Photothermal Catalytic Degradation of Lomefloxacin with Nano Au/TiO2
Abstract
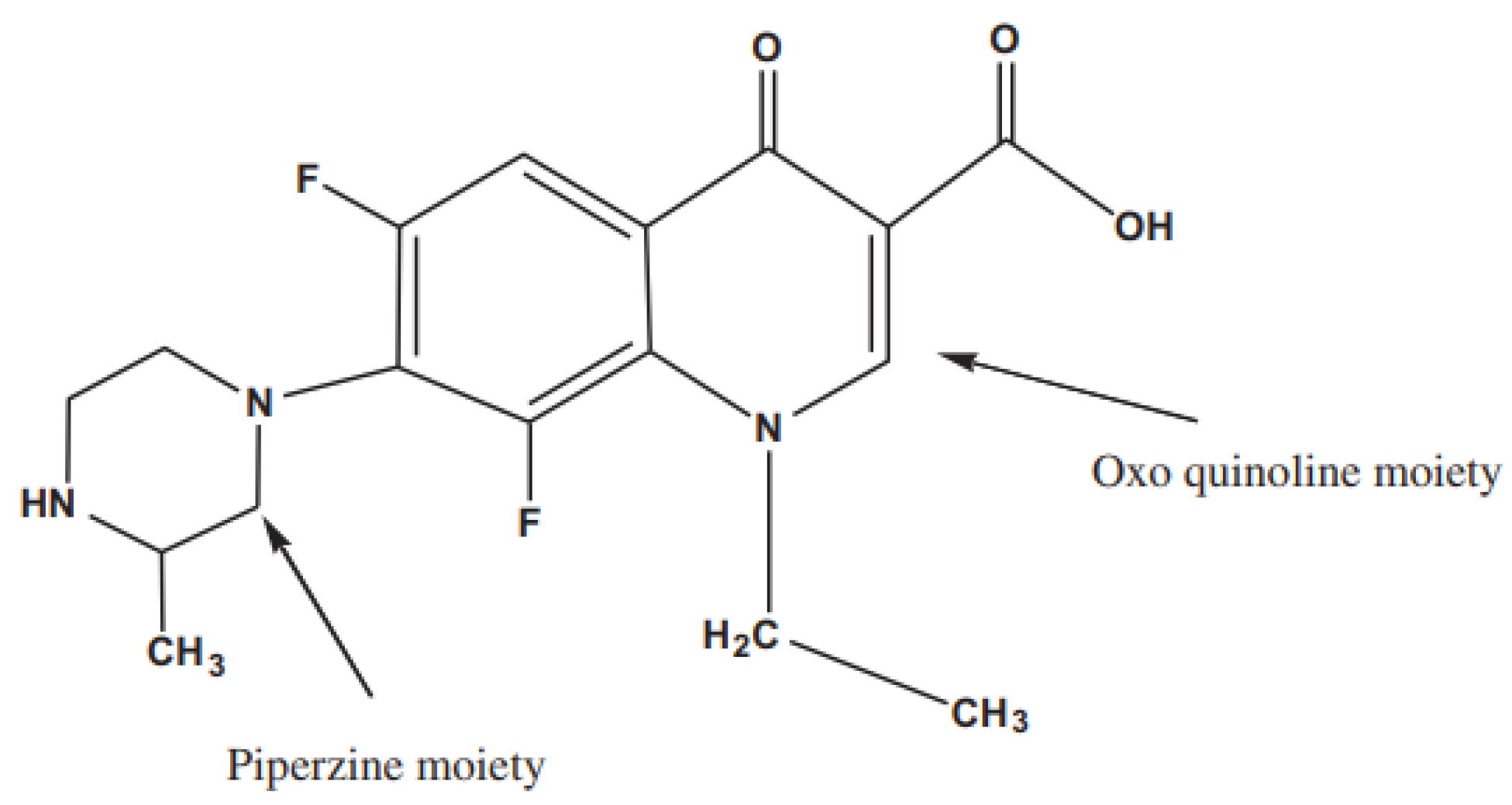
:1. Introduction
2. Materials and Methods
2.1. Materials and Method
2.2. Catalyst Preparation
2.3. Photodegradation Test
3. Results and Discussion
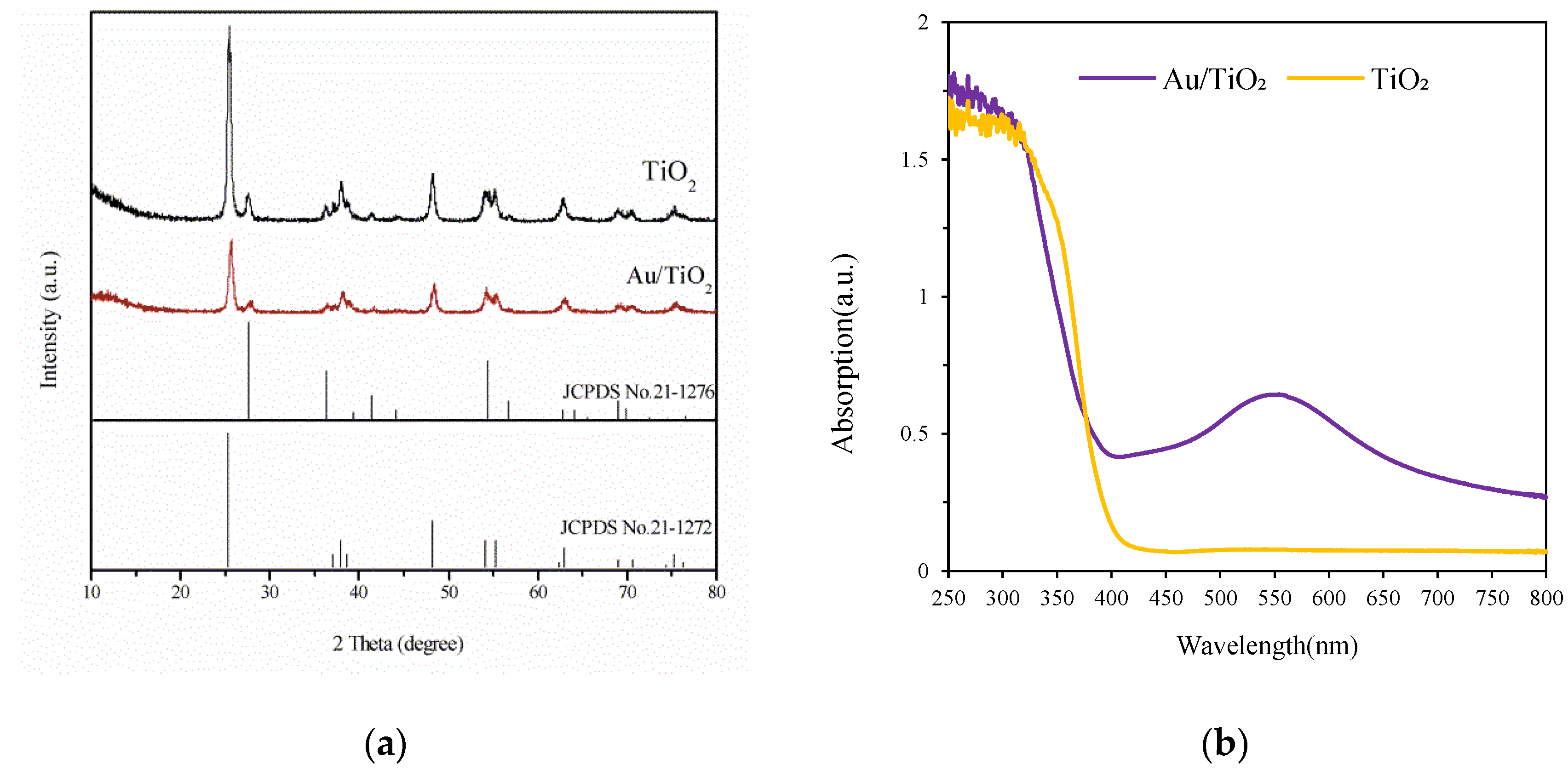
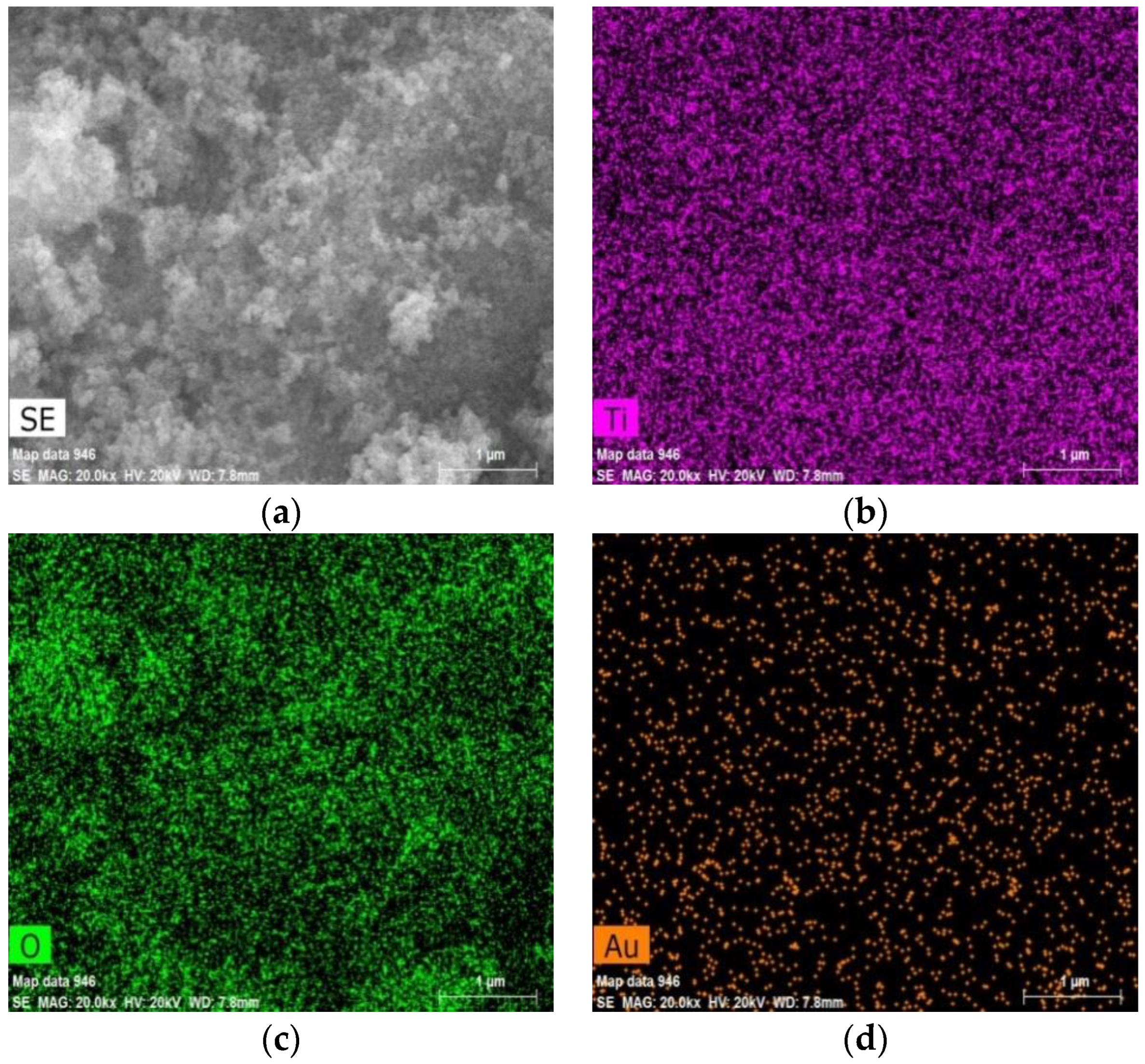
3.1. Characterization of Prepared Catalysts
3.2. Photocatalytic Degradation of LOM with Au/TiO2
3.3. The Effect of pH on the Photocatalytic Degradation of LOM
3.4. The Effect of Cl− on the Photocatalytic Degradation of LOM
3.5. The Effect of Catalyst Amount on the Photocatalytic Degradation of LOM
3.6. The Effect of LOM Initial Concentrations on the Photocatalytic Degradation of LOM
3.7. The LOM Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, E.H.; Wang, K.; Hu, Z.Q.; Wang, H. Dual-stimuli-responsive CuS-based micromotors for efficient photo-Fenton degradation of antibiotics. J. Colloid Interface Sci. 2021, 603, 685–694. [Google Scholar] [CrossRef]
- Richardson, B.J.; Lam, P.K.S.; Martin, M. Emerging chemicals of concern: Pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar. Pollut. Bull. 2005, 50, 913–920. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, Y.; Zhong, M.; Li, W.; Huang, J.; Yang, H.; Yu, G. Seasonal and spatial variations of pharmaceuticals and personal care products occurrence and human health risk in drinking water—A case study of China. Sci. Total Environ. 2019, 694, 133711. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Gaffney, P.D. Authority and the mosque in upper Egypt: The islamic preacher as image and actor. In Islam and the Political Economy of Meaning: Comparative Studies of Muslim Discourse; Taylor and Francis Inc.: London, UK, 2015; pp. 199–225. [Google Scholar]
