Nitrogen Doped Cobalt Anchored on the Used Resin-Based Catalyst to Activate Peroxymonosulfate for the Removal of Ibuprofen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
- (1)
- Main drugs and reagents: Used D001 resin (Jinkai resin company, Yancheng, China), Dicyandiamide (DCD, CAS: 461-58-5, AR, Yatai chemical reagent), Ibuprofen (IBU, CAS: 15687-27-1, Shanghai aladdln biochemical technology), Peroxymonosulfate (KHSO5, CAS: 70693-62-8, 45%, Sinopharm chemical reagent), Cobalt nitrate hexahydrate (CAS: 10026-22-9, AR, Sinopharm chemical reagent), Phosphoric acid (CAS: 7664-38-2, AR, Sinopharm chemical reagent), Methanol (MeOH, CAS: 67-56-1, AR, Sinopharm chemical reagent), Acetonitrile (CAS: 75-05-8, BR, TEDIA), Ethanol (Eth, CAS: 64-17-5, AR, Sinopharm chemical reagent), Tert butyl alcohol (TBA, CAS: 75-65-0, AR, Sinopharm chemical reagent), L-histidine (L-his, CAS: 71-00-1, AR, Sinopharm chemical reagent), Furfuryl alcohol (FFA, CAS: 98-00-0, AR, China Huixing biochemical reagent), p-BenzoQuinone (p-BQ, CAS: 106-51-4, 99%, Shanghai aladdln biochemical technology), Sodium bicarbonate (CAS: 144-55-8, GR, Nanjing chemical reagent), Anhydrous sodium sulfate (CAS: 7757-82-6, AR, Sinopharm chemical reagent), Sodium dihydrogen phosphate (CAS: 13472-35-0, AR, Shanghai Lingfeng chemical reagent), Sodium chloride (CAS: 7647-14-5, AR, Xilong scientific and chemical experimental reagent), etc.
- (2)
- Main instruments: Water bath thermostatic oscillator, Thermostatic drying oven, Tubular furnace, High-performance liquid chromatograph (HPLC), Total organic carbon analyzer (TOC), Liquid chromatography mass spectrometer (LC-MS), Inductively coupled plasma optical emission spectrometry (ICP-OES), Scanning electron microscope (SEM), Electron emission spectrometer (EDS), Transmission scanning electron microscope (TEM), X-ray diffractometer (XRD), Fourier transform infrared (FT-IR). X-ray photoelectron spectroscopy (XPS), Brunauer Emmett Teller (BET), and electron paramagnetic resonance spectroscopy (EPR) were performed.
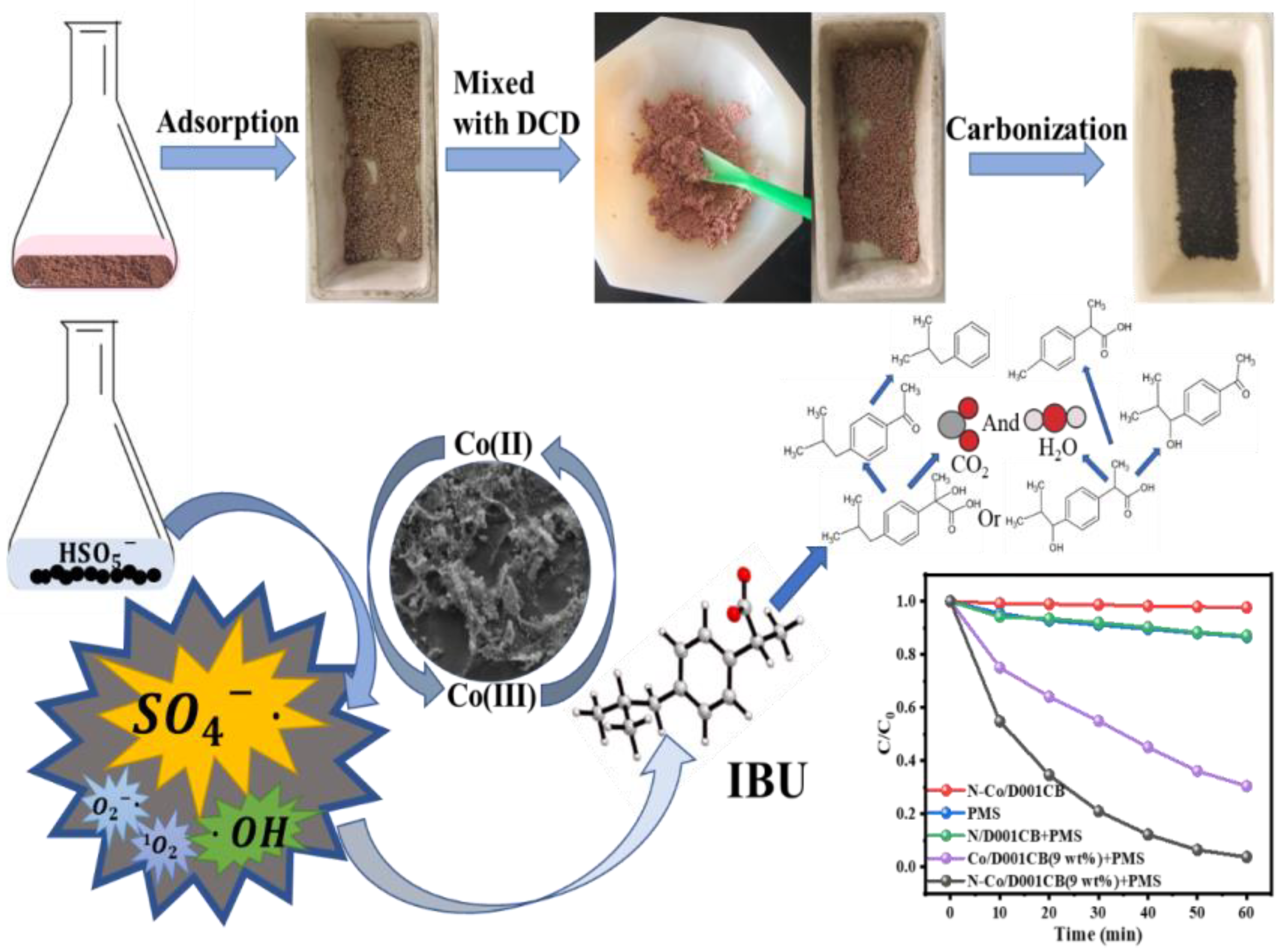
2.2. N-Co/D001CB Catalyst Preparation
2.3. Characterization and Analytical Method
2.4. Catalytic Activity Study
3. Results and Discussion
3.1. Material Characterization of N-Co/D001CB Catalyst
