Environmental Fate of Metal Nanoparticles in Estuarine Environments
Abstract
:1. Introduction
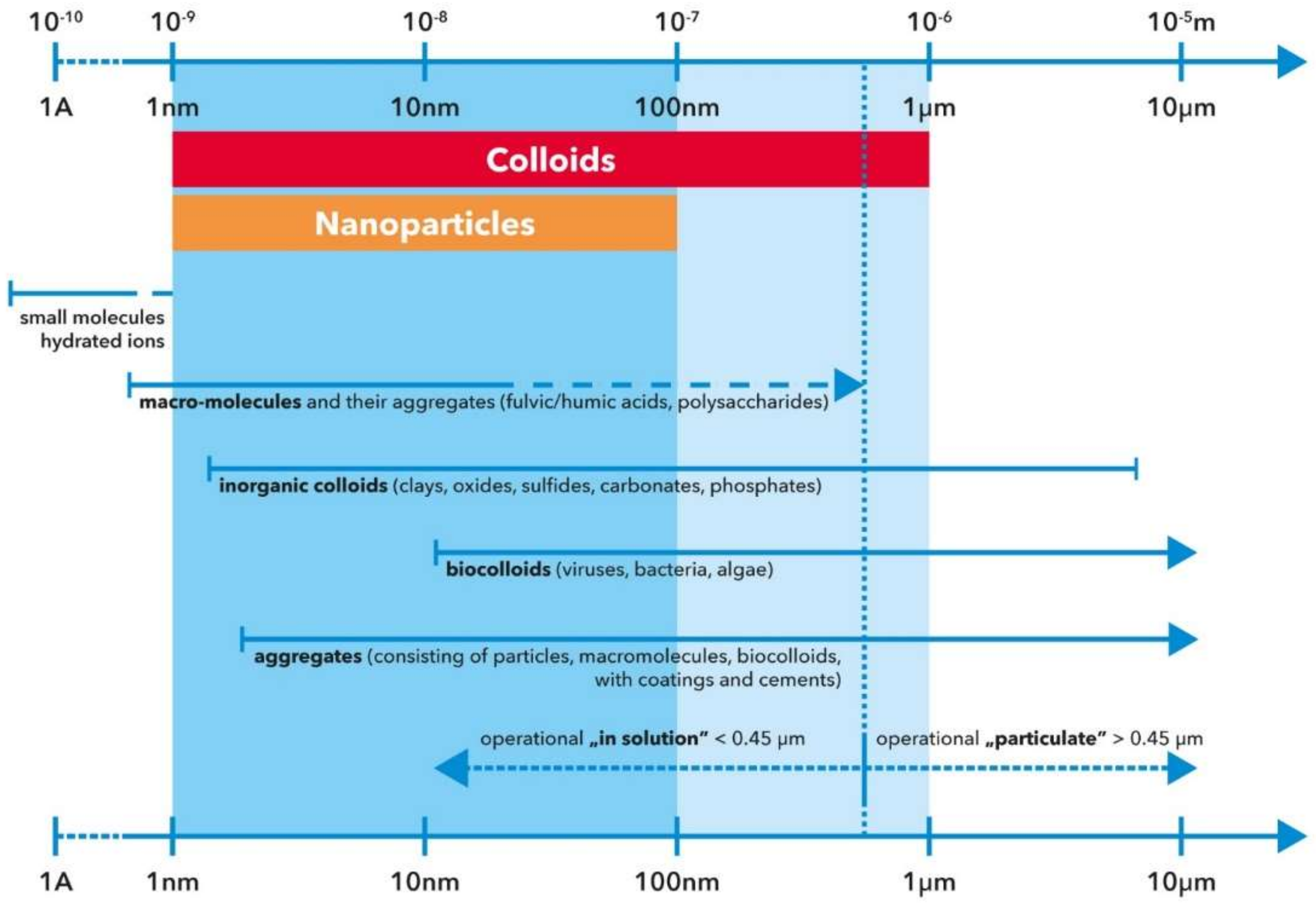
1.1. Synthesis, Properties and Nature of NPs
1.2. The Sources of ENMs
1.3. The Estimation of ENMs Release
1.4. ENMs Behaviour in Marine Environments
1.4.1. Factors Influencing Aggregation
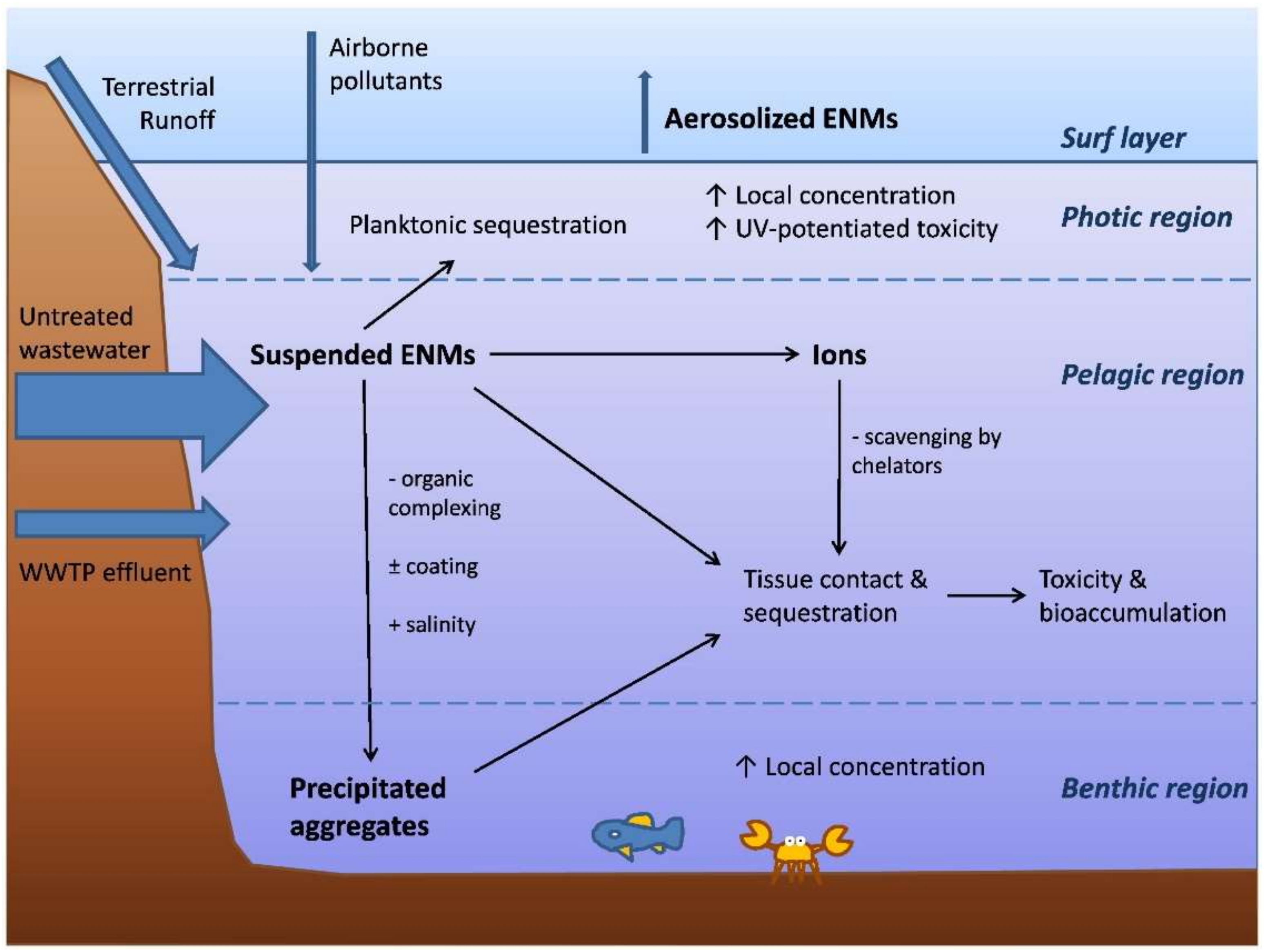
1.4.2. ENMs Behaviour in Estuary Environment
1.4.3. Interaction of ENMs with Pollutants
2. Bioavailability and Bioaccumulation of ENMs
3. Toxicity of ENMs in Marine Biota
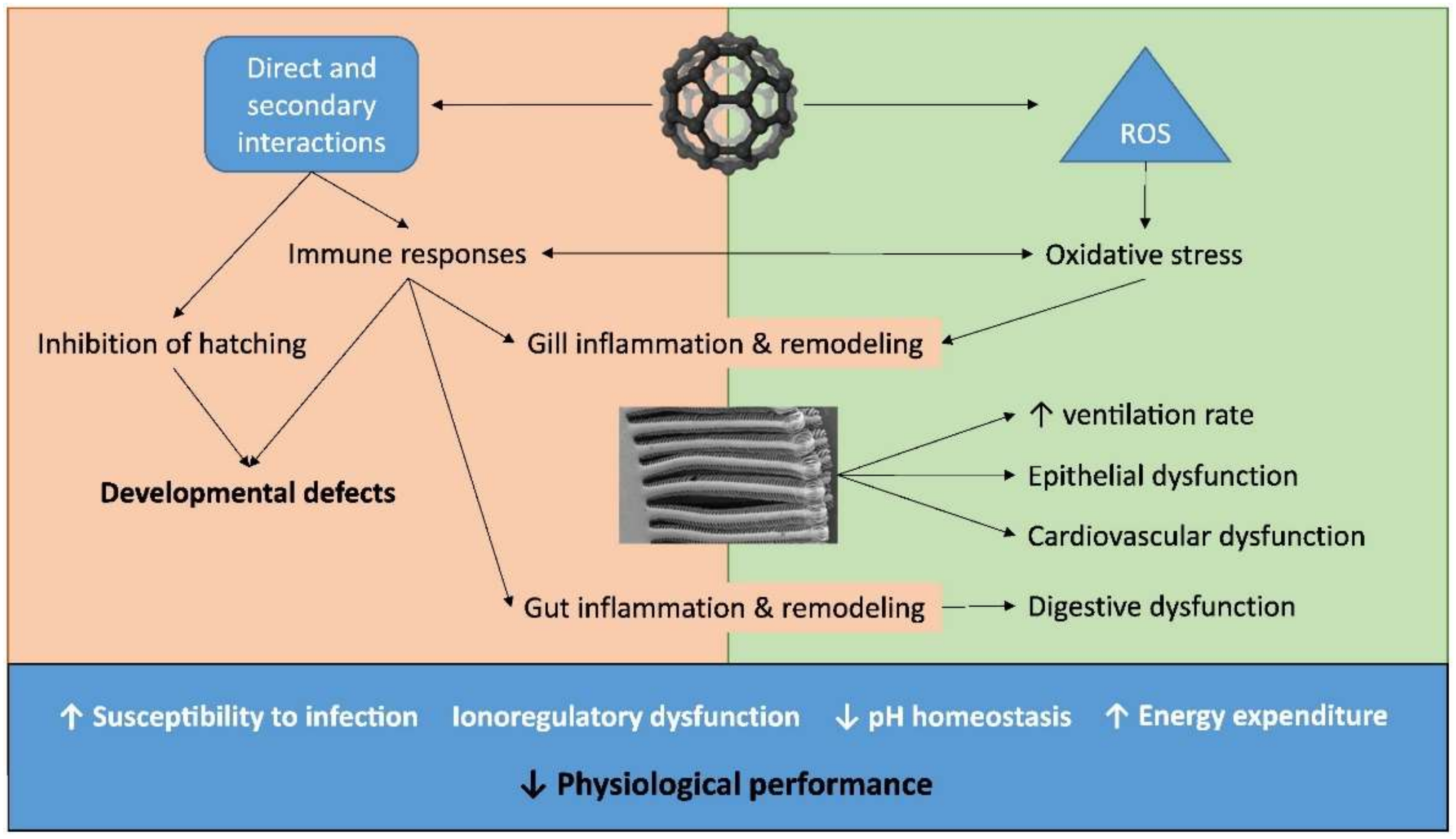
3.1. Biological ENMs Effects
3.2. Factors Influencing Marine ENM Effects
3.3. Measurements of ENMs in Aquatic System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emergen Research. Nanotechnology Market Size, Share, Trends, by Type (Nanomaterials, Nanocomposites, Nano Devices, Nano Tools), by Industry (Food and Agriculture, Healthcare, Information and Technology, Environment, Energy, Cosmetics), and by Region, Forecast to 2028. 2021, p. 250. Available online: https://www.emergenresearch.com/press-releases (accessed on 21 January 2022).