- Mompelat, S.; le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Schwab, B.W.; Hayes, E.P.; Fiori, J.M.; Mastrocco, F.J.; Roden, N.M.; Cragin, D.; Meyerhoff, R.D.; D’Aco, V.J.; Anderson, P.D. Human pharmaceuticals in US surface waters: A human health risk assessment. Regul. Toxicol. Pharmacol. 2005, 42, 296–312. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ying, G.G.; Kong, L.X.; Wang, L.; Zhao, J.L.; Zhou, L.J.; Zhang, L.J. Distribution and accumulation of endocrine-disrupting chemicals and pharmaceuticals in wastewater irrigated soils in Hebei, China. Environ. Pollut. 2011, 159, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Ahmed Siddique, M.B.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Van Den Bogaard, A.E.; Stobberingh, E.E. Antibiotic usage in animals. Impact on bacterial resistance and public health. Drugs 1999, 58, 589–607. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [Green Version]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Kuang, C.; Pan, Y. Numerical study on hydrodynamic responses to nourishment project of laohushi beach. Mar. Geol. Front. 2013, 23, 71. [Google Scholar]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef]
- Guo, R.N.; Chen, Y.; Nengzi, L.C.; Meng, L.Z.; Song, Q.Q.; Gou, J.F.; Cheng, X.W. In situ preparation of carbon-based Cu-Fe oxide nanoparticles from CuFe Prussian blue analogues for the photo-assisted heterogeneous peroxymonosulfate activation process to remove lomefloxacin. Chem. Eng. J. 2020, 398, 125556. [Google Scholar] [CrossRef]
- Huang, S.; Wang, G.; Liu, J.; Du, C.; Su, Y. A Novel CuBi2O4/BiOBr Direct Z-scheme Photocatalyst for Efficient Antibiotics Removal: Synergy of Adsorption and Photocatalysis on Degradation Kinetics and Mechanism Insight. Chem. Cat. Chem. 2020, 12, 4431–4445. [Google Scholar]
