Abstract
Abundance–Biomass Comparison (ABC) models, first described for marine benthic macrofauna, have been applied successfully to other marine and terrestrial/freshwater fauna but never to ecotonal communities. In particular, to our knowledge, ABC models have not been applied to hyporheic communities. This study represents the first application of ABC models to hyporheic assemblages. We aimed at testing the effectiveness of ABC models in describing the perturbation of hyporheic communities subjected to an existing/known disturbance. To this end, we applied the models to the hyporheic community of an Apennine creek, where the hyporheic waters of the upstream stretch were uncontaminated, whereas those of the downstream stretch were contaminated by ammonium. We also tested separated models for the summer and winter periods to account for potential variability due to season. ABC models provided a satisfactory description of the hyporheic community changes due to ammonium by showing the abundance dominance curve overlying that of the biomass in the downstream stretch contrarily to what was observed in the upstream stretch. However, ABC models did not highlight any significant seasonal effects. Our results showed that ABC models have the potential to be used as assessment tools for ecological quality of hyporheic zones in temperate regions.
1. Introduction
Abundance–Biomass Comparison (ABC) models [] are tools for detecting the effects of anthropogenic perturbation of biological communities []. ABC models are underpinned by the r- and K-selection theories and involve plotting k-dominance curves [] along with k-biomass curves on the same graph for comparative purposes. Taxa are ranked on a logarithmic scale on the x-axis, with cumulative percentage dominance (in terms of abundance and biomass) displayed on the y-axis. Taxa are displayed in a different order for the abundance and biomass curves on the x-axis. Hence, taxa identities do not match up and the model should be read by separately considering the dominance structure of the community captured for abundance and biomass []. Warwick [] first applied ABC models to marine benthic macroinvertebrates communities of soft-bottom habitats, observing that, under stable conditions (or infrequent disturbance), the biomass curve lays above that of the abundance, which, in turn, shows a typical equitable distribution. This pattern is mainly shaped by biomass, which, in pristine environments, is generally dominated by one or two K-selected (conservative) taxa, which are large in size but not dominant in numbers. r-selected (opportunist) taxa are also present, though are not dominant in terms of biomass or abundance. Warwick [] also observed that when a disturbance alters the community, the biomass curve intercepts that of abundance. In case of severe disturbance, the biomass curve even lays beneath that of abundance, indicating a drastic alteration of the community structure. The two latter patterns are determined by K-selected species, which usually decrease in numbers (some taxa might even disappear under severe disturbance), whereas r-selected taxa are not affected or might even thrive under disturbance. Hence, under perturbed environmental conditions, the k-dominance curve shows minor equitability, whereas the equitability of the k-biomass curve enhances. The result is that the two curves intermingle under an intermediate disturbance, or the biomass curve lies beneath the abundance one under severe perturbance [].
When one of the two curves is over-dependent on the single most dominant taxon, the visual information of the ABC model is challenging to interpret and may be easily misleading []. To overcome this issue, partial dominance based on the dominance of the second-ranked taxon over the remainder is recommended. Partial dominance plots ameliorate the way standard dominance curves tend to be dictated by the most abundant taxon []. ABC models based on partial dominance postulate that, with frank disturbance, dominance patterns are unaffected by successive removal of the one or two most dominant taxa in terms of abundance or biomass []. The paradigms of ABC models based on partial dominance are the same as for ABC models based on cumulative dominance: the biomass curve typically lies above the abundance curve throughout its full length in undisturbed conditions, whereas, under intermediate disturbance, there is still a change in position of partial dominance curves, with the biomass curve now below the abundance curve in one or more places, and the abundance curve becoming much more variable or “atypically erratic” []. This implies that the disturbance pervades the complete suite of taxa in the community and is not just seen in changes in a few dominant taxa []. Finally, the biomass curve might lie below the abundance curve throughout its full length under severe disturbance. Partial ABC models are less visually appealing than the original ABC models. However, they are more robust to random fluctuations in abundances of a small-sized, numerically dominant taxa [,].
ABC models, either based on cumulative or partial dominance, are effective for monitoring and surveillance [], where monitoring is the assessment of the response of a community to an existing/known disturbance (e.g., a contamination event), whereas surveillance is the observation of changing trends (i.e., the observation of the responses of a community to disturbance over time) [,]. For the models to be reliable, however, adequate replication (in terms of both spatial and temporal replicates) is essential because large-biomass dominant taxa are often represented by a few individuals []. Although first described for marine benthic macrofauna, ABC models’ paradigm also proved true for other types of communities. Examples of successful application can be found in studies concerning other marine fauna, such as fish communities (e.g., []), and terrestrial/freshwater fauna such as birds [], dragonflies, small mammals, and herpetofauna, where ABC models were used to track successional recovery after forest fires []. However, to our knowledge, ABC models have not been applied to the hyporheic communities.
