Root Functional Traits and Water Erosion-Reducing Potential of Two Indigenous C4 Grass Species for Erosion Control of Mudstone Badlands in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Growth Traits
2.3. Pullout Test
2.4. Root Tensile Test
2.5. Hydraulic Flume Experiment
2.6. Statistical Analysis
3. Results
3.1. Root System Configuration
3.2. Root Characteristics
3.3. Root Pullout Resistance
3.4. Root Tensile Strength
3.5. Water Erosion-Reducing Potential
4. Discussion
4.1. Root System Architecture
4.2. Root Traits
4.3. Root Pullout Resistance
4.4. Root Tensile Strength
4.5. Water Erosion-Reducing Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.H.; Lin, H.M.; Wu, J.H. The basic properties of mudstone slopes in southwestern Taiwan. J. GeoEngin. 2007, 2, 81–95. [Google Scholar]
- Nakata, E.; Chigira, M. Geochemistry of erosion processes on badland slopes: A case study of the Gutingkeng formation where mud volcanoes are distributed in southern Taiwan. J. GeoEngin. 2009, 118, 511–532. [Google Scholar]
- Hiramatsu, Y.; Tanahara, W.; Nakamura, S. Soil strength properties of the Shimajiri-mudstone Yonabaru layer and Shinzato layear. Geophys. Res. Abstr. 2013, 15, 6659–6669. [Google Scholar]
- Yang, C.J.; Lin, J.C.; Cheng, Y.C. Rill erosion of mudstone slope: A case study of southern Taiwan. Geophys. Res. Abstr. 2014, 16, 4598–4609. [Google Scholar]
- Lee, D.H.; Chen, P.Y.; Wu, J.H.; Chen, H.L.; Yang, Y.E. Method of mitigating the surface erosion of a high-gradient mudstone slope in southwest Taiwan. Bull. Eng. Geol. Environ. 2013, 72, 533–545. [Google Scholar]
- Higuchi, K.; Chigira, M.; Lee, D.H. High rates of erosion and rapid weathering in a Plio-Pleistocene mudstone badland, Taiwan. Catena 2013, 106, 68–82. [Google Scholar]
- Yang, C.J.; Turowski, J.M.; Hovius, N.; Lin, J.C.; Chang, K.J. Badland landscape response to individual geomorphic events. Nat. Commun. 2021, 12, 4631. [Google Scholar]
- Higuchi, K.; Chigira, M.; Lee, D.H.; Wu, J.H. Simultaneous water and vapour transfer in an unsaturated mudstone in badlands terrain, southwestern Taiwan. Earth Surf. Process. Landf. 2020, 45, 3323–3335. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lai, W.J.; Chuang, C.W. A study of placement and benefits of vegetated buffer strips in a reservoir watershed. J. Chin. Soil Water Conserv. 2010, 42, 15–34. [Google Scholar]
- Burylo, M.; Dutoit, T.; Rey, F. Species traits as practical tools for ecological restoration of marly eroded lands. Restor. Ecol. 2014, 22, 633–640. [Google Scholar]
- Ngugi, M.R.; Neldner, V.J.; Doley, D.; Kusy, B.; Moore, D.; Richter, C. Soil moisture dynamics and restoration of self-sustaining pioneer vegetation ecosystem on an open-cut coal mine. Restor. Ecol. 2015, 23, 615–624. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V. Alpine pioneer plants in soil bioengineering for slope stabilization and restoration: Results of a preliminary analysis of seed germination and future perspectives. Sustainability 2020, 12, 7190. [Google Scholar] [CrossRef]
- Clarke, M.L.; Rendell, H.M. Process-form relationships in Southern Italian badlands: Erosion rates and implications for landform evolution. Earth Surf. Process. Landf. 2006, 31, 15–29. [Google Scholar] [CrossRef]
