Calcite Dissolution and Bioneutralization of Acidic Wastewater in Biosand Reactors
Abstract
1. Introduction
2. Materials and Methods
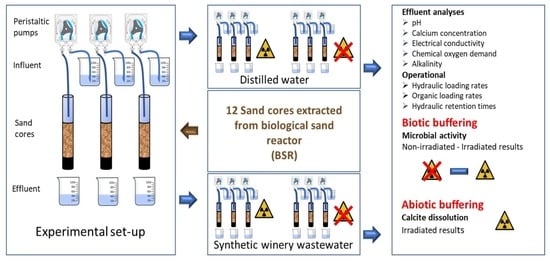
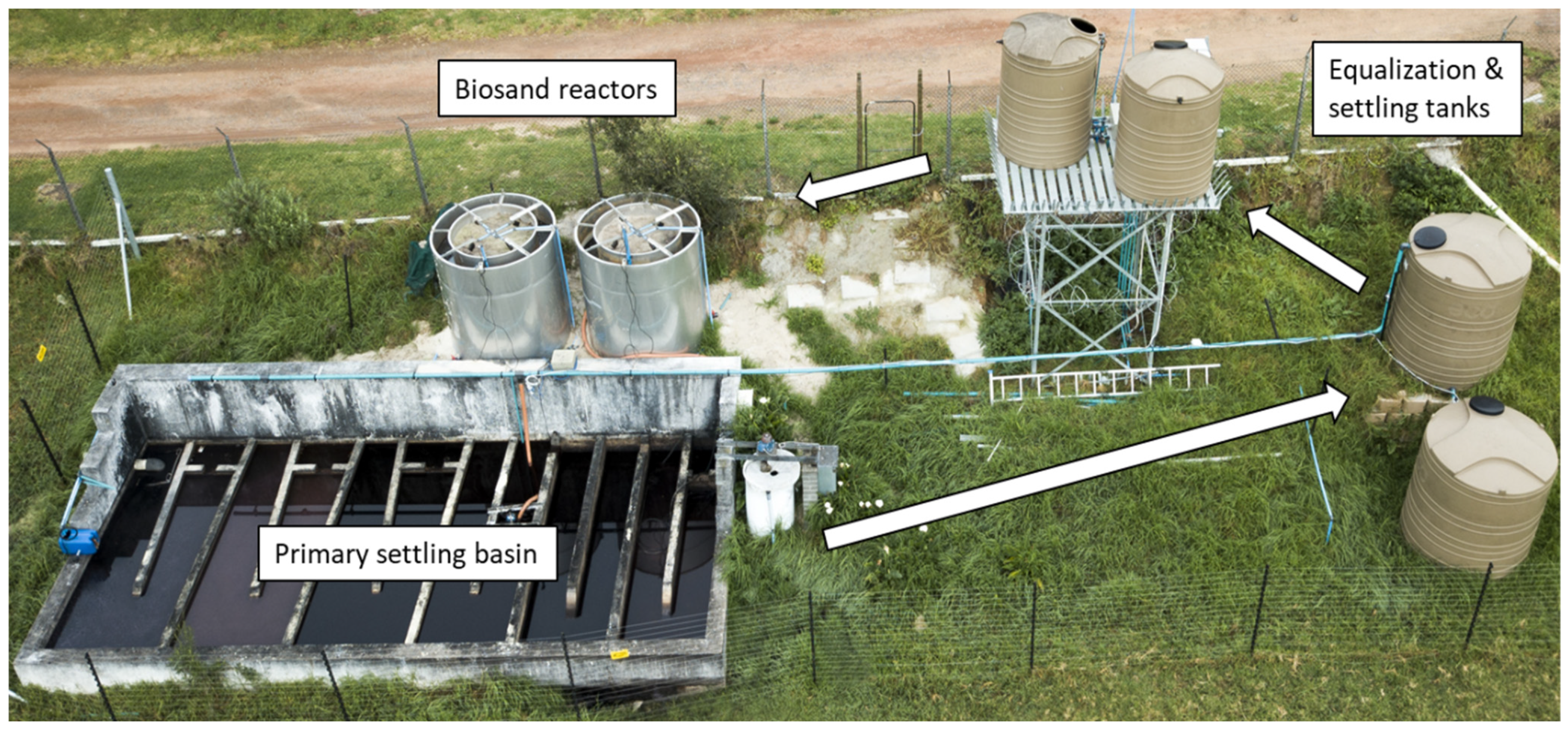
2.1. Column Experiments: Set-Up
2.2. Operation of Column Experiments
2.3. Eluant Sampling and Analytical Procedures
2.4. Statistical Analysis
3. Results and Discussion
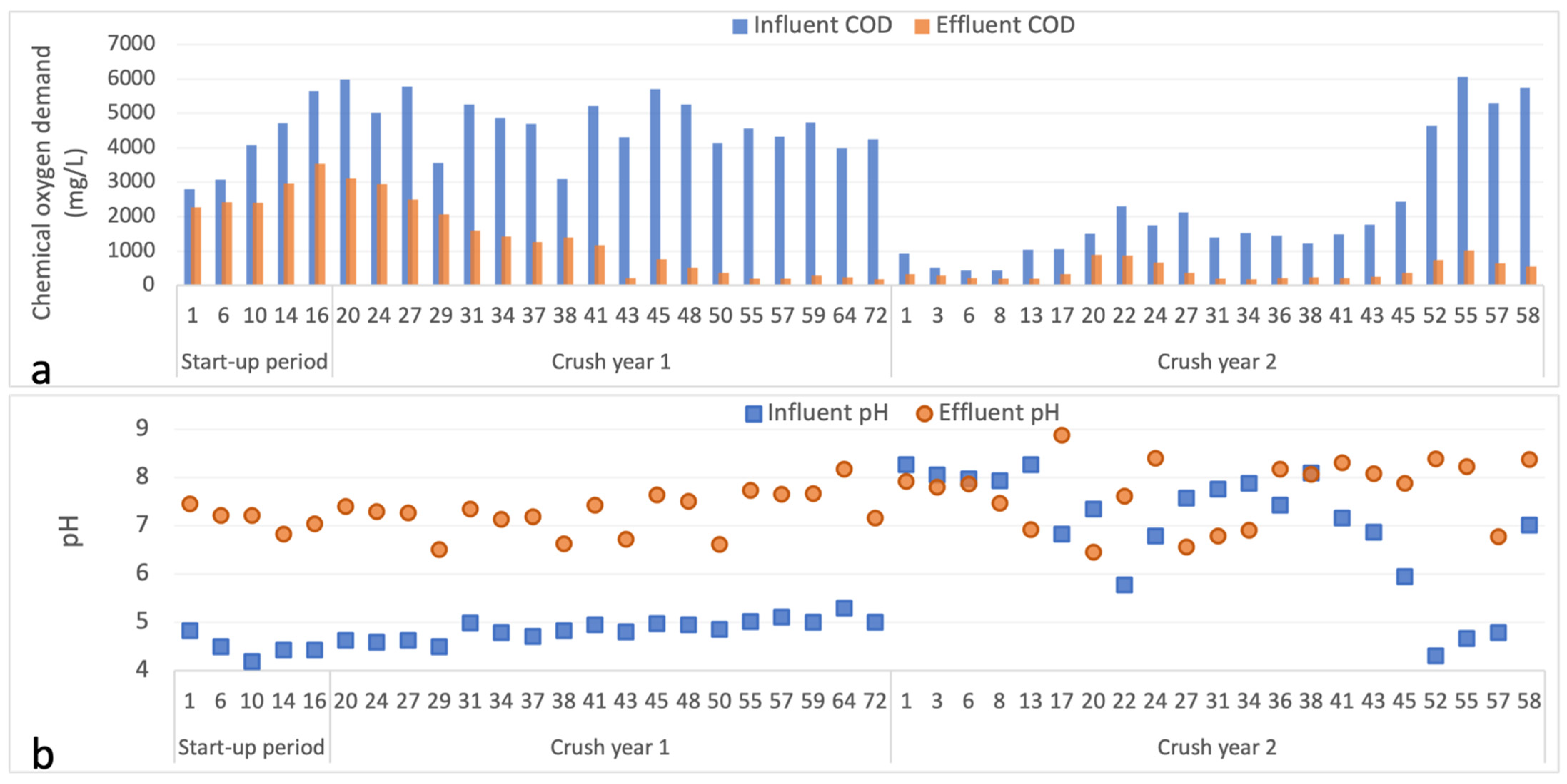
3.1. Operational Parameters
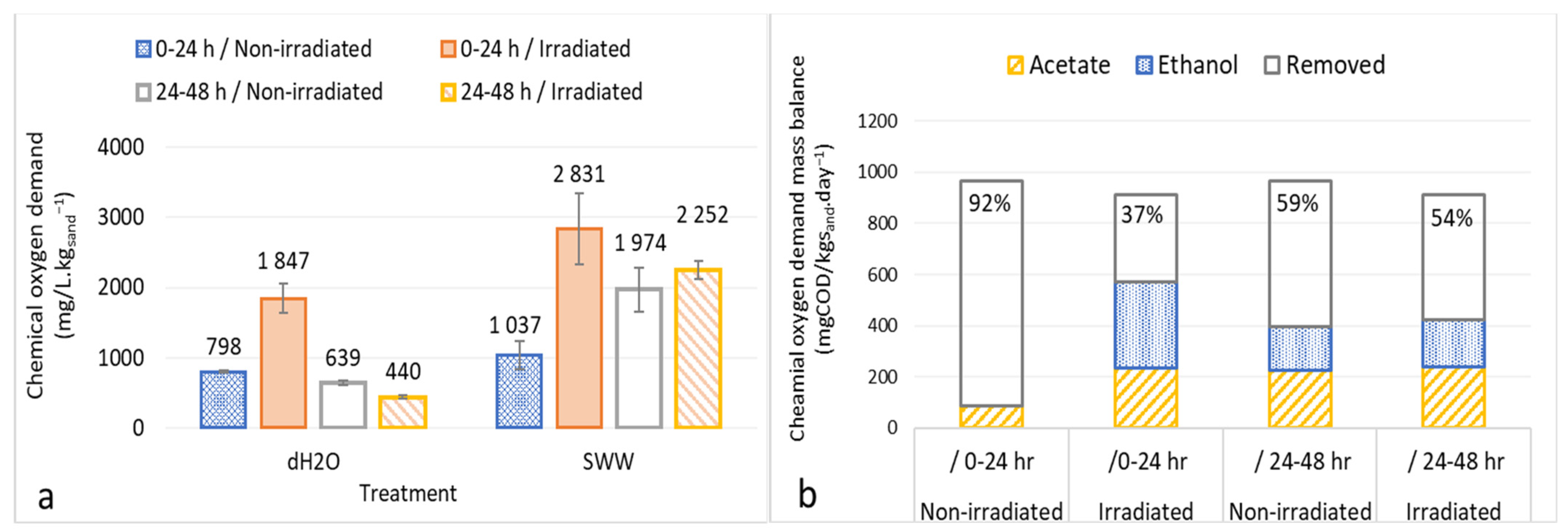
3.2. Organic Biodegradation in Irradiated and Non-Irradiated Columns
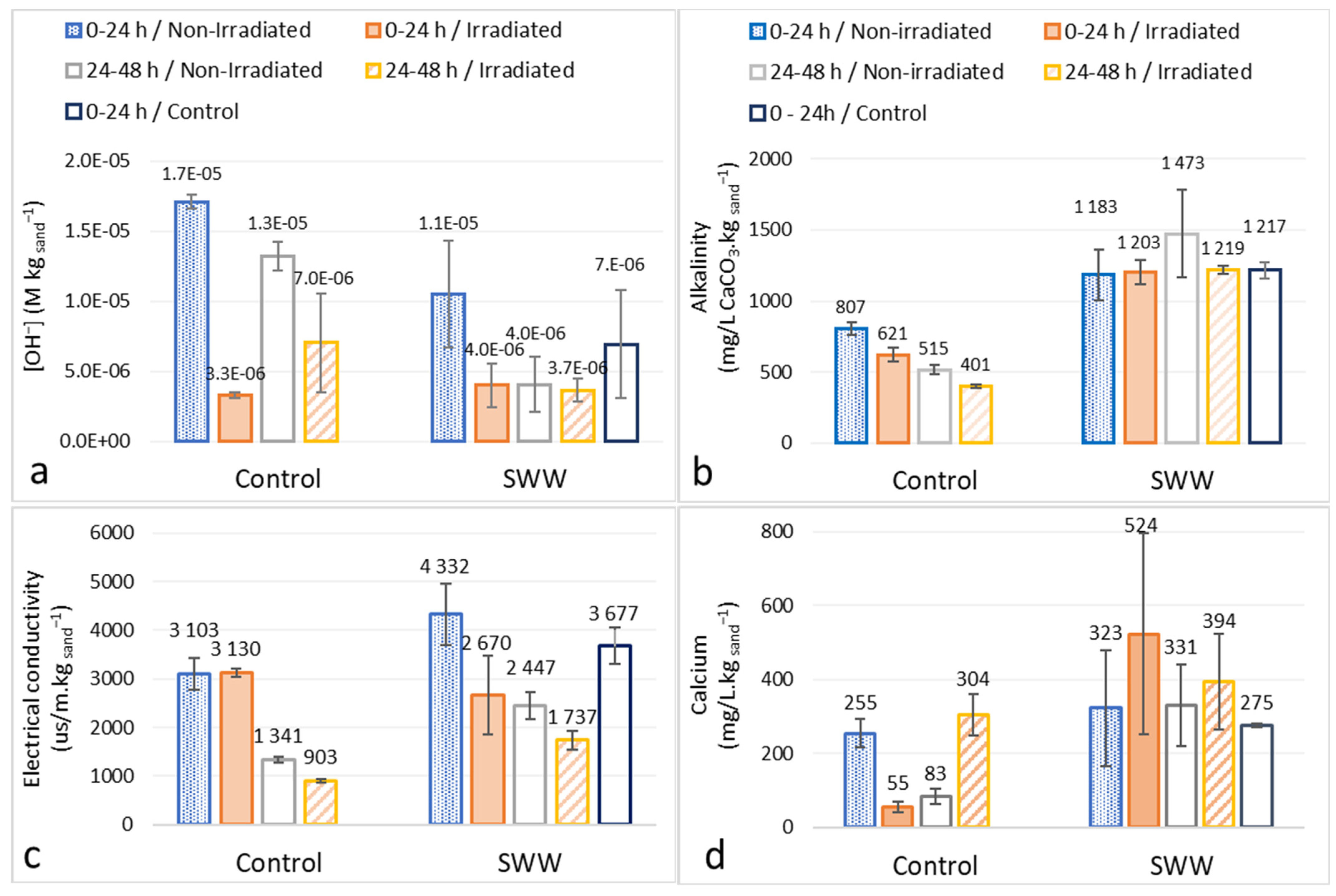
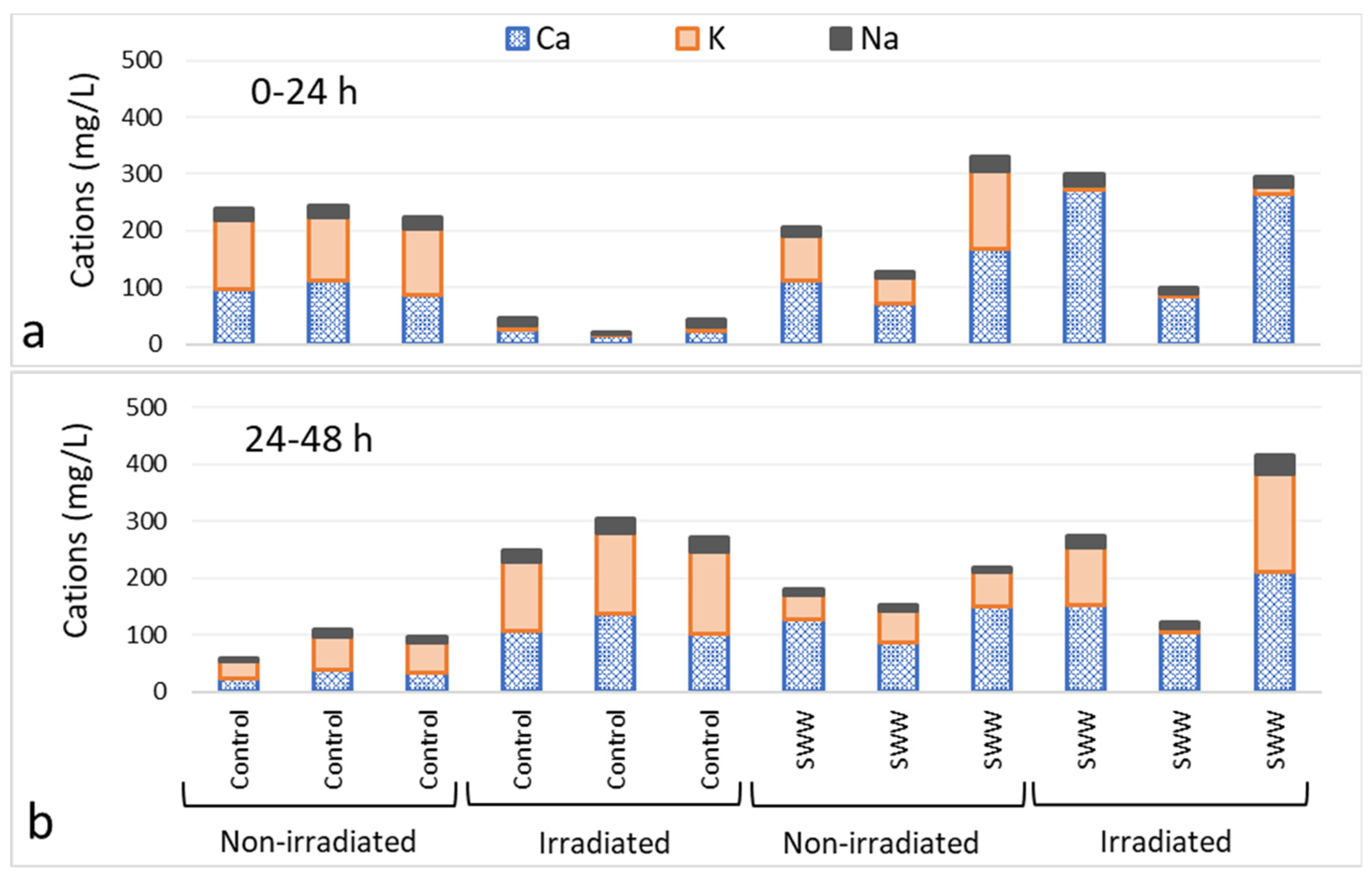
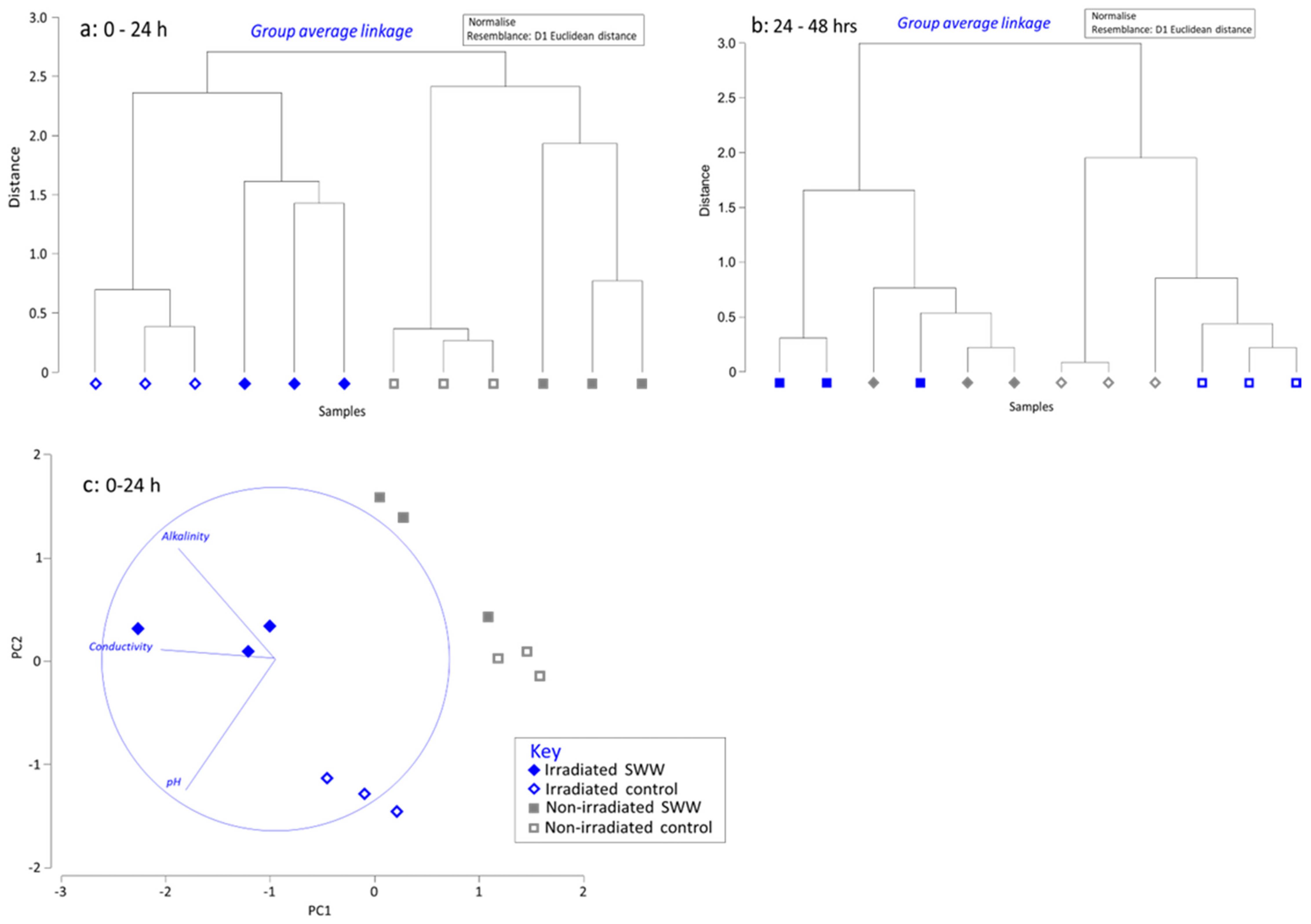
3.3. Analysis of Eluant Hydroxide Ion, Alkalinity and Calcium Concentrations and Electrical Conductivity
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtman, G.A.; Haldenwang, R.; Welz, P.J. Biological sand filter system treating winery effluent for effective reduction in organic load and pH neutralisation. J. Water Process. Eng. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Welz, P.J.; Holtman, G.; Haldenwang, R.; Le Roes-Hill, M.; Roes-hill, M. Characterisation of winery wastewater from continuous flow settling basins and waste stabilisation ponds over the course of 1 year: Implications for biological wastewater treatment and land application. Water Sci. Technol. 2016, 74, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Nadaraja, A.V.; Friesen, J.; Gill, K.; Lam, M.I.; Roberts, D.J. Narrow pH tolerance found for a microbial fuel cell treating winery wastewater. J. Appl. Microbiol. 2021, 131, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Holtman, G.A.; Haldenwang, R.; Welz, P.J. Comparison of continuous and pulse mode of operation of pilot biosand reactors treating winery effluent. Ecol. Eng. 2022, 182, 106706. [Google Scholar] [CrossRef]
- Holtman, G.A.; Haldenwang, R.; Welz, P.J. Effect of Particle Character and Calcite Dissolution on the Hydraulic Conductivity and Longevity of Biosand Filters Treating Winery and Other Acidic Effluents. Water 2022, 14, 2603. [Google Scholar] [CrossRef]
- Welz, P.J.; Mbasha, W.; Smith, I.; Holtman, G.; Terblanche, G.; Le Roes-Hill, M.; Haldenwang, R. The influence of grain physicochemistry and biomass on hydraulic conductivity in sand-filled treatment wetlands. Ecol. Eng. 2018, 116, 21–30. [Google Scholar] [CrossRef]
- Kaira, W.M.; Kimpiab, E.; Mpofu, A.B.; Holtman, G.A.; Ranjan, A.; Welz, P.J. Anaerobic digestion of primary winery wastewater sludge and evaluation of the character of the digestate as a potential fertilizer. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Yadav, R.K.; Minhas, P.S.; Saini, U. Long-Term Effect of Alkali and Partially Neutralized Irrigation Water on Soil Quality. J. Soil Sci. Plant Nutr. 2022, 22, 1252–1266. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yoon, H. Exploitation of acetic acid for calcite dissolution in small-capacity desalination plants. Desalination 2021, 516, 115227. [Google Scholar] [CrossRef]
- Larraguibel, A.; Navarrete-Calvo, A.; García, S.; Armijos, V.F.; Caraballo, M.A. Exploring sulfate and metals removal from Andean acid mine drainage using CaCO3-rich residues from agri-food industries and witherite (BaCO3). J. Clean Prod. 2020, 274, 123450. [Google Scholar] [CrossRef]