The hyporheic zone, which includes the saturated portions of streambeds, banks, and floodplains, is characterized by a mixture of groundwater and stream water [], though downwelling and upwelling might not occur throughout the year (e.g., [,]). From an ecological perspective, the hyporheic zone is an ecotone, where benthic taxa, specialized hyporheic organisms, and groundwater fauna find a suitable habitat (e.g., [,]). Hyporheic communities include microorganisms (e.g., []), meiofauna (e.g., []), macroinvertebrates (e.g., []), and fish and amphibian fry [], which provide essential ecosystem services (e.g., []).
In this study, we aimed to test the effectiveness of ABC models in describing changes in hyporheic communities. To this end, we applied the models to the hyporheic invertebrate community of an Apennine creek (Rio Gamberale; central Italy), where the upstream stretch runs across a protected area, whereas the downstream stretch runs across orchards and urban areas (Figure 1). We used the abundance and biomass data obtained in our previous study where we analyzed the hyporheic waters of the two stretches in December 2014 and June 2015 []. In our previous study, we found out that, in the upstream stretch of the creek, the hyporheic waters were uncontaminated, whereas the hyporheic waters of the downstream stretch were contaminated by ammonium []. In our previous study, we also analyzed the hyporheic community of the creek []. In detail, we carried out a permutational analysis of variance [], which showed that the hyporheic assemblage of the downstream stretch was significantly different from that of the upstream one due to a decrease in taxonomic and functional diversity []. We also performed a gradient analysis using the DistLM (Distance-based Linear Models) routine [] to model the linear relationships between the biological data and the predictor environmental variables. We examined 71 models (both uni- and multivariate) and, in about half of these, we found that ammonium was the predictor variable []. Based on these analyses and literature data, we concluded that ammonium had a detrimental effect on the hyporheic invertebrates of the Rio Gamberale creek, affecting their growth, development [], biomass, reproduction, behaviour, respiration, and survival (e.g., [,,,,,]). In this study, we used the data (abundance and biomass data) obtained in our previous study [] to test the effectiveness of ABC models in describing the alteration of the hyporheic community of the Rio Gamberale creek subjected to an existing/known disturbance, i.e., the ammonium contamination. In detail, our hypotheses were that: (i) in the upstream stretch, which was not contaminated by ammonium, the ABC models based on partial dominance would display the biomass curve typically lying above the abundance curve throughout its full length, and (ii) in the downstream stretch, which was contaminated by ammonium, the models would display the reverse, that is the biomass curve below the abundance curve in one or more points or throughout its full length.
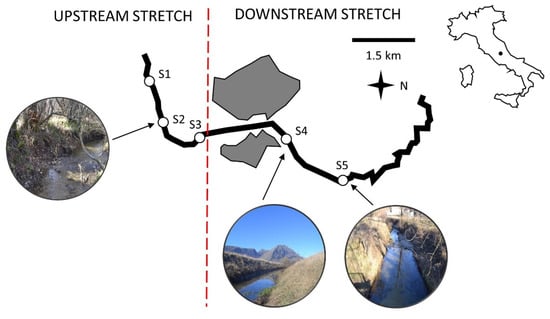
Figure 1.
The Rio Gamberale creek and sampling stations (white dots and pics). Urban areas served by wastewater treatment plants are represented in grey.
2. Materials and Methods
2.1. Study Area
The Apennine creek Rio Gamberale forms at 1500 m a.s.l. (42°14′00′′ N, 13°32′10′′ E) and runs for 10 km across a karst plateau, once the site of a Late Pleistocene-Holocene lake basin, with an average discharge of 360 L s−1 []. The upstream stretch (3 km in length) sprints across Parco Naturale Regionale Sirente-Velino (Abruzzo, Italy), a protected mountain area established in 1989. The downstream stretch (7 km in length) runs across an anthropized area where agriculture and two urban wastewater treatment plants are sources of ammonium release (Figure 1).