- Liu, Y.; Tourbin, M.; Lachaize, S.; Guiraud, P. Nanoparticles in wastewaters: Hazards, fate and remediation. Powder Technol. 2014, 255, 149–156. [Google Scholar] [CrossRef] [Green Version]
- European Commission. 2011/696/EU Commission Recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union 2011, L275, 8. [Google Scholar]
- Project on Emerging Nanotechnologies, 2013. Consumer Products Inventory [WWWDocument]. Available online: http://www.nanotechproject.org/cpi (accessed on 21 January 2022).
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Callaghan, N.I.; MacCormack, T.J. Ecophysiological perspectives on engineered nanomaterial toxicity in fish and crustaceans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 193, 30–41. [Google Scholar] [CrossRef]
- Williams, R.J.; Harrison, S.; Keller, V.; Kuenen, J.; Lofts, S.; Praetorius, A.; Svendsen, C.; Vermeulen, L.C.; van Wijnen, J. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Curr. Opin. Environ. Sustain. 2019, 36, 105–115. [Google Scholar] [CrossRef]
- Markus, A.A.; Parsons, J.R.; Roex, E.W.M.; de Voogt, P.; Laane, R.W.P.M. Modelling the transport of engineered metallic nanoparticles in the river Rhine. Water Res. 2016, 91, 214–224. [Google Scholar] [CrossRef]
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hassellov, M.; Canesi, L.; Pondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V.; et al. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano 2014, 8, 9694–9709. [Google Scholar] [CrossRef] [Green Version]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Minetto, D.; Libralato, G.; Volpi Ghirardini, A. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ. Int. 2014, 66, 18–27. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, S.; Sheehan, D. Nanomaterials as emerging environmental threats. Curr. Chem. Biol. 2010, 4, 151–160. [Google Scholar]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Chaudhry, Q.; Stone, V.; Fernandes, T.F. A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ. Toxicol. Chem. 2009, 28, 2142–2149. [Google Scholar] [CrossRef]
- Croteau, M.N.; Dybowska, A.D.; Luoma, S.N.; Valsami-Jones, E. A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures. Nanotoxicology 2011, 5, 79–90. [Google Scholar] [CrossRef]
- Zhao, C.M.; Wang, W.X. Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ. Toxicol. Chem. 2011, 30, 885–892. [Google Scholar] [CrossRef]
- Asghari, S.; Johari, S.A.; Lee, J.H.; Kim, Y.S.; Jeon, Y.B.; Choi, H.J.; Moon, M.C.; Yu, I.J. Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J. Nanobiotechnol. 2012, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Environ. Toxicol. Chem. 2008, 17, 326–343. [Google Scholar] [CrossRef]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res. 2013, 46, 854–862. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- IUPAC. IUPAC Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Science: Hoboken, NJ, USA, 1997; p. 446. [Google Scholar]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.J.; Ji, Z.X.; Zhang, H.Y. Nanomaterial testing in the 21st century: Use of a predictive toxicological approach and high through put screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, X.; Lao, Y.; Lv, X.; Tao, Y.; Huang, B.; Wang, J.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ. 2016, 565, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr. Environ. Assess. Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, F. Review of analytical studies on TiO2 nanoparticles and particle aggregation, coagulation, flocculation, sedimentation, stabilization. Chemosphere 2018, 212, 662–677. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Umebayashi, T.; Yoshikawa, M. Fabrication and characterization of C-doped anatase TiO2 photocatalysts. J. Mater. Sci. 2004, 39, 1837–1839. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef]
- Gondikas, A.P.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the Old Danube recreational lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef]
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550. [Google Scholar] [CrossRef]
- Yang, W.W.; Wang, Y.; Huang, B.; Wang, N.X.; Wei, Z.B.; Luo, J.; Miao, A.J.; Yang, L.Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate Tetrahymena thermophila. Environ. Sci. Technol. 2014, 48, 7568–7575. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Motabar, D.; Quiñones, O.; Stanford, B.; Vanderford, B.; Moss, D. Titanium distribution in swimming pool water is dominated by dissolved species. Environ. Pollut. 2013, 181, 68–74. [Google Scholar] [CrossRef]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yin, Y.; Tan, Z.; Zhang, Z.; Chen, Y.; Liu, J. Significant enrichment of engineered nanoparticles in water surface microlayer. Environ. Sci. Technol. 2016, 3, 381–385. [Google Scholar] [CrossRef]
- Tong, T.; Fang, K.; Thomas, S.A.; Kelly, J.J.; Gray, K.A.; Gaillard, J.-F. Chemical interactions between nano-ZnO and nano-TiO2 in a natural aqueous medium. Environ. Sci. Technol. 2014, 48, 7924–7932. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Müller, E.; Hilty, L.M.; Widmer, R.; Schluep, M.; Faulstich, M. Modeling Metal Stocks and Flows: A Review of Dynamic Material Flow Analysis Methods. Environ. Sci. Technol. 2014, 48, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- EPA 2009. Toxicological Review of Cerium Oxide and Cerium Compounds (2009) EPA/635/R-08/002F. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/1018tr.pdf (accessed on 21 January 2022).