- Turingan, C.O.A.; Singson, G.B.; Melchor, B.T.; Alorro, R.D.; Beltran, A.B.; Orbecido, A.H. Evaluation of efficiencies of locally available neutralizing agents for passive treatment of acid mine drainage. Minerals 2020, 10, 845. [Google Scholar] [CrossRef]
- Le Bourre, B.; Neculita, C.M.; Coudert, L.; Rosa, E. Manganese removal processes and geochemical behavior in residues from passive treatment of mine drainage. Chemosphere 2020, 259, 127424. [Google Scholar] [CrossRef]
- Offeddu, F.G.; Cama, J.; Soler, J.M.; Dávila, G.; McDowell, A.; Craciunescu, T.; Tiseanu, I. Processes affecting the efficiency of limestone in passive treatments for AMD: Column experiments. J. Environ. Chem. Eng. 2015, 3, 304–316. [Google Scholar] [CrossRef]
- Welz, P.J.; le Roes-Hill, M. Biodegradation of organics and accumulation of metabolites in experimental biological sand filters used for the treatment of synthetic winery wastewater: A mesocosm study. J. Water Process. Eng. 2014, 3, 155–163. [Google Scholar] [CrossRef]
- Coral, T.; Placko, A.-L.; Beaufort, D.; Tertre, E.; Bernier-Latmani, R.; Descostes, M.; De Boissezon, H.; Guillon, S.; Rossi, P. Biostimulation as a sustainable solution for acid neutralization and uranium immobilization post acidic in-situ recovery. Sci. Total Environ. 2022, 822, 153597. [Google Scholar] [CrossRef] [PubMed]