3.2. Catalytic Activity of N-Co/D001CB Catalyst
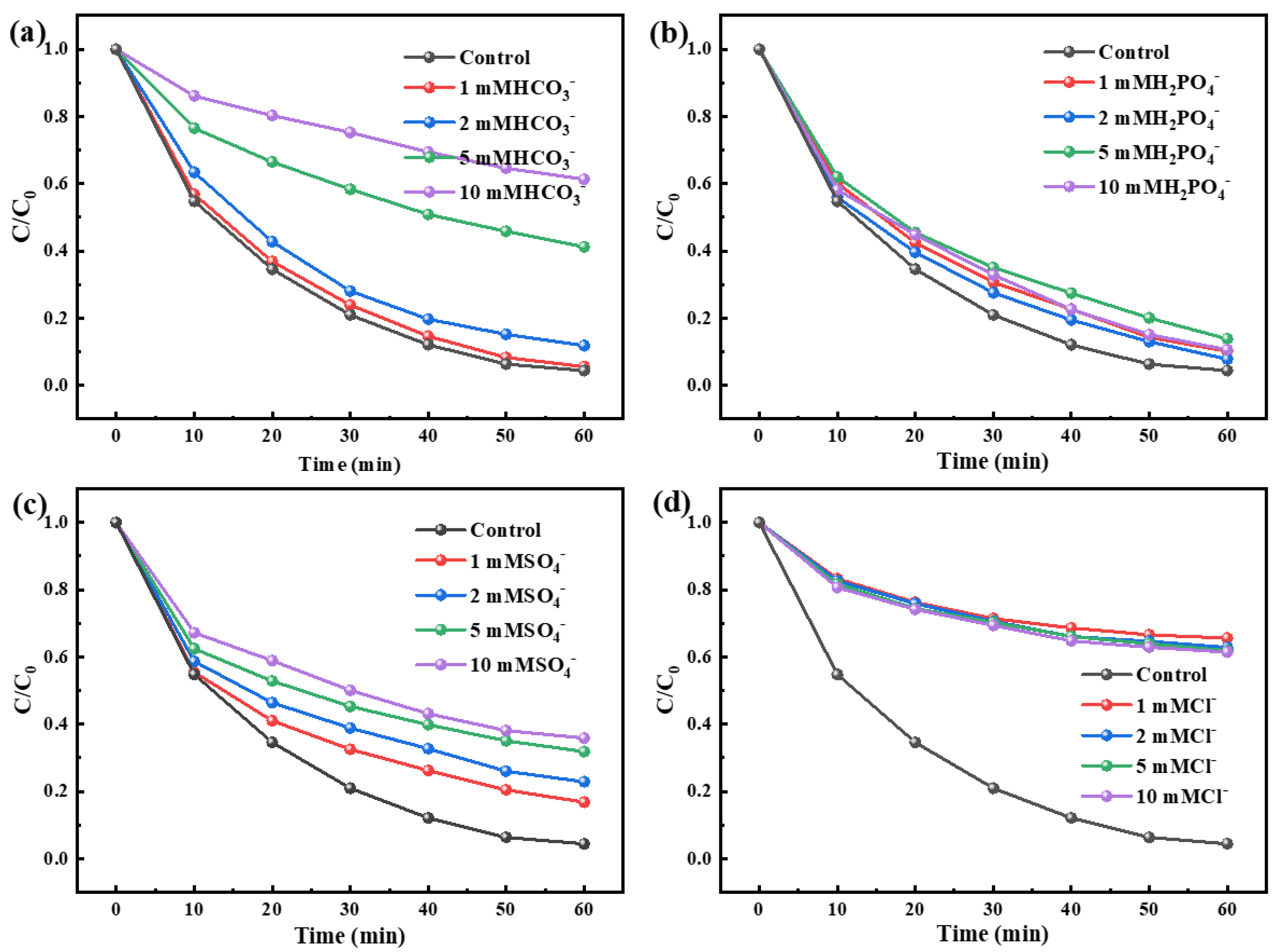
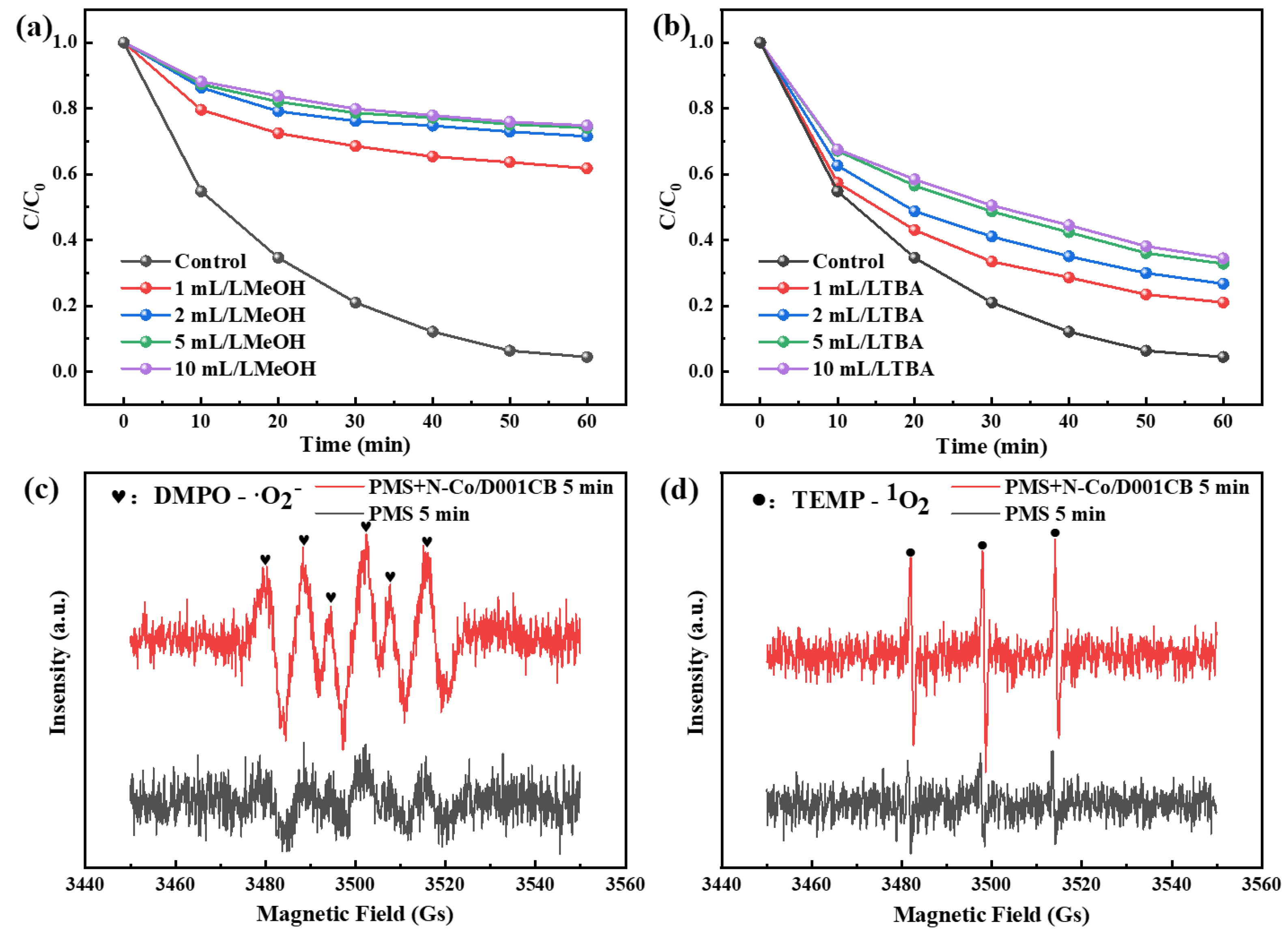
3.3. Mechanism Exploration of Ibuprofen Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Oyekunle, D.T.; Wu, B.; Luo, F.; Ali, J.; Chen, Z. Synergistic effects of Co and N doped on graphitic carbon as an in situ surface-bound radical generation for the rapid degradation of emerging contaminants. Chem. Eng. J. 2021, 421, 129818. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.H.; Xiong, W.P.; Zhou, Y.Y.; Wu, Y.; Jia, M.Y.; Sun, S.W.; Zhou, C.Y.; Zhang, Y.R.; Zhong, R.H. Peroxymonosulfate Activation of Magnetic Co Nanoparticles Relative to an N-Doped Porous Carbon Under Confinement: Boost-ing Stability and Performance. Sep. Purif. Technol. 2020, 250, 117237. [Google Scholar] [CrossRef]
- Brillas, E. A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. Chemosphere 2022, 286, 131849. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M. Degradation of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solution, a Direct Comparison of Ozonation, Photocatalysis, and Non-Thermal Plasma. Chem. Eng. J. 2017, 313, 1033–1041. [Google Scholar] [CrossRef]
- Sun, S.P.; Zeng, X.; Lemley, A.T. Nano-Magnetite Catalyzed Heterogeneous Fenton-Like Degradation of Emerging Con-taminants Carbamazepine and Ibuprofen in Aqueous Suspensions and Montmorillonite Clay Slurries at Neutral pH. J. Mol. Catal. A Chem. 2013, 371, 94–103. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.C.; Ren, Y.M.; Park, B.; Ma, J.J.; Han, Z.C.; Khim, J. Activation of Peroxodisulfate and Peroxymono-sulfate by Ultrasound with Different Frequencies: Impact On Ibuprofen Removal Efficient, Cost Estimation and Energy Analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Wu, X.; Sun, D.; Ma, H.; Ma, C.; Zhang, X.; Hao, J. Activation of peroxymonosulfate by magnetic CuFe2O4@ZIF-67 composite catalyst for the study on the degradation of methylene blue. Colloids Surfaces A 2022, 637, 128278. [Google Scholar] [CrossRef]