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef]
- Johnson, B.D.; Gilbert, S.L.; Khan, B.; Carrol, D.L.; Ringwood, A.H. Cellular responses of eastern oysters, Crassostrea virginica, to titanium dioxide nanoparticles. Mar. Environ. Res. 2015, 111, 135–143. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Luoma, S.N. PEN 15-Silver Nanotechnologies and the Environment: Old Problems or New Challenges? 2008. Available online: https://www.researchgate.net/publication/221720677_Silver_Nanotechnologies_and_the_Environment (accessed on 21 January 2022).
- Stoller, M.; Ochando-Pulido, J. ZnO Nano-particles production intensification by means of a spinning disk reactor. Nanomaterials 2020, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.W.; Leung, P.T.; Djurišić, A.B.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618. [Google Scholar] [CrossRef]
- Loosli, F.; Wang, J.; Rothenberg, S.; Bizimis, M.; Winkler, C.; Borovinskaya, O.; Flamigni, L.; Baalousha, M. Sewage spills are a major source of engineered titanium dioxide release into the environment. Environ. Sci. Nano 2019, 6, 763–777. [Google Scholar] [CrossRef]
- Saharia, A.M.; Zhu, Z.; Aich, N.; Baalousha, M.; Atkinson, J.F. Modeling the transport of titanium dioxide nanomaterials from combined sewer overflows in an urban river. Sci. Total Environ. 2019, 696, 133904. [Google Scholar] [CrossRef]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F., Jr. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557–558, 740–753. [Google Scholar] [CrossRef] [Green Version]
- ASTM D7942 15; Standard Specification for Thermoplastic Pavement Markings in Non Snow Plow Areas. ASTM: West Conshohocken, PA, USA, 2015.
- Coatingsworld. U.S. Demand for Paint & Coatings to Reach 1.4 Billion Gallons in 2019. 2019. Available online: https://www.coatingsworld.com/contents/view_breaking-news/2015-09-07/us-demand-for-paint-coatings-to-reach-14-billion-gallons-in-2019/ (accessed on 9 December 2021).
- Wang, T.; Wen, X.; Hu, Y.; Zhang, X.; Wang, D.; Yin, S. Copper nanoparticles induced oxidation stress, cell apoptosis and immune response in the liver of juvenile Takifugu fasciatus. Fish Shellfish Immunol. 2019, 84, 648–655. [Google Scholar] [CrossRef]
- Adamiec, E. Road environments: Impact of metals on human health in heavily congested cities of Poland. Int. J. Environ. Res. Public Health 2017, 14, 697. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Nabi, M.M.; Mohanty, S.K.; Nabiul Afrooz, A.R.M.; Cantando, E.; Aich, N.; Baalousha, M. Detection and quantification of engineered particles in urban runoff Jingjing. Chemosphere 2020, 248, 126070. [Google Scholar] [CrossRef]
- Yu, S.-J.; Yin, Y.-G.; Liu, J.-F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2012, 15, 78–92. [Google Scholar] [CrossRef]
- Blaser, S.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef]
- Wen, L.S.; Santschi, P.H.; Gill, G.A.; Tang, D.G. Silver concentrations in Colorado, USA, watersheds using improved methodology. Environ. Toxicol. Chem. 2002, 21, 2040–2051. [Google Scholar] [CrossRef]
- Gao, X.; Lowry, G.V. Progress towards standardized and validated characterizations for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks. NanoImpact 2018, 9, 14–30. [Google Scholar] [CrossRef]
- O’Brien, N.; Cummins, E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2010, 45, 992–1007. [Google Scholar] [CrossRef]
- Pikethly, M.J. Nanomaterials—The driving force. Mater. Today 2004, 7, 20–29. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Hischier, R.; Nowack, B.; Gottschalk, F.; Hincapie, I.; Steinfeldt, M.; Som, C. Life cycle assessment of façade coating systems containing manufactured nanomaterials. J. Nanopart. Res. 2015, 17, 68. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Bornhöft, N.A.; Sun, T.Y.; Hilty, L.M.; Nowack, B. A dynamic probabilistic material flow modeling method. Environ. Model. Softw. 2016, 76, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Guzman, A.; Sun, T.; Nowack, B. Flows of engineered nanomaterials through the recycling process in Switzerland. Waste Manag. 2015, 36, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Sendra, M.; Yeste, M.P.; Gatica, J.M.; Moreno-Garrido, I.; Blasco, J. Homo agglomeration and hetero agglomeration of TiO2 in nanoparticle and bulk form, onto freshwater and marine microalgae. Sci. Total Environ. 2017, 592, 403–411. [Google Scholar] [CrossRef]
- Bansal, P.; Deshpande, A.P.; Basavaraj, M.G. Hetero aggregation of oppositely charged nanoparticles. J. Colloid Interface Sci. 2017, 492, 92–100. [Google Scholar] [CrossRef]
- Praetorius, A.; Labille, J.; Scheringer, M.; Thill, A.; Hungerbühler, K.; Bottero, J.-Y. Hetero aggregation of titanium dioxide nanoparticles with model natural colloids under environmentally relevant conditions. Environ. Sci. Technol. 2014, 48, 10690–10698. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Zhang, L.; Zhao, J.; Xing, B.S. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Bennett, S.W.; Zhou, D.; Mielke, R.; Keller, A.A. Photoinduced disaggregation of TiO2 nanoparticles enables transdermal penetration. PLoS ONE 2012, 7, e48719. [Google Scholar]
- Jiang, C.; Cao, Y.; Xiao, G.; Zhu, R.; Lu, Y. A review on the application of inorganic nanoparticles in chemical surface coatings on metallic substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ibáñez, P.; Blanco, J.; Malato, S.; de las Nieves, F.J. Application of the colloidal stability of TiO2 particles for recovery and reuse in solar photocatalysis. Water Res. 2003, 37, 3180–3188. [Google Scholar] [CrossRef]