- Burylo, M.; Hudek, C.; Rey, F. Soil reinforcement by the roots of six dominant species on eroded mountainous marly slopes (Southern Alps, France). Catena 2011, 84, 70–78. [Google Scholar] [CrossRef]
- Hsu, C.C. Poaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of Flora of Taiwan: Taipei, Taiwan, 2000; Volume 5, pp. 406–545. [Google Scholar]
- Lin, C.Y. Revegetation of bare mudstone slopeland for a watershed in southern Taiwan. J. Chin. Soil Water Conserv. 1997, 29, 169–181. [Google Scholar]
- Lin, S.H. Vegetation and slope stability. In Vegetation Engineering of Slopeland; Lin, S.H., Ed.; Wu-Nan Culture Enterprise: Taipei, Taiwan, 2016; pp. 105–120. [Google Scholar]
- De Baets, S.; Poesen, J.; Knapen, A.; Barbera, G.G.; Navarro, J.A. Root characteristics of representative Mediterranean plant species and their erosion-reducing potential during concentrated runoff. Plant Soil 2007, 294, 169–183. [Google Scholar] [CrossRef]
- Comino, E.; Druetta, A. The effect of Poaceae roots on the shear strength of soils in the Italian alpine environment. Soil Tillage Res. 2010, 106, 194–201. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The influence of plant root systems on subsurface flow: Implications for slope stability. Bioscience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Yen, C.P. Tree root patterns and erosion control. In Proceedings of the International Workshop on Soil Erosion and Its Counter-Measures; Jantawat, S., Ed.; Soil and Water Conservation Society of Thailand: Bangkok, Thailand, 1987; pp. 92–111. [Google Scholar]
- Mickovski, S.B.; van Beek, L.P.H.; Salin, F. Uprooting of vetiver uprooting resistance of vetiver grass (Vetiveria zizanioides). Plant Soil 2005, 278, 33–41. [Google Scholar] [CrossRef]
- Ghestem, M.; Cao, K.; Ma, W.; Rowe, N.; Leclerc, R.; Gadenne, C.; Stokes, A. A framework for identifying plant species to be used as ‘ecological engineers’ for fixing soil on unstable slopes. PLoS ONE 2014, 9, e95876. [Google Scholar]
- Nwoke, H.U.; Dike, B.U.; Okoro, B.C.; Nwite, S.A. Uprooting resistance and morphological traits of plants used in erosion mitigation. Int. J. Civ. Eng. Technol. 2016, 7, 104–110. [Google Scholar]
- Noorasyikin, M.N.; Zainab, M. Mechanical root reinforcement of Bermuda grass toward slope stabiblity. Int. J. Eng. Res. Adv. Technol. 2019, 8, 426–430. [Google Scholar]
- Lee, J.T.; Lin, Y.S.; Shi, C.Y.; Lee, M.J. Morphological traits and root anchorage ability of native pioneer tree species for reforestation of mudstone badlands. J. For. Res. 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Simonato, T.; Speziali, B.; Vitali, B.; Vullo, P.; Zocco, A. Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant Soil 2005, 278, 11–22. [Google Scholar] [CrossRef]
- Bouma, T.J.; Nielsen, K.L.; Koutstaal, K. Sample preparation and scanning protocol for computerized analysis of root length and diameter. Plant Soil 2000, 218, 185–196. [Google Scholar] [CrossRef]
