO2 primary particle size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 32: TiO2 stability (chemical and physical) in sunscreen formulation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 33: Drain and replace water | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 3 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 3 | |
| 34: Use of a sequestering/chelating agent | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | |
| 35: Use of algaecide | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| 36: Use of enzymes | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 37: Use of flocculant/clarifier | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | |
| 38: Algae level | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | |
| 39: Amount of (other) living/non-living contaminants | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | |
| 40: Amount of washed-off sunscreen | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | |
| 41: Calcite crystal formation | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 42: Filter/pipe clogging | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 43: Heavy metal level | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 44: Over-stabilisation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 45: Phosphate level | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 46: Plaster etching/surface fading/pitting | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | |
| 47: Scale formation | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | |
| 48: Total TiO2 concentration in SPW | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |

| Category | Influence Rank | Dependence Rank | ||
|---|---|---|---|---|
| Direct | Indirect | Direct | Indirect | |
| Essential SPW chemistry (monitoring parameters) |
|
|
|
|
| Supplementary SPW chemistry (chemicals) |
|
|
|
|
| SP(W) conditions |
|
|
|
|
| Essential SPW property |
|
|
|
|
| Operating parameter |
|
|
|
|
| Monitoring parameter (external) |
|
|
|
|
| Bathers’ behaviour |
|
|
|
|
| Sunscreen formulation/TiO2 particle property |
|
|
|
|
References
- Rakicevic, M. 25 Shocking Swimming Pool Statistics You Need to Know. 2022. Available online: https://comfyliving.net/swimming-pool-statistics/ (accessed on 11 April 2022).
- Morgan, R. Swimming Pool Ownership Increases in Australia. 2018. Available online: http://www.roymorgan.com/findings/7811-australian-swimming-pool-ownership-september-2018-201811230555 (accessed on 27 May 2020).
- Goodwin, D. Where is Swimming Popular? 2019. Available online: https://aquamobileswim.com/blog/where-is-swimming-popular/ (accessed on 11 April 2022).
- Wood, L. Global Swimming Pool Market (2020 to 2025)-Growth, Trends and Forecasts. 2020. Available online: https://www.businesswire.com/news/home/20200416005518/en/Global-Swimming-Pool-Market-2020-to-2025---Growth-Trends-and-Forecasts---ResearchAndMarkets.com (accessed on 11 April 2022).
- Faus, J.A. Timothy. Pool Sales Skyrocket as Consumers Splash Out on Coronavirus Cocoons. 2020. Available online: https://www.reuters.com/article/us-health-coronavirus-pools-idUSKCN2520HW (accessed on 11 April 2022).
- Chomicki, C. Demand for Backyard Pools and Spas Sees Some Waiting until Next Christmas. 2021. Available online: https://www.abc.net.au/news/2021-10-30/demand-for-pools-and-spas-never-higher/100581322 (accessed on 13 April 2022).
- Analytics, C. CAPE Data Finds Pandemic-Driven, 533% Surge in New Backyard Swimming Pools. 2020. Available online: https://capeanalytics.com/blog/cape-new-swimming-pool-installations/ (accessed on 11 April 2022).
- Europe, P.G. 2020, a Big Year for Private Pools Worldwide. 2021. Available online: https://www.piscine-global-europe.com/en/blog/2021/06/2020-big-year-private-pools-worldwide (accessed on 11 April 2022).
- South Australia. Department for Health and Ageing. Guideline for the Inspection and Maintenance of Swimming Pools and Spa Pools; Department for Health and Ageing: Adelaide, Australia, 2013.
- Puetz, J.D. Swimming Pool Water Chemistry. The Care and Treatment of Swimming Pool Water; Arch Chemicals, Inc., Now a Part of Lonza: Norwalk, CT, USA, 2013. [Google Scholar]
- Victoria State Government. Water Quality Guidelines for Public Aquatic Facilities. Managing Public Health Risks; Department of Health and Human Services: Melbourne, VIC, Australia, 2019.