- Qiu, G.; Au, M.-J.; Ting, Y.-P. Impacts of nano-TiO2 on system performance and bacterial community and their removal during biological treatment of wastewater. Water Air Soil Pollut. 2016, 227, 386. [Google Scholar] [CrossRef]
- Boldrin, A.; Hansen, S.F.; Baun, A.; Hartmann, N.I.B.; Astrup, T.F. Environmental exposure assessment framework for nanoparticles in solid waste. J. Nanopart. Res. 2014, 16, 2394. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Jayalath, S.; Wu, H.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Suwannee River humic acid on TiO2 nanoparticles: A study of pH and particle size. Langmuir 2018, 34, 3136–3145. [Google Scholar] [CrossRef]
- Miller, R.J.; Lenihan, H.S.; Muller, E.B.; Tseng, N.; Hanna, S.K.; Keller, A.A. Impacts of metal oxide nanoparticles on marine phytoplankton. Environ. Sci. Technol. 2010, 44, 7329–7334. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Su, C. Effects of material properties on sedimentation and aggregation of titanium dioxide nanoparticles of anatase and rutile in the aqueous phase. J. Colloid Interface Sci. 2011, 363, 84–91. [Google Scholar] [CrossRef]
- Domingos, R.F.; Peyrot, C.; Wilkinson, K.J. Aggregation of titanium dioxide nanoparticles: Role of calcium and phosphate. Environ. Chem. 2010, 7, 61–66. [Google Scholar] [CrossRef]
- He, X.; Mitrano, D.M.; Nowack, B.; Bahk, Y.K.; Figi, R.; Schreiner, C.; Bürki, M.; Wang, J. Agglomeration potential of TiO2 in synthetic leachates made from the fly ash of different incinerated wastes. Environ. Pollut. 2017, 223, 616–623. [Google Scholar] [CrossRef]
- Li, J.; Schiavo, S.; Xiangli, D.; Rametta, G.; Miglietta, M.L.; Oliviero, M.; Changwen, W.; Manzo, S. Early ecotoxic effects of ZnO nanoparticle chronic exposure in Mytilus galloprovincialis revealed by transcription of apoptosis and antioxidant-related genes. Ecotoxicology 2018, 27, 369–384. [Google Scholar] [CrossRef]
- Yang, X.; Gondikas, A.P.; Marinakos, S.M.; Auffan, M.; Liu, J.; Hsu-Kim, H.; Meyer, J.N. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, I.; Tripathi, B.N. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 2011, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir 2007, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Vitiello, V.; Pellegrini, D.; Wu, C.; Morelli, E.; Buttino, I. Toxicological effects of CdSe/ZnS quantum dots on marine planktonic organisms. Ecotoxicol. Environ. Saf. 2016, 123, 26–31. [Google Scholar] [CrossRef]
- Vindedahl, A.M.; Stemig, M.S.; Arnold, W.A.; Penn, R.L. Character of humic substances as a predictor for goethite nanoparticle reactivity and aggregation. Environ. Sci. Technol. 2016, 50, 1200–1208. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Wang, X.; Liu, W.; Chen, G.; Ma, Y.; Xing, B. Suspension stability and aggregation of multi-walled carbon nanotubes as affected by dissolved organic matters extracted from agricultural wastes. Environ. Pollut. 2016, 210, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.X.; Liu, X.Y.; Su, C.M. Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ. Sci. Technol. 2012, 46, 7142–7150. [Google Scholar] [CrossRef]
- Louie, S.M.; Spielmansun, E.R.; Small, M.J.; Tilton, R.D.; Lowry, G.V. Correlation of the physicochemical properties of natural organic matter samples from different sources to their effects on gold nanoparticle aggregation in monovalent electrolyte. Environ. Sci. Technol. 2015, 49, 2188–2198. [Google Scholar] [CrossRef]
- Wang, M.; Gao, B.; Tang, D. Review of key factors controlling engineered nanoparticle transport in porous media. J. Hazard. Mater. 2016, 318, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xu, N.; Christodoulatos, C.; Wang, D. Synergistic effects of phosphorus and humic acid on the transport of anatase titanium dioxide nanoparticles in water-saturated porous media. Environ. Pollut. 2018, 243, 1368–1375. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Lu, T.; Wang, Y.; Zhang, H.; Shang, Z.; Li, D.; Zhou, Y.; Qi, Z. Effects of low-molecular weight organic acids on the transport of graphene oxide nanoparticles in saturated sand columns. Sci. Total Environ. 2019, 666, 94–102. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, H.; Tu, C.; Luo, Y. The limited facilitating effect of dissolved organic matter extracted from organic wastes on the transport of titanium dioxide nanoparticles in acidic saturated porous media. Chemosphere 2019, 237, 124529. [Google Scholar] [CrossRef]
- Wu, F.; Falfushynska, H.; Dellwig, O.; Piontkivska, H.; Sokolova, I.M. Photocatalytic effects of titanium dioxide nanoparticles on aquatic organisms—Current knowledge and suggestions for future research. Aquat. Toxicol. 2017, 185, 138–148. [Google Scholar]
- Verdugo, P.; Alldredge, A.L.; Azam, F.; Kirchman, D.L.; Passow, U.; Santschi, P.H. The oceanic gel phase: A bridge in the DOM-POM continuum. Mar. Chem. 2004, 92, 67–85. [Google Scholar] [CrossRef]