- Naderi, M.; Soltani, R.D.C. Hybrid of ZnFe layered double hydroxide/nano-scale carbon for activation of peroxymonosulfate to decompose ibuprofen: Thermodynamic and reaction pathways investigation. Environ. Technol. Innov. 2021, 24, 101951. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Zeng, H.X.; Deng, L.; Zhang, H.J.; Zhou, C.; Shi, Z. Development of Oxygen Vacancies Enriched Coal Hydrox-ide@Hydroxysulfide Hollow Flowers for Peroxymonosulfate Activation: A Highly Efficient Singlet Oxygen-Dominated Oxidation Process for Sulfamethoxazole Degradation. J. Hazard. Mater. 2020, 400, 123297. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Xie, G.S.; Shao, P.H.; Ren, W.; Li, M.L.; Hu, Y.F.; Yang, L.M.; Shi, H.; Luo, X.B. A Comparison of Smx Degra-dation by Persulfate Activated with Different Nanocarbons: Kinetics, Transformation Pathways, and Toxicity. Appl. Catal. B Environ. 2022, 310, 121345. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.Y.; Chen, Z.L.; Chen, Z.Q. Non-Radical Pms Activation by the Nano-hybrid Material with Periodic Confinement of Reduced Graphene Oxide (RGO) and Cu Hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Shao, B.B.; Yan, M.; Liu, Z.F.; Liang, Q.H.; He, Q.Y.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of Peroxymonosulfate by Biochar-Based Catalysts and Applications in the Degradation of Organic Contaminants: A Review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wang, J. Nitrogen, sulfur and oxygen co-doped carbon-armored Co/Co9S8 rods (Co/Co9S8@N-S-O-C) as efficient activator of peroxymonosulfate for sulfamethoxazole degradation. J. Hazard. Mater. 2020, 387, 121669. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765, 142794. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal And Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (Pms) Activation, with Focus On Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Wang, L.L.; Guo, X.; Chen, Y.Y.; Ai, S.S.; Ding, H.M. Cobalt-Doped G-C3N4 as a Heterogeneous Catalyst for Photo-Assisted Activation of Peroxymonosulfate for the Degradation of Organic Contaminants. Appl. Surf. Sci. 2019, 467, 954–962. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Li, W.; Lan, Y.; Chen, C. Performance Comparison and Mechanism Investigation of Co3O4-Modified Different Crystallographic Mno2 (A, Β, Γ, and Δ) as an Activator of Peroxymonosulfate (Pms) for Sulfisoxazole Degradation. Chem. Eng. J. 2022, 427, 130888. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Wang, J. Degradation of sulfamethoxazole using PMS activated by cobalt sulfides encapsulated in nitrogen and sulfur co-doped graphene. Sci. Total Environ. 2022, 827, 154379. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Zhao, J.; Liu, W.; Ji, H. Efficient activation of peroxymonosulfate by hollow cobalt hydroxide for degradation of ibuprofen and theoretical study. Chin. Chem. Lett. 2019, 30, 2191–2195. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, J.E.; Duan, X.; Wang, S.; Sun, D.D.; Yuan, B.; Fu, M. Engineered Co2AlO4/CoAl2O4@Al2O3 Monolithic Cata-lysts for Peroxymonosulfate Activation: Co3+/Co2+ and Odefect/Olattice Ratios Dependence and Mechanism. Chem. Eng. J. 2021, 409, 128162. [Google Scholar] [CrossRef]
- Yu, Y.; Li, N.; Wang, C.; Cheng, Z.; Yan, B.; Chen, G.; Hou, L.; Wang, S. Iron cobalt and nitrogen co-doped carbonized wood sponge for peroxymonosulfate activation: Performance and internal temperature-dependent mechanism. J. Colloid Interface Sci. 2022, 619, 267–279. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wang, H.; Cao, Y.; Yu, H.; Peng, F. Co9S8-porous carbon spheres as bifunctional electrocatalysts with high activity and stability for oxygen reduction and evolution reactions. Electrochim. Acta 2018, 265, 32–40. [Google Scholar] [CrossRef]
- Li, Q.; Song, H.; Ye, Y.; Pan, F.; Zhang, D.; Xia, D. A green designed copper-resin composite for highly efficient catalytic reduction of 4-nitrophenol. Colloids Interface Sci. Commun. 2021, 42, 100407. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, Y.; Zhang, X.; Sun, Y.; Wang, C.; Yu, P. Efficient Activation of Peroxymonosulfate by Cobalt Supported Used Resin Based Carbon Ball Catalyst for the Degradation of Ibuprofen. Materials 2022, 15, 5003. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, M.; Zhang, H.; Yan, X.; Xie, J.; Qi, J.; Sun, X.; Li, J. Dicyandiamide-assisted HKUST-1 derived Cu/N-doped porous carbon nanoarchitecture for electrochemical detection of acetaminophen. Environ. Res. 2021, 201, 111500. [Google Scholar] [CrossRef]