- Environmental Health Unit Rural and Regional Health and Aged Care Services Division. Pool Operators’ Handbook; Department of Human Services: Melbourne, VIC, Australia, 2008.
- World Health Organization; Water, S.H. Team. Guidelines for Safe Recreational Water Environments. Volume 2, Swimming Pools and Similar Environments; World Health Organization: Geneva, Switherland, 2006. [Google Scholar]
- Bonadonna, L.; Giuseppina, L.R. A Review and Update on Waterborne Viral Diseases Associated with Swimming Pools. Int. J. Environ. Res. Public Health 2019, 16, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinnola, O.O.; Ajayi, A.S.; Ogunleye, B.O.; Enueme, I.N. Chapter 10—Disinfection By-Products in Swimming Pools and Health-Related Issues. In Disinfection By-Products in Drinking Water; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 235–252. [Google Scholar]
- De Laat, J.; Feng, W.; Freyfer, D.A.; Dossier-Berne, F. Concentration Levels of Urea in Swimming Pool Water and Reactivity of Chlorine with Urea. Water Res. 2011, 45, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, X.; She, Q.; Cao, G.; Liu, Y.; Chang, V.W.C.; Tang, C.Y. Regulation, Formation, Exposure, and Treatment of Disinfection By-Products (DBPs) in Swimming Pool Waters: A Critical Review. Environ. Int. 2018, 121, 1039–1057. [Google Scholar] [CrossRef]
- Carter, R.A.A.; Joll, C.A. Occurrence and Formation of Disinfection By-Products in the Swimming Pool Environment: A Critical Review. J. Environ. Sci. 2017, 58, 19–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwiener, C.; Richardson, S.D.; DeMarini, D.M.; Grummt, T.; Glauner, T.; Frimmel, F.H. Drowning in Disinfection Byproducts? Assessing Swimming Pool Water. Environ. Sci. Technol. 2007, 41, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.L.; Coleman, H.M.; Khan, S.J. Chemical Contaminants in Swimming Pools: Occurrence, Implications and Control. Environ Int. 2015, 76, 16–31. [Google Scholar] [CrossRef]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection Byproducts in Swimming Pool: Occurrences, Implications and Future Needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef]
- Richardson Susan, D.; DeMarini David, M.; Kogevinas, M.; Fernandez, P.; Marco, E.; Lourencetti, C.; Villanueva Cristina, M. What’s in the Pool? A Comprehensive Identification of Disinfection By-products and Assessment of Mutagenicity of Chlorinated and Brominated Swimming Pool Water. Environ. Health Perspect. 2010, 118, 1523–1530. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Mao, H.; Li, H.; Wang, Q.; Yang, Z. Occurrence of and Human Exposure to Parabens, Benzophenones, Benzotriazoles, Triclosan and Triclocarban in Outdoor Swimming Pool Water in Changsha, China. Sci. Total Environ. 2017, 605–606, 1064–1069. [Google Scholar] [CrossRef]
- Weng, S.; Sun, P.; Ben, W.; Huang, C.-H.; Lee, L.T.; Blatchley, E.R., III. The Presence of Pharmaceuticals and Personal Care Products in Swimming Pools. Environ. Sci. Technol. Lett. 2014, 1, 495–498. [Google Scholar] [CrossRef]