- Vermilyea, A.W.; Voelker, B.M. Photo-fenton reaction at near neutral pH. Environ. Sci. Technol. 2009, 43, 6927–6933. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3/Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A. Visible and UV photocatalysis of aqueous perfluorooctanoic acid by TiO2 and peroxymonosulfate: Process kinetics and mechanistic insights. Chemosphere 2020, 243, 125366. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, R.; Zhu, Y.; Chen, Q. Efficient degradation of cefotaxime by a UV plus ferrihydrite/TiO2+H2O2 process: The important role of ferrihydrite in transferring photo-generated electrons from TiO2 to H2O2. J. Chem. Technol. Biotechnol. 2019, 94, 2512–2521. [Google Scholar] [CrossRef]
- Jalali, H.M.; Dezhampanah, H. Kinetic investigation of photo-degradation of amoxicillin, ampicillin, and cloxacillin by semiconductors using Monte Carlo simulation. Chem. Eng. Commun. 2021, 208, 159–165. [Google Scholar] [CrossRef]
- Xie, L.; Ren, Z.; Zhu, P.; Xu, J.; Luo, D.; Lin, J. A novel CeO2–TiO2/PANI/NiFe2O4 magnetic photocatalyst: Preparation, characterization and photodegradation of tetracycline hydrochloride under visible light. J. Solid State Chem. 2021, 300, 122208. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhang, D.; Yang, Y. Ag and N co-doped TiO2 nanostructured photocatalyst for printing and dyeing wastewater. J. Water Process Eng. 2017, 16, 14–20. [Google Scholar] [CrossRef]
- Gao, Q.; Si, F.; Zhang, S.; Fang, Y.; Chen, X.; Yang, S. Hydrogenated F-doped TiO2 for photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrog. Energy 2019, 44, 8011–8019. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Deng, Y.; Peng, X.; Wang, X.; Yuan, H.; Yang, Z.; Wang, Y.; Wang, H. Strategic modulation of energy transfer in Au-TiO2-Pt nanodumbbells: Plasmon-enhanced hydrogen evolution reaction. Nanoscale 2020, 12, 7035–7044. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Control of Surface Plasmon Resonance of Au/SnO2 by Modification with Ag and Cu for Photoinduced Reactions under Visible-Light Irradiation over a Wide Range. Chem.-A Eur. J. 2016, 22, 4592–4599. [Google Scholar] [CrossRef]
- Pal, N.K.; Kryschi, C. Improved photocatalytic activity of gold decorated differently doped TiO2 nanoparticles: A comparative study. Chemosphere 2016, 144, 1655–1664. [Google Scholar] [CrossRef]
- Zhang, H.X.; Nengzi, L.C.; Wang, Z.; Zhang, X.; Li, B.; Cheng, X. Construction of Bi2O3/CuNiFe LDHs composite and its enhanced photocatalytic degradation of lomefloxacin with persulfate under simulated sunlight. J. Hazard. Mater. 2020, 383, 121236. [Google Scholar] [CrossRef]
- Gezgin, S.Y.; Kepceoglu, A.; Gundogdu, Y.; Zongo, S.; Zawadzka, A.; Kilic, H.S.; Sahraoui, B. Effect of Ar Gas Pressure on LSPR Property of Au Nanoparticles: Comparison of Experimental and Theoretical Studies. Nanomaterials 2020, 10, 1071. [Google Scholar] [CrossRef]
- Gezgin, S.Y.; Kilic, H.S. An improvement on the conversion efficiency of Si/CZTS solar cells by LSPR effect of embedded plasmonic Au nanoparticles. Opt. Mater. 2020, 101, 109760. [Google Scholar] [CrossRef]
- Kim, S.; Cheng, N.; Jeong, J.R.; Jang, S.G.; Yang, S.M.; Huck, W.T.S. Localized surface plasmon resonance (LSPR) sensitivity of Au nanodot patterns to probe solvation effects in polyelectrolyte brushes. Chem. Commun. 2008, 31, 3666–3668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, Y.; Nengzi, L.C.; Gou, J.; Li, B.; Cheng, X. Activation of persulfate by a novel magnetic CuFe2O4/Bi2O3 composite for lomefloxacin degradation. Chem. Eng. J. 2020, 379, 122362. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Reish, M.E.; Zhang, D.; Su, N.Q.; Gutierrez, Y.; Moreno, F.; Yang, W.; Everitt, H.O.; Liu, J. Plasmon-Enhanced Catalysis: Distinguishing Thermal and Nonthermal Effects. Nano Lett. 2018, 18, 1714–1723. [Google Scholar] [CrossRef]
- Qu, Y.H.; Liu, F.; Wei, Y.; Gu, C.L.; Zhang, L.H.; Liu, Y. Forming ceria shell on Au-core by LSPR photothermal induced interface reaction. Appl. Surf. Sci. 2015, 343, 207–211. [Google Scholar] [CrossRef]
- Zhong, H.X.; Wei, Y.; Yue, Y.Z.; Zhang, L.H.; Liu, Y. Preparation of core-shell Ag@CeO2 nanocomposite by LSPR photothermal induced interface reaction. Nanotechnology 2016, 27, 135701. [Google Scholar] [CrossRef]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Zhang, R.M.; Song, C.; Kou, M.; Yin, P.; Jin, X.; Wang, L.; Deng, Y.; Wang, B.; Xia, D.; Wong, P.K.; et al. Sterilization of Escherichia coli by Photothermal Synergy of WO3-x/C Nanosheet under Infrared Light Irradiation. Environ. Sci. Technol. 2020, 54, 3691–3701. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duo, J.; Li, W.; Wang, Y.; Wang, S.; Wufuer, R.; Pan, X. Photothermal Catalytic Degradation of Lomefloxacin with Nano Au/TiO2. Water 2022, 14, 339. https://doi.org/10.3390/w14030339
Duo J, Li W, Wang Y, Wang S, Wufuer R, Pan X. Photothermal Catalytic Degradation of Lomefloxacin with Nano Au/TiO2. Water. 2022; 14(3):339. https://doi.org/10.3390/w14030339
Chicago/Turabian StyleDuo, Jia, Wenfeng Li, Yingji Wang, Shuzhi Wang, Rehemanjiang Wufuer, and Xiangliang Pan. 2022. "Photothermal Catalytic Degradation of Lomefloxacin with Nano Au/TiO2" Water 14, no. 3: 339. https://doi.org/10.3390/w14030339