- Li, Y.; Ji, C.; Lu, Y.; Wu, L.; Sun, S.; Qu, R.; Sun, C.; Zhang, Y.; Xue, Z. In situ synthesis of carbon/g-C3N4 composites for visible light catalysis by facile one-step pyrolysis of partially formaldehyde-modified dicyandiamide. Mater. Chem. Phys. 2018, 214, 28–33. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, C.; Liu, Y.; Jiang, H.; Chen, R. Selective hydrogenation of phenol to cyclohexanone in water over Pd@N-doped carbons derived from ZIF-67: Role of dicyandiamide. Appl. Surf. Sci. 2017, 425, 484–491. [Google Scholar] [CrossRef]
- Yang, L.; Chen, W.; Sheng, C.; Wu, H.; Mao, N.; Zhang, H. Fe/N-codoped carbocatalysts loaded on carbon cloth (CC) for activating peroxymonosulfate (PMS) to degrade methyl orange dyes. Appl. Surf. Sci. 2021, 549, 149300. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. High Efficient Activation of Peroxymonosulfate by Co9S8 Anchored in N, S, O Co-Doped Carbon Composite for Degradation of Sulfamethoxazole: Effect of Sulfur Precursor and Sulfur Doping Content. Chem. Eng. J. 2022, 434, 134824. [Google Scholar] [CrossRef]
- Han, X.; Zhang, W.; Li, S.; Cheng, C.; Zhou, L.; Jia, Q.; Xiu, G. Efficient activation of peroxymonosulfate by MnS/Fe-MOF hybrid catalyst for sulfadiazine degradation: Synergistic effects and mechanism. Sep. Purif. Technol. 2022, 287, 120509. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Wang, J.; Wang, H.; Chen, Z.; Wang, S.; Chen, Z. Recycling application of modified waste electrolytic manganese anode slag as efficient catalyst for PMS activation. Sci. Total Environ. 2021, 762, 143120. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Brahma, S.; Hou, S.-C.; Chang, C.-C.; Huang, J.-L. Petroleum waste hydrocarbon resin as a carbon source modified on a Si composite as a superior anode material in lithium ion batteries. Mater. Chem. Phys. 2020, 259, 124011. [Google Scholar] [CrossRef]
- Yu, H.J.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.; Zhao, Y.F.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Nitro-gen-Doped Porous Carbon Nanosheets Templated From G-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Ding, Y.B.; Hu, Y.; Peng, X.Q.; Xiao, Y.W.; Huang, J. Micro-Nano Structured Cos: An Efficient Catalyst for Peroxymonosulfate Activation for Removal of Bisphenol A. Sep. Purif. Technol. 2020, 233, 116022. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.; Chen, S.; Zhang, D. Metal organic framework derived P-doping CoS@C with sulfide defect to boost high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2022, 624, 385–393. [Google Scholar] [CrossRef]