- Vila, M.; Celeiro, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Simultaneous In-Vial Acetylation Solid-Phase Microextraction Followed by Gas Chromatography Tandem Mass Spectrometry for the Analysis of Multiclass Organic UV Filters in Water. J. Hazard. Mater. 2017, 323, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, D.R.; Motabar, D.; Quinones, O.; Stanford, B.; Vanderford, B.; Moss, D. Titanium Distribution in Swimming Pool Water is Dominated by Dissolved Species. Environ. Pollut. 2013, 181, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.K.; Reed, R.B.; Lee, S.; Bi, X.; Hanigan, D.; Yang, Y.; Westerhoff, P. Detection and Sizing of Ti-Containing Particles in Recreational Waters Using Single Particle ICP-MS. Bull. Environ. Contam. Toxicol. 2018, 100, 120–126. [Google Scholar] [CrossRef]
- Hadioui, M.; Knapp, G.; Azimzada, A.; Jreije, I.; Frechette-Viens, L.; Wilkinson, K.J. Lowering the Size Detection Limits of Ag and TiO2 Nanoparticles by Single Particle ICP-MS. Anal. Chem. 2019, 91, 13275–13284. [Google Scholar] [CrossRef]
- Trivedi, M.; Murase, J. Titanium Dioxide in Sunscreen; IntechOpen: Rijeka, Croatia, 2017; Volume 26, pp. 61–71. [Google Scholar]
- Chaiyabutr, C.; Sukakul, T.; Kumpangsin, T.; Bunyavaree, M.; Charoenpipatsin, N.; Wongdama, S.; Boonchai, W. Ultraviolet Filters in Sunscreens and Cosmetic Products—A Market Survey. Contact Dermat. 2021, 85, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials; ACS Publications: Washington, DC, USA, 2014; pp. 9281–9282. [Google Scholar]
- EFSA. Titanium Dioxide: E171 No Longer Considered Safe When Used as a Food Additive. 2021. Available online: https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive (accessed on 12 April 2022).
- Bernauer, U.; Bodin, L.; Chaudhry, Q.; Coenraads, P.; Dusinska, M.; Ezendam, J.; Panteri, E. SCCS OPINION on Titanium Dioxide (TiO2) Used in Cosmetic Products That Lead to Exposure by Inhalation-SCCS/1617/20, Final Opinion; European Commission: Luxembourg, 2020.
- Rashid, M.M.; Forte Tavčer, P.; Tomšič, B. Influence of Titanium Dioxide Nanoparticles on Human Health and the Environment. Nanomaterials 2021, 11, 2354. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Reed, R.B.; Martin, D.P.; Bednar, A.J.; Montano, M.D.; Westerhoff, P.; Ranville, J.F. Multi-Day Diurnal Measurements of Ti-containing Nanoparticle and Organic Sunscreen Chemical Release During Recreational Use of a Natural Surface Water. Environ. Sci.-Nano 2017, 4, 69–77. [Google Scholar] [CrossRef]
- Gondikas, A.; von der Kammer, F.; Kaegi, R.; Borovinskaya, O.; Neubauer, E.; Navratilova, J.; Hofmann, T. Where Is the Nano? Analytical Approaches for the Detection and Quantification of TiO2 Engineered Nanoparticles in Surface Waters. Environ. Sci.-Nano 2018, 5, 313–326. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Milani, N.B.L.; den Hertog, G.C.T.; Undas, A.K.; van der Lee, M.; Bouwmeester, H. Detection of Nanoparticles in Dutch Surface Waters. Sci. Total Environ. 2018, 621, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Rand, L.N.; Bi, Y.; Poustie, A.; Bednar, A.J.; Hanigan, D.J.; Westerhoff, P.; Ranville, J.F. Quantifying Temporal and Geographic Variation in Sunscreen and Mineralogic Titanium-Containing Nanoparticles in Three Recreational Rivers. Sci. Total Environ. 2020, 743, 140845. [Google Scholar] [CrossRef]
- Bland, G.D.; Battifarano, M.; Pradas del Real, A.E.; Sarret, G.; Lowry, G.V. Distinguishing Engineered TiO2 Nanomaterials from Natural Ti Nanomaterials in Soil Using spICP-TOFMS and Machine Learning. Environ. Sci. Technol. 2022, 56, 2990–3001. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Bertone, E.; Beal, C.D. A Systems Approach for Assessing Water Conservation Potential Through Demand-Based Water Tariffs. J. Clean. Prod. 2017, 148, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Sahin, O.; Stewart, R.A.; Giurco, D.; Porter, M.G. Renewable Hydropower Generation as a Co-Benefit of Balanced Urban Water Portfolio Management and Flood Risk Mitigation. Renew. Sustain. Energy Rev. 2017, 68, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Meadows, D.H. Thinking in Systems: A Primer; Earthscan: London, UK; Sterling, VA, USA, 2009. [Google Scholar]