2.2. Sample Collection and Processing
Hereafter, we report the methodology used in our previous study [] to monitor the hyporheic zone of the Rio Gamberale creek. We monitored the upstream stretch in three sampling stations and the downstream stretch in two stations []. The stations were at least 1.5 km apart (Figure 1). In each sampling station, we collected three spatial replicates across a transect oblique to the stream channel, at the hydrographic right, in the middle of the channel and at the hydrographic left. Prior to the sampling survey, we analyzed the granulometry of the five sampling stations by visually estimating the sediment composition of the streambed according to the Wentworth [] scale. Sand (63 μm < φ ≤ 2 mm) accounted for most of the hyporheic sediment composition (60–81%), followed by clay-silt (φ ≤ 63 μm; range: 18–40%) and gravel (2 mm < φ ≤ 64 mm; range: 0–3%) []. The granulometric composition of the streambed in each station is reported in the Supplementary File in our previous study []. The sediment composition did not vary significantly between the up- and downstream stretches []. We also collected biological and water samples from the hyporheic zone of the two stretches in winter (December 2014; mean rainfall < 20 mm) and summer (June 2015; mean rainfall: 190 mm). We collected the samples following the methods in Malard et al. []. Mobile steel piezometers with a 5 mm-hole-screened tip [] were hammered in the hyporheic zone at a depth of max 40 cm and connected to a Bou-Rouch pump [].
To collect the biological samples, ten litres of interstitial waters and sediments were pumped and filtered by a 60 µm-mesh net. The samples were fixed in the field in a 70% alcohol solution. In the laboratory, we sorted the samples under a stereomicroscope at 16×, without staining. Next, we picked up the invertebrate individuals by using a glass pipette and identified each specimen to the lowest possible taxonomic resolution based on the updated literature (e.g., [,,,,,]). Afterwards, we measured the body dimensions of each specimen (length and width) using a LEICA M205C stereomicroscope provided with an integrated camera and the LAS software (Leica Application Suite, version 4.7.1). We converted the body size into biomass (mg of dry carbon) using the equations in Reiss and Schmid-Araya [], assuming that the dry carbon content accounted for 40% of the dry mass with a dry/wet mass ratio equal to 0.25.
We collected the hyporheic water samples immediately after the biological sampling by pumping 2 L of hyporheic water. The samples were analyzed for screening 99 chemical compounds (metals, pesticides, volatile organic compounds, and hydrocarbons). We also measured eight environmental parameters (temperature, electrical conductivity, pH, dissolved oxygen, particulate matter, total and dissolved organic carbon) and nine ions (ammonium, nitrite, nitrate, phosphorous, sulphates, calcium, potassium, chloride, and sodium). The mean values of the environmental parameters measured in our previous study [] are reported for the up- and downstream stretches in Table S1. The analyses performed in our previous study highlighted a gradient of ammonium contamination in the hyporheic zone of the Rio Gamberale creek []. In the upstream stretch, the mean concentration of ammonium was below the European Quality Standard (0.03 mg L−1 vs. 0.5 mg L−1) [], whereas in the downstream stretch, ammonium was twice the legal threshold in both summer and winter (Table S1). We did not detect any other pollutant (i.e., their concentrations were consistently below the instrumental limit of detection) in both the up- and downstream stretches [].
2.3. ABC Models
The abundance and biomass data collected as previously described were used in this study to perform ABC models. In detail, we ranked the collected taxa in decreasing order of abundance and biomass for two sample pools, one for the upstream stretch (pool of 18 samples) and the other for the downstream stretch (pool of 12 samples). Cumulative relative abundances and biomasses of each pool were plotted against increasing ranks (x-axis on a log scale). We obtained partial dominance plots, in which the y-axis was the abundance (or the biomass) of each taxon relative to the total of its abundance (or biomass) plus that of all other less-abundant taxa in the pool. Finally, the cumulative partial dominance curves, referred to as k-dominance curves, were reported on the same plots to obtain the ABC models. We also built separated models for the summer and winter periods to account for the potential variability due to the two different sampling seasons. We used PRIMER v7 to build all the curves and models [].
3. Results
In the upstream stretch, we collected 32 taxa (Table 1), represented by 292 individuals. Chironomidae were the most abundant taxon (40% of the overall abundances), followed by the plecopteran Leuctra fusca fusca (13%), Ostracoda (9%), and the harpacticoid Bryocamptus pygmaeus (7%). The remaining 28 taxa accounted for less than 5% of the total abundances. The biomass was equal to 22 g dry C and was dominated by Ecdyonurus gr. venosus (52%), followed by L. fusca fusca (21%), and the ephemeropteran Siphlonurus lacustris (7%). The remnant taxa accounted for less than 6% of the total biomass (Table 1). We collected 21 taxa in winter (134 individuals) and 25 in summer (158 individuals). Twenty taxa were present in both seasons, whereas 6 taxa were exclusive of winter and 10 of summer. Chironomidae dominated the abundances in winter (56%) and summer (27%). The total biomass was 16 g dry C in winter (dominated by E. gr. venosus: 72%) and 6 g in summer (dominated by L. fusca fusca: 44%; Table 1).