- Pang, W.; Crow, W.T.; Luc, J.E.; McSorley, R.; Giblin-Davis, R.M.; Kruse, J.K. Comparison of water displacement and WinRHIZO software for plant root parameter assessment. Plant Dis. 2011, 95, 1308–1310. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Yen, L.Z.; Chu, M.Y.; Lin, Y.S.; Chang, C.C.; Lin, R.S.; Chao, K.H.; Lee, M.J. Growth characteristics and anti-wind erosion ability of three tropical foredune pioneer species for sand dune stabilization. Sustainability 2020, 12, 3353. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Yen, L.Z.; Lee, M.J. Wind affects the growth, root anchorage and tensile strength of Australian pine (Casuarina equisetifolia) seedlings. J. For. Res. 2019, 24, 219–229. [Google Scholar] [CrossRef]
- Boldrin, D.; Leung, A.K.; Bengough, A.G. Effects of root dehydration on biomechanical properties of woody roots of Ulex europaeus. Plant Soil 2018, 431, 347–369. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Tsai, S.M.; Wu, Y.J.; Lin, Y.S.; Chu, M.Y.; Lee, M.J. Root characteristics and water erosion-reducing ability of alpine silver grass and Yushan cane for alpine grassland soil conservation. Sustainability 2021, 13, 7633. [Google Scholar] [CrossRef]
- Sidorchuk, A.; Sidorchuk, A. Model for estimating gully morphology. In Modelling Soil Erosion, Sediment Transport and Closely Related Hydrological Processes (Proceedings of a Symposium Held at Vienna, July 1998); IAHS Publications: Vienna, Austria, 1998. [Google Scholar]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. 2005, 29, 189–217. [Google Scholar] [CrossRef] [Green Version]
- Carmo-Silva, A.E.; Soares, A.S.; da Silva, J.M.; da Silva, A.B.; Alfred, J.; Keys, A.J.; Arrabaça, M.C. Photosynthetic responses of three C4 grasses of different metabolic subtypes to water deficit. Funct. Plant Biol. 2007, 34, 204–213. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Zong, J.; Chen, J.; Li, D.; Liu, J. Genetic diversity in centipedegrass [Eremochola ophiuroides (Munro) Hack.]. Hortic. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Truong, P.; Loch, R. Vetiver system for erosion and sediment control. In Proceedings of the 13th International Soil Conservation Organization Conference, Brisbane, Australia, 1–4 July 2004; pp. 1–6. [Google Scholar]
- Burylo, M.; Rey, F.; Roumet, C.; Buisson, E.; Dutoit, T. Linking plant morphological traits to uprooting resistance in eroded marly lands (Southern Alps, France). Plant Soil 2009, 324, 31–42. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surf. Process. Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- Katuwal, S.; Vermang, J.; Cornelis, W.M.; Gabriels, D.; Moldrup, P.; De Jonge, L.W. Effect of root density on erosion and erodibility of a loamy soil under simulated rain. Soil Sci. 2013, 178, 29–36. [Google Scholar] [CrossRef]
- Guo, M.M.; Wang, W.L.; Kang, H.L.; Yang, B. Changes in soil properties and erodibility of gully heads induced by vegetation restoration on the Loess Plateau, China. J. Arid Land 2018, 10, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Chu, M.Y.; Lin, Y.S.; Kung, K.N.; Lin, W.C.; Lee, M.J. Root traits and biomechanical properties of three tropical pioneer tree species for forest restoration in landslide areas. Forests 2020, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Roering, J.J.; Schmidt, K.M.; Stock, J.D.; William, E.D.; David, R.M. Shallow landsliding, root reinforcement, and the spatial distribution of trees in the Oregon Coast Range. Can. Geotech. J. 2003, 40, 237–253. [Google Scholar] [CrossRef] [Green Version]