- Senge, P.M. The Fifth Discipline; Measuring Business Excellence; Emerald Publishing Limited (Previously MCB UP Ltd.): Bradford, UK, 1997. [Google Scholar]
- Arcade, J.; Godet, M.; Meunier, F.; Roubelat, F. Structural Analysis with the MICMAC Method & Actor’s Strategy with MACTOR Method. In The Millennium Project 1999: Futures Research Methodology; American Council for the United Nations University: Washington, DC, USA, 2010. [Google Scholar]
- Godet, M. Creating Futures: Scenario Planning as a Strategic Management Tool; Economica Brookings diffusion; Economica: Washington, DC, USA, 2006; 280p. [Google Scholar]
- Sterman, J. Business Dynamics; McGraw-Hill, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Voinov, A. Systems Science and Modeling for Ecological Economics, 1st ed.; Elsevier Academic Press: London, UK, 2008. [Google Scholar]
- Videira, N.; Schneider, F.; Sekulova, F.; Kallis, G. Improving Understanding on Degrowth Pathways: An Exploratory Study Using Collaborative Causal Models. Futures 2014, 55, 58–77. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M. Systems Thinking: What, Why, When, Where, and How. Syst. Think. 1997, 8, 6–7. [Google Scholar]
- Kim, D.H. Systems Thinking Tools: A User’s Reference Guide; Pegasus Communications, Inc.: Waltham, MA, USA, 1995. [Google Scholar]
- Monat, J.P.; Gannon, T.F. What is Systems Thinking? A Review of Selected Literature Plus Recommendations. Am. J. Syst. Sci. 2015, 4, 11–26. [Google Scholar]
- Kirkwood, C.W. System Dynamics Methods; College of Business Arizona State University USA: Tempe, AZ, USA, 1998. [Google Scholar]
- Kim, D.H. Introduction to Systems Thinking; Pegasus Communications: Waltham, MA, USA, 1999; Volume 16. [Google Scholar]
- Currie, D. Taking the ‘Poo’out of ‘Pool’: Participatory Systems Modelling as a Decision-Support Tool for Even the Messiest Public Environmental Health Problems; The Univeristy of Queensland: Brisbane, Australia, 2019. [Google Scholar]
- Suprun, E.; Sahin, O.; Stewart, R.A.; Panuwatwanich, K. Model of the Russian Federation Construction Innovation System: An Integrated Participatory Systems Approach. Systems 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-Y.; Chiu, S.-I.; Yen, T.-M. Modified IPA for Order-Winner Criteria Improvement: A MICMAC Approach. J. Appl. Sci. 2009, 9, 3792–3803. [Google Scholar] [CrossRef] [Green Version]
- La Prospective. Micmac. Structural Analysis. 2010. Available online: http://en.laprospective.fr/methods-of-prospective/softwares/59-micmac.html (accessed on 18 April 2022).
- Godet, M. Micmac. Software V 6.1.2; Lipsor: Paris, France, 2004. [Google Scholar]
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding Nano: Challenges Involved in Monitoring the Presence and Fate of Engineered Titanium Dioxide Nanoparticles in Aquatic Environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Orenda. Understanding LSI: The Langelier Saturation Index. Orenda. 2022. Available online: https://blog.orendatech.com/langelier-saturation-index (accessed on 18 April 2022).