Table 1.
Percentages of abundance (Abb) and biomass (Bio) of the taxa collected in the upstream stretch of the Rio Gamberale creek. Percentage data reported overall, and separately for winter (December 2014: W) and summer (June 2015: S) seasons.
In the downstream stretch, we collected 19 taxa (Table 2), represented by 501 individuals. Chironomidae were the most abundant taxon (22% of the overall abundance). The remaining abundances were equally distributed mainly among six taxa, namely: Nematoda Mermithidae (15%), Ostracoda (13%), the copepods Paracyclops fimbriatus (11%), Attheyella crassa (9%), B. pygmaeus (9%), and Eucyclops subterraneus intermedius (7%). Each of the remaining 12 taxa represented less than 5% of the overall abundances. The biomass was equal to 6 g dry C and was dominated by the copepod Diacyclops bisetosus (89%), followed by Chironomidae (9%). Except for Ceratopogonidae (2% of the overall biomass), the remaining taxa accounted for less than 1% of the biomasses (Table 2). More specifically, in the downstream stretch, we collected 10 taxa in winter (188 individuals) and 16 in summer (313 individuals). In winter, three taxa were the most abundant (Nematoda Mermithidae: 25%; Ostracoda: 22%; A. crassa 23%), whereas the abundances were dominated by Chironomidae (27%) and P. fimbriatus (17%) in summer. The biomass was 0.05 g dry C in winter (dominated by Chironomidae: 99%) and 5.95 g in summer (dominated by D. bisetosus: 83%).

Table 2.
Percentages of abundance (Abb) and biomass (Bio) of the taxa collected in the downstream stretch of the Rio Gamberale. Percentage data are also reported according to the season: winter (December 2014: W) and summer (June 2015: S).
The ABC models detected significant spatial differences in the hyporheic assemblages of the up- and downstream stretches. In the upstream stretch, the curve of the biomass lay above that of the abundance throughout its full length (Figure 2a). In the downstream stretch, the curve of the biomass was above the abundance one for the first 5 ranked taxa, crossed the abundance curve between taxon 5 and 6 and 6–7, and showed a trend similar to the abundance one for taxa 7–14; from taxa 15 to 19, the two curves showed approximately an opposite trend (Figure 2b). This pattern was consistent in both winter and summer. In winter, the biomass curve lay above that of the abundance throughout its full length in the upstream stretch (Figure 2c), whereas it was below the abundance curve at the ranked taxa 5 and 6 (Figure 2d). Similar patterns were observed in summer (Figure 2e,f).
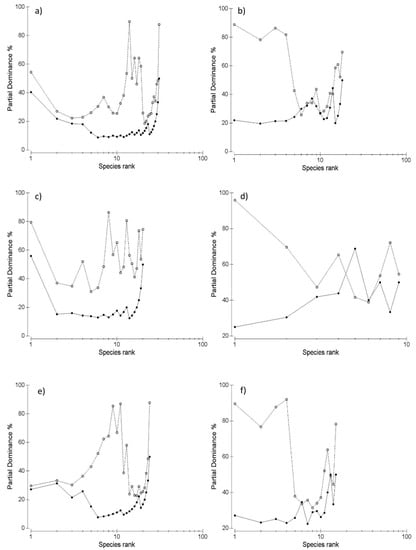
Figure 2.
Abundance–Biomass Comparison models of the hyporheic community of the Rio Gamberale creek (Italy): (a) overall data of the upstream stretch; (b) overall data of the downstream stretch; (c) data of the upstream stretch in the winter period (December 2014); (d) data of the downstream stretch in the winter period (December 2014); (e) data of the upstream stretch in the summer period (June 2015); (f) data of the downstream stretch in the summer period (June 2015). The biomass curve is white-dotted, whereas the abundance curve is black-dotted.
4. Discussion
According to the ABC models’ theory, the biomass curve is above the abundance curve when the community is likely dominated by K-selected taxa, with one or two taxa at high biomass level characterized by large body size, slow growth, and late maturation. They would be rarely dominant in terms of abundance though dominant in terms of biomass []. On the contrary, in perturbed communities, the paradigm of ABC models indicates that the biomass and abundance curve tend to overlap when K-selected taxa are replaced by r-selected taxa [], which show fast growth and are dominant in terms of both biomass and abundance [].