- Biermann, V.; Lillicrap, A.M.; Magana, C.; Price, B.; Bell, R.W.; Oldham, C.E. Applicability of passive compost bioreactors for treatment of extremely acidic and saline waters in semi-arid climates. Water Res. 2014, 55, 83–94. [Google Scholar] [CrossRef][Green Version]
- Gomes, H.I.; Rogerson, M.; Burke, I.T.; Stewart, D.I.; Mayes, W.M. Hydraulic and biotic impacts on neutralisation of high-pH waters. Sci. Total Environ. 2017, 601, 1271–1279. [Google Scholar] [CrossRef]
- You, F.; Ma, Y.; Huang, L. Pre-culturing soil microbial inoculum in plant residues enhanced the resilience of tolerant bacteria and bioneutralization efficacy in alkaline bauxite residues. Sci. Total Environ. 2022, 822, 153627. [Google Scholar] [CrossRef]
- Santini, T.C.; Wang, J.C.; Warren, K.L.; Pickering, G.; Raudsepp, M.J. Simple Organic Carbon Sources and High Diversity Inocula Enhance Microbial Bioneutralization of Alkaline Bauxite Residues. Environ. Sci. Technol. 2021, 55, 3929–3939. [Google Scholar] [CrossRef]
- Rakotonimaro, T.V.; Neculita, C.M.; Bussière, B.; Zagury, G.J. Comparative column testing of three reactive mixtures for the bio-chemical treatment of iron-rich acid mine drainage. Miner. Eng. 2017, 111, 79–89. [Google Scholar] [CrossRef]
- Kölbl, A.; Bucka, F.; Marschner, P.; Mosley, L.; Fitzpatrick, R.; Schulz, S.; Lueders, T.; Kögel-Knabner, I. Consumption and alteration of different organic matter sources during remediation of a sandy sulfuric soil. Geoderma 2019, 347, 220–232. [Google Scholar] [CrossRef]
- Ramond, J.B.; Welz, P.J.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Assessment of temporal and spatial evolution of bacterial communities in a biological sand filter mesocosm treating winery wastewater. J. Appl. Microbiol. 2013, 115, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Welz, P.J.; Ramond, J.B.; Cowan, D.A.; Burton, S.G. Phenolic removal processes in biological sand filters, sand columns and microcosms. Bioresour. Technol. 2012, 119, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Welz, P.J.; Ramond, J.B.; Cowan, D.A.; Prins, A.; Burton, S.G. Ethanol degradation and the benefits of incremental priming in pilot-scale constructed wetlands. Ecol. Eng. 2011, 37, 1453–1459. [Google Scholar] [CrossRef]