- Quigg, A.; Chin, W.C.; Chen, C.S.; Zhang, S.; Jiang, Y.; Miao, A.J.; Schwehr, K.A.; Xu, C.; Santschi, P.H. Direct and indirect toxic effects of engineered nanoparticles on algae: Role of natural organic matter. ACS Sustain. Chem. Eng. 2013, 1, 686–702. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Y.; Chen, C.; Spurgin, J.; Schwehr, K.A.; Quigg, A.; Chin, W.; Santschi, P.H. Aggregation, dissolution, and stability of quantum dots in marine environments: Importance of extracellular polymeric substances. Environ. Sci. Technol. 2012, 46, 8764–8772. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Perez, T.; Rutten, P.; Keller, A.A. Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles. Environ. Sci. Technol. 2014, 48, 12561–12568. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Culloty, S.; Darmody, G.; Lynch, S.; Davenport, J.; Ramirez-Garcia, S.; Dawson, K.; Lynch, I.; Doyle, H.; Sheehan, D. Neutral red retention time assay in determination of toxicity of nanoparticles. Mar. Environ. Res. 2015, 111, 158–161. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Xu, Z.; Guo, W.; Li, Q. Impact of natural organic matter on the physicochemical properties of aqueous C60 nanoparticles. Environ. Sci. Technol. 2008, 42, 2853–2859. [Google Scholar] [CrossRef]
- Matranga, V.; Corsi, I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar. Environ. Res. 2012, 76, 32–40. [Google Scholar] [CrossRef]
- Tappin, A.D.; Barriada, J.L.; Braungardt, C.B.; Evans, E.H.; Patey, M.D.; Achterberg, E.P. Dissolved silver in European estuarine and coastal waters. Water Res. 2010, 44, 4204–4216. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M.; Lead, J.R. Characterization of natural aquatic colloids (<5 nm) by flow-field flow fractionation and atomic force microscopy. Environ. Sci. Technol. 2007, 41, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Tipping, E.; Higgins, D.C. The effect of adsorbed humic substances on the colloid stability of haematite particles. Colloids Surf. 1982, 5, 85–92. [Google Scholar] [CrossRef]
- Jekel, M.R. The stabilization of dispersed mineral particles by adsorption of humic substances. Water Res. 1986, 20, 1543–1554. [Google Scholar] [CrossRef]
- Buffle, J.; Wilkinson, K.J.; Stoll, S.; Filella, M.; Zhang, J. A generalized description of aquatic colloidal interactions: The three-colloidal component approach. Environ. Sci Technol. 1998, 32, 2887–2899. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- Zhang, R.C.; Zhang, H.B.; Tu, C.; Hu, X.F.; Li, L.Z.; Luo, Y.M.; Christie, P. Facilitated transport of titanium dioxide nanoparticles by humic substances in saturated porous media under acidic conditions. J. Nanopart. Res. 2015, 17, 165. [Google Scholar] [CrossRef]
- Louie, S.M.; Tilton, R.D.; Lowry, G.V. Critical review: Impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials. Environ. Sci. Nano 2016, 3, 283–310. [Google Scholar] [CrossRef]
- Lyven, B.; Hassellov, M.; Turner, D.R.; Haraldsson, C.; Andersson, K. Competition between iron- and carbon-based colloidal carriers for trace metals in a freshwater assessed using flow field flow fractionation coupled to ICPMS. Geochim. Cosmochim. Acta 2003, 67, 3791–3802. [Google Scholar] [CrossRef]
- Obare, S.O.; Meyer, G.J. Nanostructured materials for environmental remediation of organic contaminants in water. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2004, 39, 2549–2582. [Google Scholar] [CrossRef]
- Giammar, D.E.; Maus, C.J.; Xie, L. Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ. Eng. Sci. 2007, 24, 85–95. [Google Scholar] [CrossRef]
- Madden, A.S.; Hochella, J.; Luxton, T.P. Insights for size dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim. Cosmochim. Acta 2006, 70, 4095–4104. [Google Scholar] [CrossRef]
- Bustamante, P.; Miramand, P. Subcellular and body distributions of 17 trace elements in the variegated scallop Chlamys varia from the French coast of the Bay of Biscay. Sci. Total Environ. 2005, 337, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Bai, J.; Jiang, X.; Nienhaus, G.U. Cellular uptake of nanoparticles by membrane penetration: A study combining confocal microscopy with FTIR Spectro electrochemistry. ACS Nano 2012, 6, 1251–1259. [Google Scholar] [CrossRef]
- Ferry, J.L.; Craig, P.; Hexel, C.; Sisco, P.; Frey, R.; Pennington, P.L.; Fulton, M.H.; Scott, I.G.; Decho, A.W.; Kashiwada, S.; et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol. 2009, 4, 441–444. [Google Scholar] [CrossRef]
- Cleveland, D.; Long, S.E.; Pennington, P.L.; Cooper, E.; Fulton, M.H.; Scott, G.I.; Brewer, T.; Davis, J.; Petersen, E.J.; Wood, L. Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products. Sci. Total Environ. 2012, 421–422, 267–272. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Bielmyer, G.K.; Grosell, M.; Brix, K.V. Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet. Environ. Sci. Technol. 2006, 40, 2063–2068. [Google Scholar] [CrossRef]
- Baalousha, M.; Ju-Nam, Y.; Cole, P.A.; Hriljac, J.A.; Jones, I.P.; Tyler, C.R.; Stone, V.; Fernandes, T.F.; Jepson, M.A.; Lead, J.R. Characterization of cerium oxide nanoparticles—Part 2: Non size measurements. Environ. Toxicol. Chem. 2012, 31, 994–1003. [Google Scholar] [CrossRef]
- Choi, O.; Clevenger, T.E.; Deng, B.; Surampalli, R.Y.; Ross, L., Jr.; Hu, Z. Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res. 2009, 43, 1879–1886. [Google Scholar] [CrossRef]
- Roma, J.; Matos, A.R.; Vinagre, C.; Duarte, B. Engineered metal nanoparticles in the marine environment: A review of the effects on marine fauna. Mar. Environ. Res. 2020, 161, 105110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. National Recommended Water Quality Criteria; EPA-822-R-02-047; Office of Science and Technology: Washington, DC, USA, 2002.