The paradigm of ABC models accurately fits the hyporheic assemblages of the Rio Gamberale creek. In the upstream stretch, biomasses were dominated by K-selected taxa such as E. gr. venosus [], which, however, occurred with low abundances. Ecdyonorus gr. venosus has large body size and the individuals collected in the Rio Gamberale were the largest (0.9–18 mm) as compared to the individuals of other taxa composing the hyporheic community. Furthermore, Ecdyonurus species are reported to be bioindicators of unpolluted small and slow-flowing streams []. In particular, E. venosus is very sensitive to organic pollution []. On the contrary, in the downstream stretch, the biomass and abundance curves overlapped, identifying a disturbed community, where the drop of the biomass curve was due to the dominance of small-sized, opportunistic taxa such as the cyclopoid D. bisetosus. This species is cosmopolitan, metal-tolerant, and highly resistant to changes in environmental conditions, thriving even in hypersaline waters []. Copepods, which have a relatively small biomass (as compared to the macroinvertebrate species which dominated the upstream stretch) seemed to have a relevant influence on the structure of the hyporheic community of the Rio Gamberale creek, similarly to that of small polychetes in sandy substrates of sea littoral [].
The community impairment highlighted by ABC models in the downstream stretch of the Rio Gamberale is consistent with the findings of our previous study [], where we observed a severe erosion of the taxonomic and functional diversities in the downstream stretch. In detail, we measured the traits of the whole invertebrate community of the hyporheic zone of the Rio Gamberale creek and assessed that 12 taxa and 11 trait modalities related to size and body form, fecundity and reproduction, resistance and respiration, diet, locomotion, and feeding habits, occurred in the upstream stretch but not in the downstream stretch []. The results of this new study are also in line with the expectations based on previous studies where ammonium proved to impact the fitness of several hyporheic species (e.g., [,,,,,]). In particular, the absence of the ephemeropterans (Siphlonurus lacustris, Ecdyonurus gr. venosus, Baetis sp. and Rhyacophila foliacea) in the downstream stretch due to ammonium contamination is in agreement with previous investigations [,]. Finally, the alteration of the hyporheic community found in this study due to a nitrogenous compound is in agreement with the findings of Pacioglu et al. [] and Iepure et al. [] who demonstrated that eutrophication and urban pressure change hyporheic communities, with microcrustaceans replacing other taxa, such as insect larvae in downstream river sectors.
The ABC models were relatively identical in summer and winter, indicating no change in the abundance-biomass relationship because of the season. On the contrary, the outcomes of our previous study indicated that summer is the period during which ammonium has the most substantial detrimental effect on the functionality of the hyporheic zone of the Rio Gamberale creek []. However, this missed seasonal effect does not represent a limitation of ABC models. Rather, it reflects their rationale that relates abundance and biomass relationships and does not consider the morpho-physio-phenological characteristics of taxa.
This study represents the first application of ABC models to hyporheic assemblages. Our results suggest that the models can be conveniently applied to groundwater-surface water ecotonal communities and that hyporheic invertebrates have the potential to be bioindicators of human impacts related to ammonium release as postulated in other studies [,,]. According to the results of this study, ABC models have a good performance in assessing the effects of an existing/known disturbance (the ammonium contamination, in this study). Hence, the models can be used in Control-Impact or also in Before-After-Control-Impact designs [].
5. Conclusions
Our results showed that ABC models have the potential to be used as assessment tools for ecological quality of hyporheic zones in temperate regions. In our study, ABC models were easy to interpret. However, they required vast taxonomic knowledge covering several aquatic invertebrate taxa. Possible errors in the interpretation of curves might arise from insufficient knowledge of the ecological aspects of the taxa that form the hyporheic community. For this reason, we suggest using ABC models in combination with other ecological indicators and analyses, such as those used in our previous study [], to provide a coherent and accurate characterization of the quality of the hyporheic zone of temperate freshwater streams.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w14182934/s1, Table S1: Mean and standard deviation of the environmental variables measured in the up- and downstream stretches of the Rio Gamberale (Italy).