- Salter, C.; Langhus, D.L. The Chemistry of Swimming Pool Maintenance. J. Chem. Educ. 2007, 84, 1124. [Google Scholar] [CrossRef]
- De La Matter, D. Swimming Pool Chemistry. Available online: http://www.dougdelamatter.com/website1/science/chemistry/pool/pool1.pdf (accessed on 28 April 2022).
- Queensland Studies Authority. Extended Experimental Investigation: The Effect of Sunlight on the Chlorine Levels in Pools. Queensland Studies Authority. 2013. Available online: https://www.qcaa.qld.edu.au/downloads/senior/snr_chemistry_07_ass_chlorine.pdf (accessed on 28 April 2022).
- U.S. Department of Health and Human Services-Centers for Disease Control and Prevention. Fecal Incident Response Recommendations for Aquatic Staff. Healthy Swimming. 2018. Available online: https://www.cdc.gov/healthywater/swimming/pdf/fecal-incident-response-guidelines.pdf (accessed on 28 April 2022).
- Orenda. The Cyanuric Acid Limit for Public Swimming Pools. Orenda. 2020. Available online: https://blog.orendatech.com/cyanuric-acid (accessed on 28 April 2022).
- Falk, R.A. Chlorine/Cyanuric Acid Relationship and Implications for Nitrogen Trichloride. Available online: https://standards.nsf.org/apps/group_public/download.php/5891/Chlorine-CYA.pdf (accessed on 28 April 2022).
- Falk, R.A.; Blatchley, E.R.; Kuechler, T.C.; Meyer, E.M.; Pickens, S.R.; Suppes, L.M. Assessing the Impact of Cyanuric Acid on Bather’s Risk of Gastrointestinal Illness at Swimming Pools. Water 2019, 11, 1314. [Google Scholar] [CrossRef] [Green Version]
- Williams, K. Cyanurics-Benefactor or Bomb? 1997. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.175.9147&rep=rep1&type=pdf (accessed on 22 April 2022).
- Meyer, E. Over-Stabilization Explained. 2020. Available online: https://blog.orendatech.com/experts-blog/avoid-over-stabilization (accessed on 28 April 2022).
- Orenda. How to Reduce Cyanuric Acid (CYA) in a Swimming Pool. Orenda. 2020. Available online: https://blog.orendatech.com/how-to-reduce-cyanuric-acid-cya-in-a-swimming-pool (accessed on 28 April 2022).
- Missouri Department of Health and Senior Services Section for Environmental Public Health. Swimming Pool and Spa Water Chemistry. Available online: https://www.nitt.edu/home/students/facilitiesnservices/sportscenter/swimmingpool/Swim-pool-chemistry.pdf (accessed on 5 May 2022).
- Orenda. Understanding Algae. Orenda. 2022. Available online: https://blog.orendatech.com/understanding-algae (accessed on 5 May 2022).
- Askins, A. Cyanuric Acid in Commercial Swimming Pools and Its Effects on Chlorine’s “Staying Power” and Oxidation Reduction Potentails; North Carolina State University: Raleigh, NC, USA, 2013. [Google Scholar]
- Orenda. Phosphates, Algae, and Chlorine Demand. Orenda. 2022. Available online: https://blog.orendatech.com/phosphates-algae-chlorine-demand (accessed on 5 May 2022).
- Orenda. Phosphate Removal|Pillar 3. Orenda. 2022. Available online: https://blog.orendatech.com/phosphate-removal-pillar-3 (accessed on 5 May 2022).