- Wu, F.; Falfushynska, H.; Dellwig, O.; Piontkivska, H.; Sokolova, I.M. Interactive effects of salinity variation and exposure to ZnO nanoparticles on the innate immune system of a sentinel marine bivalve, Mytilus edulis. Sci. Total Environ. 2020, 712, 136473. [Google Scholar] [CrossRef] [PubMed]
- Johari, S.A.; Sarkheil, M.; Tayemeh, M.B.; Veisi, S. Influence of salinity on the toxicity of silver nanoparticles (AgNPs) and silver nitrate (AgNO3) in halophilic microalgae, Dunaliella salina. Chemosphere 2018, 209, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.-J.; Luo, Z.; Chen, C.-S.; Chin, W.-C.; Santschi, P.-H.; Quigg, A. Intracellular uptake: A possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Zhang, Y.; Guo, J.; Han, B.; Yang, X.; Yuan, J. Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus. J. Environ. Sci. 2010, 22, 155–160. [Google Scholar] [CrossRef]
- Truong, L.; Zaikova, T.; Richman, E.K.; Hutchison, J.E.; Tanguay, R.L. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology 2012, 6, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Ward, T.J.; Kramer, J.R. Silver speciation during chronic toxicity tests with the mysid, Americamysis bahia. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 75–86. [Google Scholar] [CrossRef]
- Mouneyrac, C.; Buffet, P.-E.; Poirier, L.; Zalouk-Vergnoux, A.; Guibbolini, M.; Risso-de Faverney, C.; Gilliland, D.; Berhanu, D.; Dybowska, A.; Châtel, A.; et al. Fate and effects of metal-based nanoparticles in two marine invertebrates, the bivalve mollusc Scrobicularia plana and the annelid polychaete Hediste diversicolor. Environ. Sci. Pollut. Res. 2014, 21, 7899–7912. [Google Scholar] [CrossRef]
- McCarthy, M.P.; Carroll, D.L.; Ringwood, A.H. Tissue specific responses of oysters, Crassostrea virginica, to silver nanoparticles. Aquat. Toxicol. 2013, 138, 123–128. [Google Scholar] [CrossRef]
- D’Agata, A.; Fasulo, S.; Dallas, L.J.; Fisher, A.S.; Maisano, M.; Readman, J.W.; Jha, A.N. Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 2014, 8, 549–558. [Google Scholar] [CrossRef]
- Ward, J.E.; Kach, D.J. Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Mar. Environ. Res. 2009, 68, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Marcornini, A.; Pojano, G.; Gallo, G. Bivalve molluscs as a unique target group for nanoparticle toxicity. Mar. Environ. Res. 2012, 76, 16–21. [Google Scholar] [CrossRef]
- Moore, M.N.; Viarengo, A.; Donkin, P.; Hawkins, A.J.S. Autophagic and lysosomal reactions to stress in the hepatopancreas of blue mussels. Aquat. Toxicol. 2007, 84, 80–91. [Google Scholar] [CrossRef]
- Cong, Y.; Jin, F.; Wanga, J.; Mu, J. The embryotoxicity of ZnO nanoparticles to marine medaka, Oryzias melastigma. Aquat. Toxicol. 2017, 185, 11–18. [Google Scholar] [CrossRef]
- Noor, M.N.; Wu, F.; Sokolov, E.P.; Falfushynska, H.; Timm, S.; Haider, F.; Sokolova, I.M. Salinity-dependent effects of ZnO nanoparticles on bioenergetics and intermediate metabolite homeostasis in a euryhaline marine bivalve, Mytilus edulis. Sci. Total Environ. 2021, 774, 145195. [Google Scholar] [CrossRef]
- Cong, Y.; Banta, G.T.; Selck, H.; Berhanu, D.; Valsami-Jones, E.; Forbes, V.E. Toxic effects and bioaccumulation of nano-, micron- and ionic-Ag in the polychaete, Nereis diversicolor. Aquat. Toxicol. 2011, 105, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Differential protein expression in mussels Mytilus galloprovincialis exposed to nano and ionic Ag. Aquat. Toxicol. 2013, 136, 79–90. [Google Scholar] [CrossRef]
- García-Alonso, J.; Khan, F.R.; Misra, S.K.; Turmaine, M.; Smith, B.D.; Rainbow, P.S.; Luoma, S.N.; Valsami-Jones, E. Cellular Internalization of Silver Nanoparticles in Gut Epithelia of the Estuarine Polychaete Nereis diversicolor. Environ. Sci. Technol. 2011, 45, 4630–4636. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cipelli, R.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; Money, C.; Mccormack, C.; Melzer, D. Daily bisphenol A excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 2010, 118, 1603–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrega, J.; Tantra, R.; Amer, A.; Björn, S. Sequestration of Zinc from Zinc Oxide Nanoparticles and Life Cycle Effects in the Sediment Dweller Amphipod Corophium volutator. Environ. Sci. Technol. 2012, 46, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Blasco, J.; Araújo, C.V.M. Is the cell wall of marine phytoplankton a protective barrier or a nanoparticle interaction site? Toxicological responses of Chlorella autotrophica and Dunaliella salina to Ag and CeO2 nanoparticles. Ecol. Indic. 2018, 95, 1053–1067. [Google Scholar] [CrossRef]
- Hanna, S.K.; Miller, R.J.; Zhou, D.; Keller, A.A.; Lenihan, H.S. Accumulation and toxicity of metal oxide nanoparticles in a soft-sediment estuarine amphipod. Aquat. Toxicol. 2013, 142–143, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burić, P.; Jakšić, Z.; Štajner, L.; Sikirić, M.D.; Jurašin, D.; Cascio, C.; Calzolai, L.; Mark Lyons, D. Effect of silver nanoparticles on Mediterranean Sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Peng, Q.; Li, Y. Controlled synthesis and self-assembly of highly monodisperse Ag and Ag2S nanocrystals. Chemistry 2011, 17, 941–946. [Google Scholar]
- Turner, A.; Brice, B.; Brown, M.T. Interactions of silver nanoparticles with the marine macroalga, Ulva lactuca. Ecotoxicology 2012, 21, 148–154. [Google Scholar] [CrossRef]
- Snell, T.W.; Hicks, D.G. Assessing toxicity of nanoparticles using Brachionus manjavacas (Rotifera). Environ. Toxicol. 2011, 26, 146–152. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Amiard, J.C.; Mouneyrac, C. Aquatic Ecotoxicology. Advancing Tools for Dealing with Emerging Risks; Academic Press: London, UK, 2015; p. 504. [Google Scholar]