Author Contributions
Conceptualization, T.D.L.; methodology, T.D.L., B.F., M.D.C., A.T.D.C., I.V. and D.M.P.G.; software, T.D.L.; validation, D.M.P.G.; formal analysis, T.D.L. and S.C.; investigation, B.F., M.D.C., A.T.D.C., I.V., S.C. and D.M.P.G.; resources, D.M.P.G.; data curation, B.F. and D.M.P.G.; writing—original draft preparation, T.D.L.; writing—review and editing, D.M.P.G.; visualization, project administration, B.F. and D.M.P.G.; funding acquisition, D.M.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was granted by the European Commission AQUALIFE LIFE12 BIO/IT/000231 “Development of an innovative and userfriendly indicator system for biodiversity in groundwater dependent ecosystems” and by MUR-FOE-Project Capitale Naturale-Task Biodiversità.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Marco Cifoni for helping in the sampling survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Warwick, R.M. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Lambshead, P.J.D.; Platt, H.M.; Shaw, K.M. The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J. Nat. Hist. 1983, 17, 859–874. [Google Scholar] [CrossRef]
- Clarke, K.R. Comparisons of dominance curves. J. Exp. Mar. Biol. Ecol. 1990, 138, 143–157. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial.; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Dauer, D.M.; Luckenbach, M.W.; Rodi, A.J. Abundance biomass comparison (ABC method): Effects of an estuarine gradient, anoxic/hypoxic events and contaminated sediments. Mar. Biol. 1993, 116, 507–518. [Google Scholar] [CrossRef]
- Warwick, R.M.; Pearson, T.H. Detection of pollution effects on marine macrobenthos: Further evaluation of the species abundance/biomass method. Mar. Biol. 1987, 95, 193–200. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Relearning the ABC: Taxonomic changes and abundance/biomass relationships in disturbed benthic communities. Mar. Biol. 1994, 118, 739–744. [Google Scholar] [CrossRef]
- Xu, S.; Guo, J.; Liu, Y.; Fan, J.; Xiao, Y.; Xu, Y.; Li, C.; Barati, B. Evaluation of Fish Communities in Daya Bay Using Biomass Size Spectrum and ABC Curve. Front. Environ. Sci. 2021, 9, 663169. [Google Scholar] [CrossRef]
- Meire, P.M.; Dereu, J. Use of the abundance/biomass comparison method for detecting environmental stress: Some considerations based on intertidal macrozoobenthos and bird communities. J. Appl. Ecol. 1990, 27(1), 210–223. [Google Scholar] [CrossRef]
- Smith, W.H.; Rissler, L.J. Quantifying disturbance in terrestrial communities: Abundance–biomass comparisons of herpetofauna closely track forest succession. Restor. Ecol. 2010, 18, 195–204. [Google Scholar] [CrossRef]
- Woessner, W.W. Chapter 8—Hyporheic Zones. In Methods in Stream Ecology, 3rd ed.; Hauer, R.F., Lamberti, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 129–157. [Google Scholar]
- Brunke, M.; Gonser, T.O.M. The ecological significance of exchange processes between rivers and groundwater. Freshwat. Biol. 1997, 37, 1–33. [Google Scholar] [CrossRef]
- Boulton, A.J.; Datry, T.; Kasahara, T.; Mutz, M.; Stanford, J.A. Ecology and management of the hyporheic zone: Stream–groundwater interactions of running waters and their floodplains. J. N. Am. Benthol. Soc. 2010, 29, 26–40. [Google Scholar] [CrossRef]
- Williams, D.D.; Febria, C.M.; Wong, J.C. Ecotonal and other properties of the hyporheic zone. Fundam. Appl. Limnol. 2010, 176, 349. [Google Scholar] [CrossRef]
- Peralta-Maraver, I.; Galloway, J.; Posselt, M.; Arnon, S.; Reiss, J.; Lewandowski, J.; Robertson, A.L. Environmental filtering and community delineation in the streambed ecotone. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.G.; Fulthorpe, R.R.; Williams, D.D. Perspectives and predictions on the microbial ecology of the hyporheic zone. Freshwat. Biol. 1999, 41, 119–130. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Palmer, M.A. The ecology of hyporheic meiofauna. In Streams and Ground Waters; Jones, J.B., Mulholland, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 307–336. [Google Scholar] [CrossRef]