- Domga; Karnan, M.; Oladoyinbo, F.; Noumi, G.B.; Tchatchueng, J.B.; Sieliechi, M.J.; Sathish, M.; Pattanayak, D.K. A Simple, Economical One-Pot Microwave Assisted Synthesis of Nitrogen and Sulfur Co-Doped Graphene for High Energy Supercapacitors. Electrochim. Acta 2020, 341, 135999. [Google Scholar] [CrossRef]
- Wei, J.; Jiang, C.; Chen, B.; Li, X.; Zhang, H. Hollow C/Co9S8 hybrid polyhedra-modified carbon nanofibers as sulfur hosts for promising Li–S batteries. Ceram. Int. 2021, 47, 25387–25397. [Google Scholar] [CrossRef]
- Arunpandian, M.; Selvakumar, K.; Raja, A.; Rajasekaran, P.; Ramalingan, C.; Nagarajan, E.R.; Pandikumar, A.; Arunachalam, S. Rational Design of Novel Ternary Sm2Wo6/Zno/Go Nanocomposites: An Affordable Photocatalyst for the Mitigation of Carcinogenic Organic Pollutants. Colloids Surfaces A 2020, 596, 124721. [Google Scholar]
- Huang, H.; Yang, G.; Yu, J.; Zhang, J.; Xia, Y.; Wang, K.; Liang, C.; Gan, Y.; He, X.; Zhang, W. One-Pot Synthesis of Nano-crystalline Sns@Tremella-Like Porous Carbon by Supercritical Co2 Method for Excellent Sodium Storage Performance. Electrochim. Acta 2021, 373, 137933. [Google Scholar] [CrossRef]
- Jiang, J.B.; Zhu, L.Y.; Sun, Y.X.; Chen, Y.K.; Chen, H.T.; Han, S.; Lin, H.L. Fe2O3 Nanocatalysts On N-Doped Carbon Nanomaterial for Highly Efficient Electrochemical Hydrogen Evolution in Alkaline. J. Power Sources 2019, 426, 74–83. [Google Scholar] [CrossRef]
- Liu, X.X.; Wu, R.; Wang, Y.; Xiao, S.H.; He, Q.; Bin Niu, X.; Blackwood, D.J.; Chen, J.S. Self-supported core/shell Co3O4@Ni3S2 nanowires for high-performance supercapacitors. Electrochim. Acta 2019, 311, 221–229. [Google Scholar] [CrossRef]
- Chen, P.; Gou, Y.J.; Ni, J.M.; Liang, Y.M.; Yang, B.Q.; Jia, F.F.; Song, S.X. Efficient Ofloxacin Degradation with Co(Ii)-Doped Mos2 Nano-Flowers as Pms Activator Under Visible-Light Irradiation. Chem. Eng. J. 2020, 401, 125978. [Google Scholar] [CrossRef]