- Paterson, G.; Ataria, J.M.; Hoque, M.E.; Burns, D.C.; Metcalfe, C.D. The toxicity of titanium dioxide nanopowder to early life stages of the Japanese medaka (Oryzias latipes). Chemosphere 2011, 82, 1002–1009. [Google Scholar] [CrossRef]
- Canesi, L.; Corsi, I. Effects of nanomaterials on marine invertebrates. Sci. Total Environ. 2016, 565, 933–940. [Google Scholar] [CrossRef]
- Cherchi, C.; Gu, A.Z. Impact of titanium dioxide nanomaterials on nitrogen fixation rate and intracellular nitron storage in Anabaena variabilis. Environ. Sci. Technol. 2010, 44, 8302–8307. [Google Scholar] [CrossRef]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Barhoumi, L.; Dewez, D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. BioMed Res. Int. 2013, 2013, 647974. [Google Scholar] [CrossRef] [Green Version]
- Morelli, E.; Gabellieri, E.; Bonomini, A.; Tognotti, D.; Grassi, G.; Corsi, I. TiO2 nanoparticles in seawater: Aggregation and interactions with the green alga Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2018, 148, 184–193. [Google Scholar] [CrossRef]
- Antizar-Ladisla, B.; Bhattacharya, B.D.; Ray Chaudhuri, S.; Sarkar, S.K. Impact of silver nanoparticles on benthic prokaryotes in heavy metal-contaminated estuarine sediments in a tropical environment. Mar. Pollut. Bull. 2015, 99, 104–111. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.X. Comparative contributions of copper nanoparticles and ions to copper bioaccumulation and toxicity in barnacle larvae. Environ. Pollut. 2019, 249, 116–124. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal accumulation in marine invertebrates: Marine biology or marine chemistry? J. Mar. Biol. Assoc. U. K. 1997, 77, 195–210. [Google Scholar] [CrossRef]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Ploetz, E.; Zimpel, A.; Cauda, V.; Bauer, D.; Lamb, D.C.; Haisch, C.; Zahler, S.; Vollmar, A.M.; Wuttke, S.; Engelke, H. Metal–Organic Framework Nanoparticles Induce Pyroptosis in Cells Controlled by the Extracellular pH. Adv. Mater. 2020, 32, 1907267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volland, M.; Hampel, M.; Martos-Sitcha, J.A.; Trombini, C.; Martínez-Rodríguez, G.; Blasco, J. Citrate gold nanoparticle exposure in the marine bivalve Ruditapes philippinarum: Uptake, elimination and oxidative stress response. Environ. Sci. Pollut. Res. 2015, 22, 17414–17424. [Google Scholar] [CrossRef]
- Jebali, J.; Khedher, S.B.; Sabbagh, M.; Kamel, N.; Banni, M.; Boussetta, H. Cholinesterase activity as biomarker of neurotoxicity: Utility in the assessment of aquatic environment contamination. Rev. Gest. Costeira Integr.—J. Integr. Coast. Zone Manag. 2013, 13, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Pryor, W.A.; Stanley, J.P. Letter: A suggested mechanism for the production of malondialdehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975, 40, 3615–3617. [Google Scholar] [CrossRef]
- Ng, C.T.; Li, J.J.; Bay, B.H.; Yung, L.Y.L. Current studies into the genotoxic effects of nanomaterials. J. Nucleic Acids 2010, 2010, 947859. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Rather, M.A.; Ajima, M.N.; Gireesh-Babu, P.; Kumar, K.; Sharma, R. Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nanoparticles. Food Chem. Toxicol. 2016, 96, 122–132. [Google Scholar] [CrossRef]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat. Res. Rev. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Ivanina, A.V.; Khoma, V.V.; Stoliar, O.B.; Sokolova, I.M. Bioenergetic responses of freshwater mussels Unio tumidus to the combined effects of nano-ZnO and temperature regime. Sci. Total Environ. 2019, 650, 1440–1450. [Google Scholar] [CrossRef]
- Blanco-Rayón, E.; Ivanina, A.V.; Sokolova, I.M.; Marigómez, I.; Izagirre, U. Food-type may jeopardize biomarker interpretation in mussels used in aquatic toxicological experimentation. PLoS ONE 2019, 14, e0220661. [Google Scholar] [CrossRef] [Green Version]
- Roznere, I.; Watters, G.T.; Wolfe, B.A.; Daly, M. Nontargeted metabolomics reveals biochemical pathways altered in response to captivity and food limitation in the freshwater mussel Amblema plicata. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 12, 53–60. [Google Scholar] [CrossRef]
- Cabral, H.; Fonseca, V.; Sousa, T.; Costa Leal, M. Synergistic effects of climate change and marine pollution: An overlooked interaction in coastal and estuarine areas. Int. J. Environ. Res. Public Health 2019, 16, 2737. [Google Scholar] [CrossRef] [Green Version]
- Heithmar, E.M. Screening Methods for Metal-Containing Nanoparticles in Water; EPA/600/R-11/096; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Sadik, O.A.; Du, N.; Yazgan, I.; Okello, V. Nanotechnology Applications for Clean Water Nanostructured. In Membranes for Water Purification, 2nd ed.; William Andrew: Norwich, NY, USA, 2014; pp. 95–108. [Google Scholar]
- Dumont, E.; Johnson, A.C.; Keller, V.D.; Williams, R.J. Nano silver and nano zinc-oxide in surface waters—Exposure estimation for Europe at high spatial and temporal resolution. Environ. Pollut. 2015, 196, 341–349. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arienzo, M.; Ferrara, L. Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water 2022, 14, 1297. https://doi.org/10.3390/w14081297
Arienzo M, Ferrara L. Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water. 2022; 14(8):1297. https://doi.org/10.3390/w14081297
Chicago/Turabian StyleArienzo, Michele, and Luciano Ferrara. 2022. "Environmental Fate of Metal Nanoparticles in Estuarine Environments" Water 14, no. 8: 1297. https://doi.org/10.3390/w14081297
APA StyleArienzo, M., & Ferrara, L. (2022). Environmental Fate of Metal Nanoparticles in Estuarine Environments. Water, 14(8), 1297. https://doi.org/10.3390/w14081297