- Wood, P.J.; Boulton, A.J.; Little, S.; Stubbington, R. Is the hyporheic zone a refugium for macroinvertebrates during severe low flow conditions? Fund. Appl. Limnol. 2010, 176, 377–390. [Google Scholar] [CrossRef]
- Kawanishi, R.; Inoue, M.; Dohi, R.; Fujii, A.; Miyake, Y. The role of the hyporheic zone for a benthic fish in an intermittent river: A refuge, not a graveyard. Aquat. Sci. 2013, 75, 425–431. [Google Scholar] [CrossRef]
- Lewandowski, J.; Arnon, S.; Banks, E.; Batelaan, O.; Betterle, A.; Broecker, T.; Coll, C.; Drummond, J.D.; Gaona Garcia, J.; Galloway, J.; et al. Is the hyporheic zone relevant beyond the scientific community? Water 2019, 11, 2230. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Fiasca, B.; Di Cicco, M.; Cifoni, M.; Galassi, D.M.P. Taxonomic and functional trait variation along a gradient of ammonium contamination in the hyporheic zone of a Mediterranean stream. Ecol. Indic. 2021, 132, 108268. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J.; Robinson, J. Permutation tests for linear models. Aust. N. Z. J. Stat. 2001, 43, 7588. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Cifoni, M.; Sáenz, M.E.; Galassi, D.M.P.; Di Lorenzo, T. The ecotoxicity of binary mixtures of Imazamox and ionized ammonia on freshwater copepods: Implications for environmental risk assessment in groundwater bodies. Ecotoxicol. Environ. Safe. 2018, 149, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, W.D.; Castaldo, D.; Pantani, C.; Di Cioccio, A.; Di Lorenzo, T.; Sáenz, M.E.; Galassi, D.M.P. Relative sensitivity of hyporheic copepods to chemicals. Bull. Environ. Contam. Toxicol. 2009, 82, 488–491. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Castaldo, D.; Di Lorenzo, T.; Di Cioccio, A.; Sáenz, M.E.; Galassi, D.M.P. Developmental endpoints of chronic exposure to suspected endocrine-disrupting chemicals on benthic and hyporheic freshwater copepods. Ecotoxicol. Environ. Safe. 2013, 96, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, T.; Di Marzio, W.D.; Cifoni, M.; Fiasca, B.; Baratti, M.; Sáenz, M.E.; Galassi, D.M.P. Temperature effect on the sensitivity of the copepod Eucyclops serrulatus (Crustacea, Copepoda, Cyclopoida) to agricultural pollutants in the hyporheic zone. Curr. Zool. 2015, 61, 629–640. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Cifoni, M.; Lombardo, P.; Fiasca, B.; Galassi, D.M.P. Ammonium threshold values for groundwater quality in the EU may not protect groundwater fauna: Evidence from an alluvial aquifer in Italy. Hydrobiologia 2015, 743, 139–150. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Cannicci, S.; Spigoli, D.; Cifoni, M.; Baratti, M.; Galassi, D.M.P. Bioenergetic cost of living in polluted freshwater bodies: Respiration rates of the cyclopoid Eucyclops serrulatus under ammonia-N exposures. Fundam. Appl. Limnol. 2016, 18, 147–156. [Google Scholar] [CrossRef]
- Arenas-Sánchez, A.; Dolédec, S.; Vighi, M.; Rico, A. Effects of anthropogenic pollution and hydrological variation on macroinvertebrates in Mediterranean rivers: A case-study in the upper Tagus river basin (Spain). Sci. Total Environ. 2021, 766, 144044. [Google Scholar] [CrossRef]
- Scorzini, A.R.; Leopardi, M. River basin planning: From qualitative to quantitative flood risk assessment: The case of Abruzzo Region (central Italy). Nat. Hazards 2017, 88, 71–93. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1992, 30, 377–392. [Google Scholar] [CrossRef]
- Malard, F.; Dole-Olivier, M.-J.; Mathieu, J.; Stoch, F. Sampling manual for the assessment of regional groundwater biodiversity. In European Project PASCALIS (Protocols for the Assessment and Conservation of Aquatic Life in the Subsurface); Fift Framework Programme Key Action 2: Global Change, Climate and Biodiversity 2.2.3 Assessing and Conserving Bio-diversity Contract n◦ EVK2–CT–2001–00121; Available online: https://www.researchgate.net/publication/267567541_Sampling_Manual_for_the_Assessment_of_Regional_Groundwater_Biodiversity#fullTextFileContent (accessed on 12 July 2022).