- Ren, X.; Du, Y.Y.; Song, M.Y.; Zhou, Y.H.; Chen, Y.J.; Ma, F.W.; Wan, J.F. In-Situ Transformation of Ni Foam Into Sandwich Nanostructured Co1.29Ni1.71O4 Nanoparticle@Coni2S4 Nanosheet Networks for High-Performance Asymmetric Supercapacitors. Chem. Eng. J. 2019, 375, 122063. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Ji, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Nickel/cobalt bimetallic metal-organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. J. Alloys Compd. 2020, 825, 154069. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Li, C.; Cao, H.L.; Wang, C.Z.; Cheng, S.Y.; Zhang, Q. A Novel Strategy of Multi-Element Nanocomposite Synthesis for High Performance Zno-Cose2 Supercapacitor Material Development. Chin. J. Chem. 2021, 39, 2441–2450. [Google Scholar] [CrossRef]
- Qiu, W.D.; Xiao, H.B.; Yu, M.H.; Li, Y.; Lu, X.H. Surface Modulation of NiCo2O4 Nanowire Arrays with Significantly Enhanced Reactivity for Ultrahigh-Energy Supercapacitors. Chem. Eng. J. 2018, 352, 996–1003. [Google Scholar] [CrossRef]
- Fang, Z.; Qi, J.; Xu, Y.; Liu, Y.; Qi, T.; Xing, L.; Dai, Q.; Wang, L. Promoted generation of singlet oxygen by hollow-shell CoS/g-C3N4 catalyst for sulfonamides degradation. Chem. Eng. J. 2022, 441, 136051. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, M.; Xie, C.; Wang, H.; Wang, J.; Chen, Z.; Xiao, J.; Chen, Z. Tunable S doping from Co3O4 to Co9S8 for peroxymonosulfate activation: Distinguished Radical/Nonradical species and generation pathways. Appl. Catal. B Environ. 2020, 282, 119605. [Google Scholar] [CrossRef]
- Ren, Z.; Romar, H.; Varila, T.; Xu, X.; Wang, Z.; Sillanpää, M.; Leiviskä, T. Ibuprofen degradation using a Co-doped carbon matrix derived from peat as a peroxymonosulphate activator. Environ. Res. 2021, 193, 110564. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.J.; Pi, Z.J.; Chen, F.; He, L.; Yao, F.B.; Chen, S.J.; Li, X.M.; Wang, D.B.; Dong, H.R.; Yang, Q. Peroxymonosulfate (PMS) Activation by Mackinawite for the Degradation of Organic Pollutants: Underappreciated Role of Dissolved Sulfur Derivatives. Sci. Total Environ. 2022, 834, 151421. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, N.; Wang, D.; Wang, L.; Song, Y.; Chen, Z.; Ma, J.; Zhang, T. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways. Appl. Catal. B Environ. 2020, 263, 118350. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.; Li, R.; Cao, X.; Kan, Y.; Wei, B.; Sun, X. Efficient degradation of ibuprofen by Co/Fe@CNFs catalyst in the presence of peroxymonosulfate and persulfate: Characterization, performance, and mechanism comparison. J. Taiwan Inst. Chem. Eng. 2022, 131, 104161. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Y.; Nie, W.; Tang, H. Efficient degradation of drug ibuprofen through catalytic activation of peroxymonosulfate by Fe3C embedded on carbon. J. Environ. Sci. 2019, 78, 1–12. [Google Scholar] [CrossRef]
- Li, M.; Zhong, H.; He, Z.; Hu, L.; Sun, W.; Loganathan, P.; Xiong, D. Degradation of various thiol collectors in simulated and real mineral processing wastewater of sulfide ore in heterogeneous modified manganese slag/PMS system. Chem. Eng. J. 2021, 413, 127478. [Google Scholar] [CrossRef]
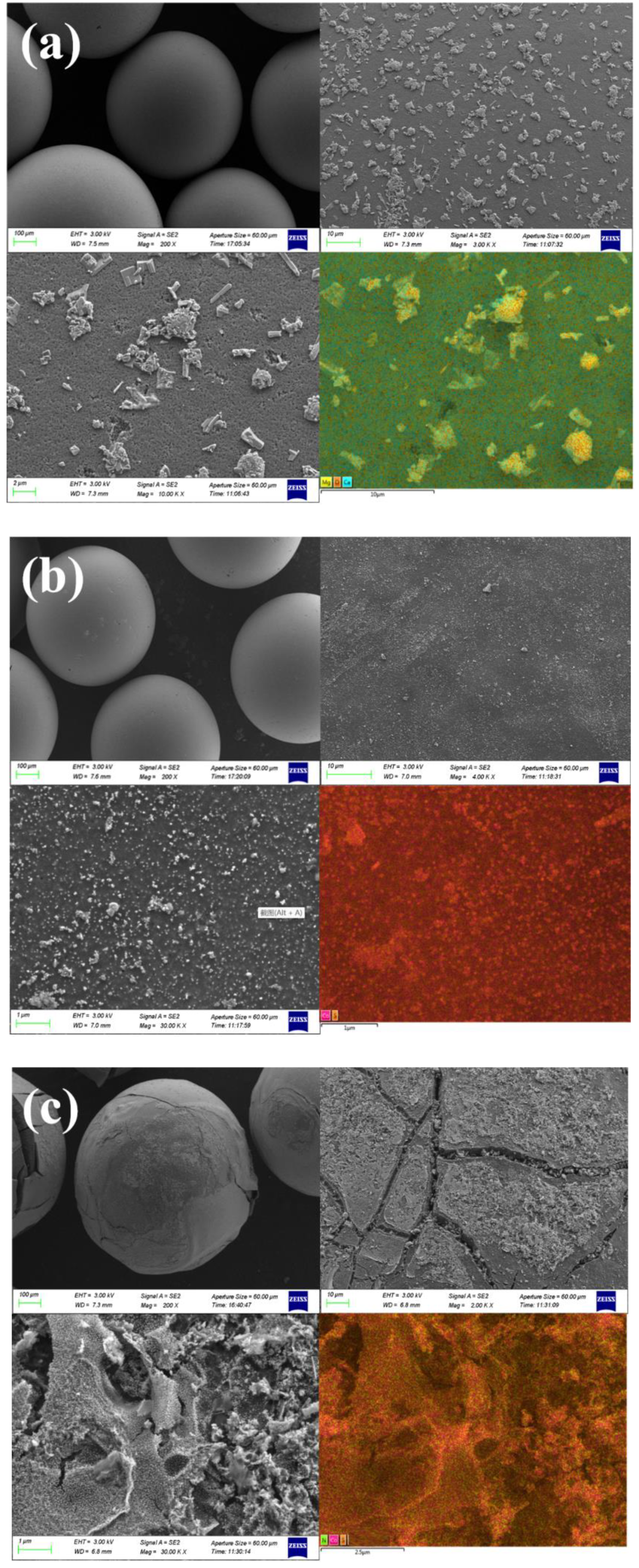


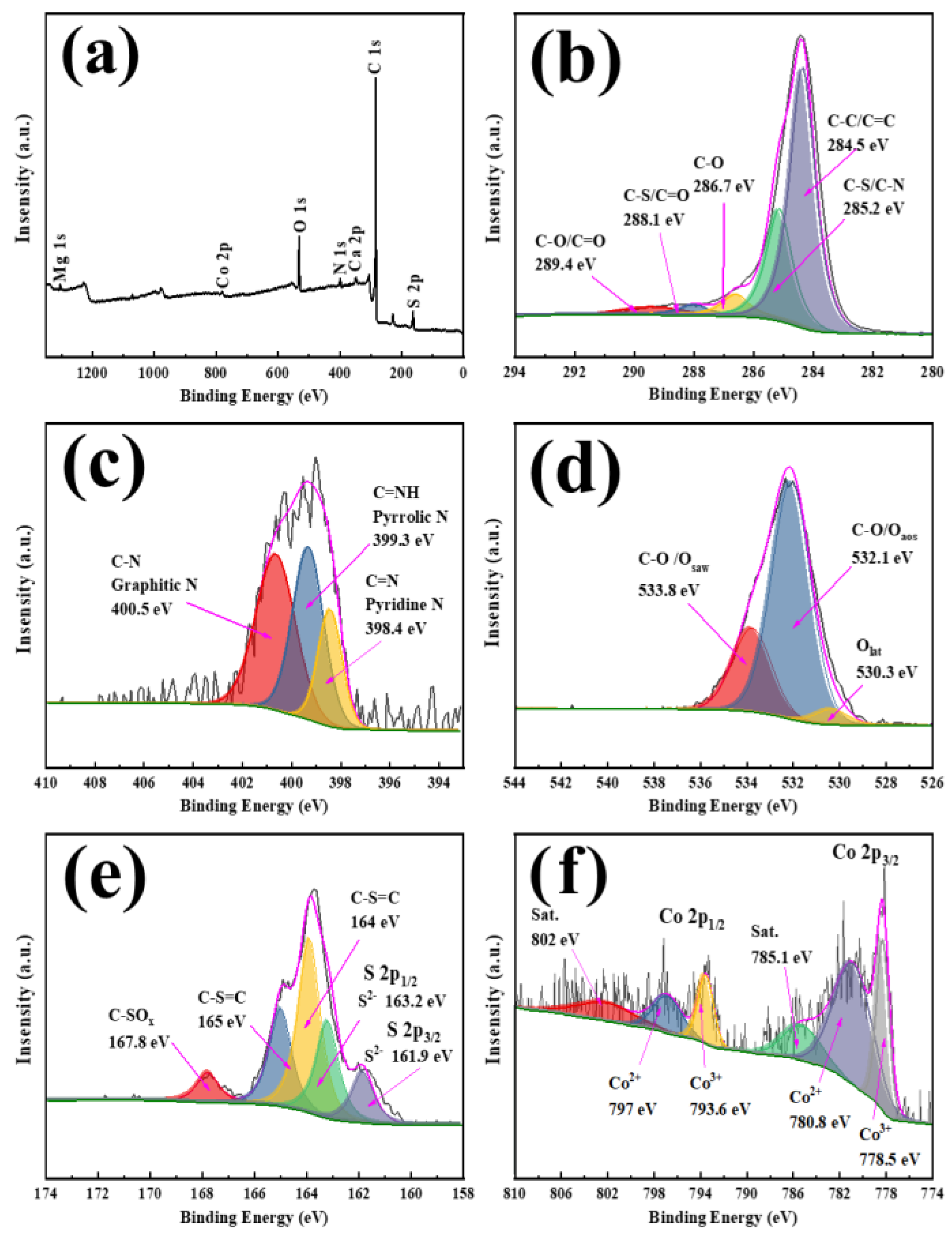



| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Siameter (nm) |
|---|---|---|---|
| Fresh D001 carbon ball | 97.6269 | 0.057726 | 2.36517 |
| Used D001 carbon ball | 67.4460 | 0.046196 | 2.73971 |
| Co/D001CB (3 wt%) | 31.2506 | 0.021145 | 2.70650 |
| Co/D001CB (9 wt%) | 22.8031 | 0.015183 | 2.66338 |
| Co/D001CB (15 wt%) | 6.9895 | 0.004999 | 2.86088 |
| N/D001CB (DCD 5 mmol/g) | 177.9066 | 0.113703 | 2.55646 |
| N-Co/D001CB (DCD 3 mmol/g) | 166.3956 | 0.109752 | 2.63834 |
| N-Co/D001CB (DCD 5 mmol/g) | 269.3317 | 0.161926 | 2.43256 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhou, G.; Xu, Y.; Yu, P.; Sun, Y. Nitrogen Doped Cobalt Anchored on the Used Resin-Based Catalyst to Activate Peroxymonosulfate for the Removal of Ibuprofen. Water 2022, 14, 3754. https://doi.org/10.3390/w14223754
Wang C, Zhou G, Xu Y, Yu P, Sun Y. Nitrogen Doped Cobalt Anchored on the Used Resin-Based Catalyst to Activate Peroxymonosulfate for the Removal of Ibuprofen. Water. 2022; 14(22):3754. https://doi.org/10.3390/w14223754
Chicago/Turabian StyleWang, Cheng, Guangzhen Zhou, Yanhua Xu, Peng Yu, and Yongjun Sun. 2022. "Nitrogen Doped Cobalt Anchored on the Used Resin-Based Catalyst to Activate Peroxymonosulfate for the Removal of Ibuprofen" Water 14, no. 22: 3754. https://doi.org/10.3390/w14223754
APA StyleWang, C., Zhou, G., Xu, Y., Yu, P., & Sun, Y. (2022). Nitrogen Doped Cobalt Anchored on the Used Resin-Based Catalyst to Activate Peroxymonosulfate for the Removal of Ibuprofen. Water, 14(22), 3754. https://doi.org/10.3390/w14223754