- Lehutso, R.F.; Thwala, M. Assessment of Nanopollution in Water Environments from Commercial Products. Nanomaterials 2021, 11, 2537. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.J.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Schirmer, K. Nanomaterials in the Environment: BEHAVIOR, Fate, Bioavailability, and Effects—An Updated Review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Wagner, S. Nanoparticles in the Environment: Where Do We Come From, Where Do We Go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar]
- Yuan, S.; Huang, J.; Jiang, X.; Huang, Y.; Zhu, X.; Cai, Z. Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials 2022, 12, 699. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf Amina, B.; Ali, M.U.; Munir, M.A.M.; El-Naggar, A.; Naushad, M. Transformation Pathways and Fate of Engineered Nanoparticles (ENPs) in Distinct Interactive Environmental Compartments: A Review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef]
- Al-Abed, S.R.; Virkutyte, J.; Ortenzio, J.N.R.; McCarrick, R.M.; Degn, L.L.; Zucker, R.; Boyes, W.K. Environmental Aging Alters Al(OH)(3) Coating of TiO2 Nanoparticles Enhancing their Photocatalytic and Phototoxic Activities. Environ. Sci.-Nano 2016, 3, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Virkutyte, J.; Al-Abed, S.R.; Dionysiou, D.D. Depletion of the Protective Aluminum Hydroxide Coating in TiO2-Based Sunscreens by Swimming Pool Water Ingredients. Chem. Eng. J. 2012, 191, 95–103. [Google Scholar] [CrossRef]
- In The Swim. Swimming Pool Clarifiers. In The Swim. 2022. Available online: https://intheswim.com/eguides/swimming-pool-clairifiers.html (accessed on 13 May 2022).
- Dhirasasna, N.; Suprun, E.; MacAskill, S.; Hafezi, M.; Sahin, O. A Systems Approach to Examining PhD Students’ Well-Being: An Australian Case. Systems 2021, 9, 17. [Google Scholar] [CrossRef]
- Omran, A.; Khorish, M.; Saleh, M. Structural Analysis with Knowledge-Based MICMAC Approach. Int. J. Comput. Appl. 2014, 86, 39–43. [Google Scholar] [CrossRef]
- Wojtowicz, J.A. Calcium Carbonate Precipitation Potential. J. Swim. Pool Spa Ind. 2001, 2, 51–57. [Google Scholar]
- Orenda. Calcium Crystals vs. Scale. Orenda. 2022. Available online: https://blog.orendatech.com/calcium-crystals (accessed on 21 May 2022).
- Wojtowicz, J.A. The Carbonate System in Swimming Pool Water. J. Swim. Pool Spa Ind. 2001, 4, 54–59. [Google Scholar]
- Wojtowicz, J.A. Effect of Cyanuric Acid on Swimming Pool Maintenance. J. Swim. Pool Spa Ind. 2004, 5, 15. [Google Scholar]
- Wojtowicz, J.A. The Effect of Cyanuric Acid and Other Interferences on Carbonate Alkalinity Measurement. J. Swim. Pool Spa Ind. 2001, 1, 7–13. [Google Scholar]
- Lewis, M.J.; Bamforth, C.W. pH. In Essays in Brewing Science; Lewis, M.J., Bamforth, C.W., Eds.; Springer: Boston, MA, USA, 2007; pp. 13–19. [Google Scholar]
- Covington, A.K.; Bates, R.; Durst, R. Definition of pH Scales, Standard Reference Values, Measurement of pH and Related Terminology (Recommendations 1984). Pure Appl. Chem. 1985, 57, 531–542. [Google Scholar] [CrossRef] [Green Version]
- Van Isle Water. What is Over Stabilization in a Swimming Pool or Spa. 2015. Available online: https://www.vanislewater.com/what-is-over-stabilization-in-a-swimming-pool-or-spa (accessed on 22 May 2022).
- Orenda. Different Types of Phosphates. Orenda. 2022. Available online: https://blog.orendatech.com/different-phosphates (accessed on 22 May 2022).
- In The Swim. Phosphate Contamination in Pools. In The Swim. 2014. Available online: https://blog.intheswim.com/phosphate-contamination-in-pools/ (accessed on 22 May 2022).
- Gonzalez, P. Understanding Phosphorus. Water Talk. 2021. Available online: https://www.cheminc.com/post/understanding-phosphorus (accessed on 11 April 2022).