- Mugnai, R.; Sousa, F.N.F.; Di Lorenzo, T. Monitoring hyporheic habitats: Techniques for unclogging minipiezometers. Pan-Am. J. Aquat. Sci. 2015, 10, 168171. [Google Scholar]
- Bou, C.; Rouch, R. Un nouveau champ de recherches sur le faune aquatique souterraine. C. R. Acad. Sci. Paris 1967, 265, 369370. [Google Scholar]
- Dussart, B.H. Les copépodes des Eaux Continentales d’Europe Occidentale, Tome I: Calanoïdes et Harpacticoïdes; Boubée et Cie: Paris, France, 1967; pp. 1–500. [Google Scholar]
- Dussart, B.H. Les Copépodes des Eaux Continentales d’Europe Occidentale, Tome 2: Cyclopöides et Biologie; Boubée et Cie: Paris, France, 1969; pp. 1–292. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il riconoscimento dei macroinvertebrati delle Acque Dolci Italiane. Volumes I & II; Provincia Autonoma di Trento: Trento, Italy, 1994; pp. 1–484. [Google Scholar]
- Di Sabatino, A.; Boggero, A.; Miccoli, F.P.; Cicolani, B. Diversity, distribution and ecology of water mites (Acari: Hydrachnidia and Halacaridae) in high Alpine lakes (Central Alps, Italy). Exp. Appl. Acarol. 2004, 34, 199–210. [Google Scholar] [CrossRef]
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters. II—Cyclopiformes; Backhuys Publishers: Leiden, The Netherlands, 2006; pp. 1–354. [Google Scholar]
- Puente, A.; Diaz, R.J. Is it possible to assess the ecological status of highly stressed natural estuarine environments using macroinvertebrates indices? Mar. Pollut. Bull. 2008, 56, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Schmid-Araya, J.M. Existing in plenty: Abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshw. Biol. 2008, 53, 652–668. [Google Scholar] [CrossRef]
- European Commission. Water Framework Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy. OJ 2000, 327, 1–73. [Google Scholar]
- Woodward, G.; Bonada, N.; Feeley, H.B.; Giller, P.S. Resilience of a stream community to extreme climatic events and long-term recovery from a catastrophic flood. Freshwat. Biol. 2015, 60, 2497–2510. [Google Scholar] [CrossRef]
- Iliopoulou-Georgudaki, J.; Kantzaris, V.; Katharios, P.; Kaspiris, P.; Georgiadis, T.; Montesantou, B. An application of different bioindicators for assessing water quality: A case study in the rivers Alfeios and Pineios (Peloponnisos, Greece). Ecol. Indic. 2003, 2, 345360. [Google Scholar] [CrossRef]
- Radojević, A.; Mirčić, D.; Živić, M.; Perić Mataruga, V.; Božanić, M.; Stojanović, K.; Lukicic, J.; Živić, I. Influence of trout farm effluents on selected oxidative stress biomarkers in larvae of Ecdyonurus venosus (Ephemeroptera, Heptageniidae). Arch. Biol. Sci. 2019, 71, 225–233. [Google Scholar] [CrossRef]
- Burton, S.M.; Rundle, S.D.; Jones, M.B. The relationship between trace metal contamination and stream meiofauna. Environ. Pollut. 2001, 111, 159–167. [Google Scholar] [CrossRef]
- Beketov, M. Different sensitivity of mayflies (Insecta, Ephemeroptera) to ammonia, nitrite and nitrate: Linkage between experimental and observational data. Hydrobiologia 2004, 528, 209–216. [Google Scholar] [CrossRef]
- Pacioglu, O.; Pârvulescu, L. The chalk hyporheic zone: A true ecotone? Hydrobiologia 2017, 790, 1–12. [Google Scholar] [CrossRef]
- Iepure, S.; Martinez-Hernandez, V.; Herrera, S.; Rasines-Ladero, R.; de Bustamante, I. Response of microcrustacean communities from the surface—groundwater interface to water contamination in urban river system of the Jarama basin (central Spain). Environ. Sci. Pollut. Res. 2013, 20, 5813–5826. [Google Scholar] [CrossRef]
- Notenboom, J.; Plénet, S.; Turquin, M.-J. Groundwater contamination and its impact on groundwater animals and ecosystems. In Groundwater ecology; Gibert, J., Danielopol, D.L., Standford, J.A., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 477–504. [Google Scholar]
- Lafont, M.; Vivier, A. Oligochaete assemblages in the hyporheic zone and coarse surface sediments: Their importance for understanding of ecological functioning of watercourses. Hydrobiologia 1998, 334, 147–155. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Meleg, I.; Levei, E.; Terente, M. A simple method for assessing biotic indicators and predicting biodiversity in the hyporheic zone of a river polluted with metals. Ecol. Indic. 2013, 24, 412–420. [Google Scholar] [CrossRef]
- Green, R.H. Sampling Design and Statistical Methods for Environmental Biologists; John Wiley & Sons: New York, NY, USA, 1979; pp. 1–272. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).