- Bank, T.L.; Kukkadapu, R.K.; Madden, A.S.; Ginder-Vogel, M.A.; Baldwin, M.E.; Jardine, P.M. Effects of gamma-sterilization on the physico-chemical properties of natural sediments. Chem. Geol. 2008, 251, 1–7. [Google Scholar] [CrossRef]
- McNamara, N.P.; Black, H.I.J.; Beresford, N.A.; Parekh, N.R. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 2003, 24, 117–132. [Google Scholar] [CrossRef]
| Influent | Flow Rate (mL/h) | HLR (L/m3sand.day−1) | OLR (gCOD/m3sand.day−1) | HRT (h) |
|---|---|---|---|---|
| Control | 8.3 | 808 | NA | 8.7 |
| SWW | 8.3 | 808 | NA | 8.7 |
| Sand Height (mm) | Sand Weight (g) | HLR (L/m3sand.day−1) | HRT (h) | |
|---|---|---|---|---|
| Control | 356 ± 8.4 | 383 ± 9.7 | 796 ± 19 | 8.81 ± 0.21 |
| (346–361) | (375–394) | (784–818) | (8.57–8.94) | |
| Control IR | 352 ± 6.8 | 380 ± 8.7 | 805 ± 16 | 8.71 ± 0.17 |
| (344–357) | (370–386) | (793–822) | (8.52–8.84) | |
| SWW | 358 ± 22 | 373 ± 30 | 792 ± 48 | 8.87 ± 0.53 |