- APSP Recreational Water Quality Committee. Total Dissolved Solids: Explained. Aqua Magazine, February 2019; Volume 44, pp. 34–37. [Google Scholar]
- Malvern Instruments. Zeta Potential: An Introduction in 30 Minutes. Zetasizer Nano Serles Tech. 2015, 1, 1–6. [Google Scholar]





| Category | Variables (Long Label) | Variables (Short Label) |
|---|---|---|
| Essential SPW chemistry (monitoring parameters) | Langelier saturation index | LSI |
| Water temperature | T | |
| Calcium hardness | CaH | |
| Total dissolved solids | TDS | |
| Total alkalinity | TA | |
| pH value | pH | |
| Free (active) chlorine | FC | |
| Cyanuric acid | CYA | |
| Carbon dioxide (CO2) level in water | CO2 (aq) | |
| Supplementary SPW chemistry (chemicals) | Use of sequestering/chelating agent | SEQ Agent |
| Use of flocculant/clarifier | FLOC/CLAR | |
| Use of algaecide | Algaecide | |
| Use of enzymes | Enzymes | |
| Drain and replace water | ReplaceH2O | |
| SP/SPW conditions | Over-stabilisation | Over-stab |
| Scale formation | Scale | |
| Calcite crystal formation | Calcite | |
| Plaster etching/surface fading/pitting | Etching | |
| Filter/pipe clogging | Clogging | |
| Heavy metal level | HMetal Lev | |
| Algae level | Algae | |
| Phosphate level | Phosphate | |
| Amount of washed-off sunscreen | WashedOffS | |
| Total TiO2 concentration in SPW | TiO2concSP | |
| Amount of (other) non-living/living contaminants | CONTAM | |
| Essential SPW property | Calcium carbonate/calcium phosphate compounds solubility | Solubility |
| Heteroagglomeration potential | AggloPot | |
| Ionic strength | IS | |
| Zeta potential | ZetaPot | |
| Operating parameter | Physical pool maintenance | Maintain |
| Turnover rate | Turnover | |
| Water agitation | Agitation | |
| Filter efficacy | FilterEffi | |
| Swimming pool holding capacity | SP HCap | |
| Debris/precipitates/contaminants removal capacity | RemovalCap | |
| Disinfection/sanitisation capacity | DisinfeCap | |
| Monitoring parameter (external) | Sunlight exposure time | Sunlight |
| Amount of rain/overflow | RainOverfl | |
| Bathers’ behaviour | Bather load | Batherload |
| Shower duration before pool use | ShowerDur | |
| Activity duration in swimming pool | ActivDur | |
| Reapplication frequency/day | ReappliSS | |
| Amount of sunscreen applied to skin | AppliedSS | |
| Sunscreen formulation/TiO2 particle property | TiO2 stability (chemical and physical) in sunscreen formulation | TiO2stabSS |
| TiO2 concentration in applied sunscreen | TiO2concSS | |
| TiO2 primary particle size | TiO2PPS | |
| TiO2 particle surface chemistry | TiO2SChem | |
| TiO2 particle surface (coating) dissolution | TiO2SDis |
| Influence Rank | Dependence Rank | ||
|---|---|---|---|
| Direct | Indirect | Direct | Indirect |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heilgeist, S.; Sahin, O.; Sekine, R.; Stewart, R.A. Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach. Water 2022, 14, 2062. https://doi.org/10.3390/w14132062
Heilgeist S, Sahin O, Sekine R, Stewart RA. Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach. Water. 2022; 14(13):2062. https://doi.org/10.3390/w14132062
Chicago/Turabian StyleHeilgeist, Simone, Oz Sahin, Ryo Sekine, and Rodney A. Stewart. 2022. "Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach" Water 14, no. 13: 2062. https://doi.org/10.3390/w14132062
APA StyleHeilgeist, S., Sahin, O., Sekine, R., & Stewart, R. A. (2022). Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach. Water, 14(13), 2062. https://doi.org/10.3390/w14132062