| (337–380) | (344–404) | (745–840) | (8.35–9.41) | |
| SWW IR | 363 ± 4 | 396 ± 4.5 | 779 ± 8.6 | 8.99 ± 0.10 |
| (359–367) | (391–400) | (771–788) | (8.89–9.09) | |
| SWW cont. | 316 ± 5 | 376 ± 0 | 896 ± 14.2 | 7.83 ± 0.15 |
| (311–321) | (376–376) | (881–910) | (7.70–7.95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holtman, G.A.; Haldenwang, R.; Welz, P.J. Calcite Dissolution and Bioneutralization of Acidic Wastewater in Biosand Reactors. Water 2022, 14, 3482. https://doi.org/10.3390/w14213482
Holtman GA, Haldenwang R, Welz PJ. Calcite Dissolution and Bioneutralization of Acidic Wastewater in Biosand Reactors. Water. 2022; 14(21):3482. https://doi.org/10.3390/w14213482
Chicago/Turabian StyleHoltman, Gareth Alistair, Rainer Haldenwang, and Pamela Jean Welz. 2022. "Calcite Dissolution and Bioneutralization of Acidic Wastewater in Biosand Reactors" Water 14, no. 21: 3482. https://doi.org/10.3390/w14213482
APA StyleHoltman, G. A., Haldenwang, R., & Welz, P. J. (2022). Calcite Dissolution and Bioneutralization of Acidic Wastewater in Biosand Reactors. Water, 14(21), 3482. https://doi.org/10.3390/w14213482