- De Baets, S.; Poeson, J.; Reubens, B.; Wemans, K.; Baerdemaeker, J.D.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Ettbeb, A.E.; Rahman, Z.A.; Idris, W.M.R.; Jumaat, A.J.; Rahim, S.A.; Tarmidzi, S.N.A.; Lihan, T. Root tensile resistance of selected Pennisetum species and shear strength of root-permeated soil. Appl. Environ. Soil Sci. 2020, 2020, 3484718. [Google Scholar] [CrossRef]
- Lateh, H.; Avani, N.; Bibalani, G. Tensile strength and root distribution of Acacia mangium and Macaranga tanarius at spatial variation (Case study: East-West highway, Malaysia). Int. J. Biosci. 2015, 6, 18–28. [Google Scholar]
- Comino, E.; Marengo, P.; Rolli, V. Root reinforcement effect of different grass species: A comparison between experimental and models results. Soil Tillage Res. 2010, 110, 60–68. [Google Scholar] [CrossRef]
- Su, X.; Zhou, Z.; Liu, J.; Cao, L.; Liu, J.; Wang, P. Estimating slope stability by the root reinforcement mechanism of Artemisia sacrorum on the Loess Plateau of China. Ecol. Model. 2021, 444, 109473. [Google Scholar] [CrossRef]
- Hsu, C.L.; Dai, S.Y. A study of ventilating and watertight resin on mudstone soil erosion control. Taiwan J. For. Sci. 2010, 25, 291–301. [Google Scholar]
- Zhang, G.H.; Tang, K.; Ren, Z.; Zhang, X.C. Impact of grass root mass density on soil detachment capacity by concentrated flow on steep slopes. Trans. ASABE 2013, 56, 927–934. [Google Scholar]
- Guo, M.; Wang, W.; Shi, Q.; Chen, T.; Kang, H.; Li, J. An experimental study on the effects of grass root density on gully headcut erosion in the gully region of China’s Loess Plateau. Land Degrad. Dev. 2019, 30, 2107–2125. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Zhang, M.; Wang, J. Effect of Cynodon dactylon community on the conservation and reinforcement of riparian shallow soil in the Three Gorges Reservoir area. Ecol. Process. 2015, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Capobianco, M.; Stive, M.J.F. Soft intervention technology as a tool for integrated coastal zone management. J. Coast. Conserv. 2000, 6, 33–40. [Google Scholar] [CrossRef]




| Species | Root Area Ratio (%) | ||||
|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 10–15 cm | 15–20 cm | 20–25 cm | |
| Cynodon dactylon Eremochloa ophiuroides | 1.34 ± 0.23 a 0.46 ± 0.14 b | 1.64 ± 0.34 a 0.18 ± 0.03 b | 0.63 ± 0.07 a 0.13 ± 0.02 b | 0.12 ± 0.03 a 0.04 ± 0.01 b | 0.02 ± 0.01 a 0.01 ± 0.01 a |
| Root Traits | C. Dactylon | E. Ophiuroides | p |
|---|---|---|---|
| RB (kg) | 0.012 ± 0.002 a | 0.006 ± 0.0004 b | 0.001 *** |
| RT | 5111.75 ± 492.3 a | 3715.63 ± 200.05 b | 0.020 * |
| RV (cm3) | 46.5 ± 1.48 a | 36.63 ± 1.24 b | 0.000 *** |
| TRL (cm) | 4751.13 ± 408.014 a | 4195.88 ± 398.57 a | 0.34 ns |
| RD (kg m−3) | 0.22 ± 0.02 a | 0.1 ± 0.01 b | 0.001 *** |
| RLD (km m−3) | 0.88 ± 0.08 a | 0.78 ± 0.07 a | 0.32 ns |
| RSA (cm2) | 4830.85 ± 513.94 a | 3297.77 ± 230.35 b | 0.017 * |
| RTD (g cm−3) | 0.26 ± 0.06 a | 0.15 ± 0.01 b | 0.001 *** |
| Biomechanical Properties | C. Dactylon | E. Ophiuroides | t-Value |
|---|---|---|---|
| Maximal pullout resistance (kN) | 0.31 ± 0.03 a | 0.16 ± 0.02 b | 5.301 ** |
| Parameters | C. Dactylon | E. Ophiuroides | p |
|---|---|---|---|
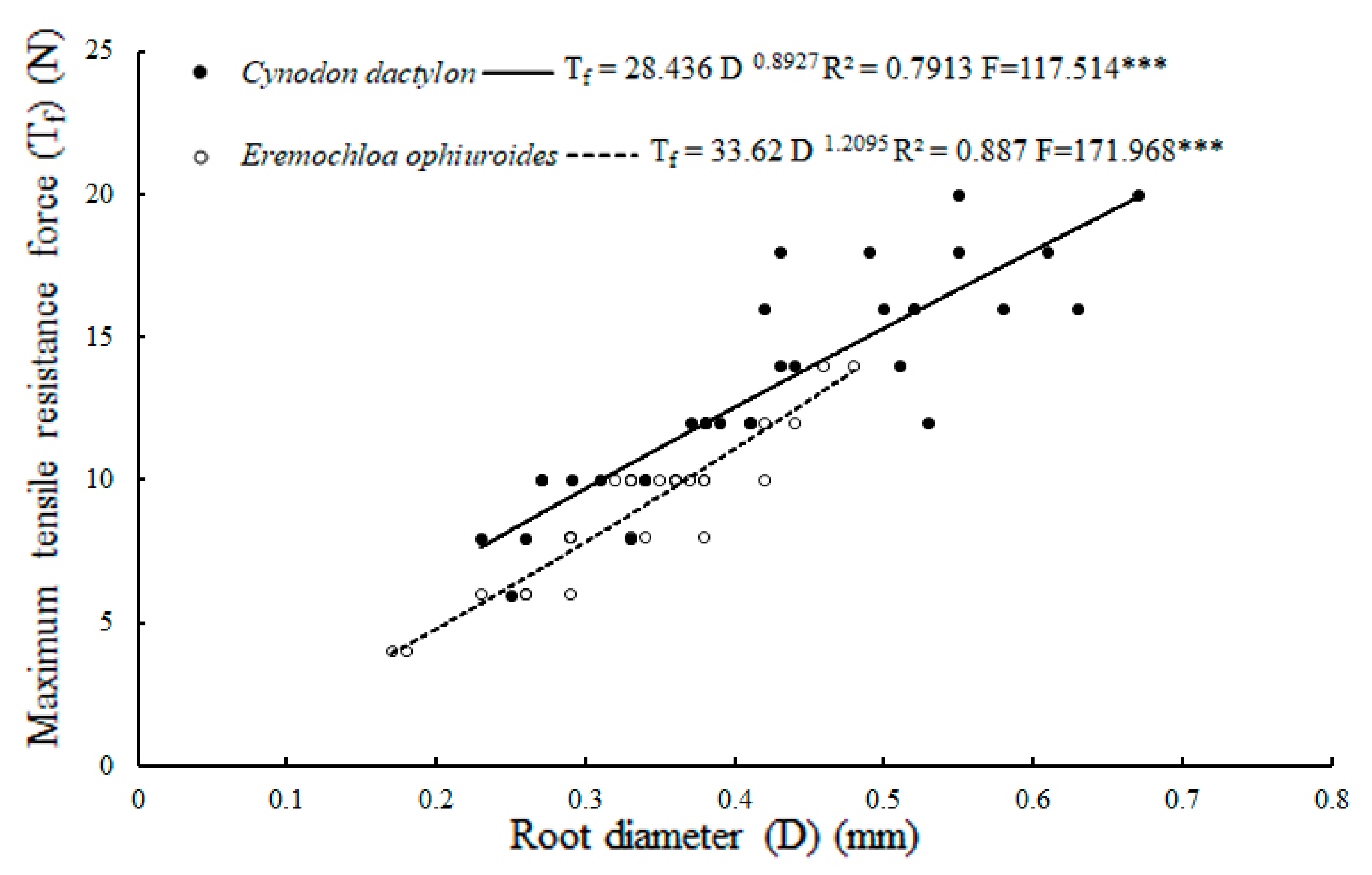
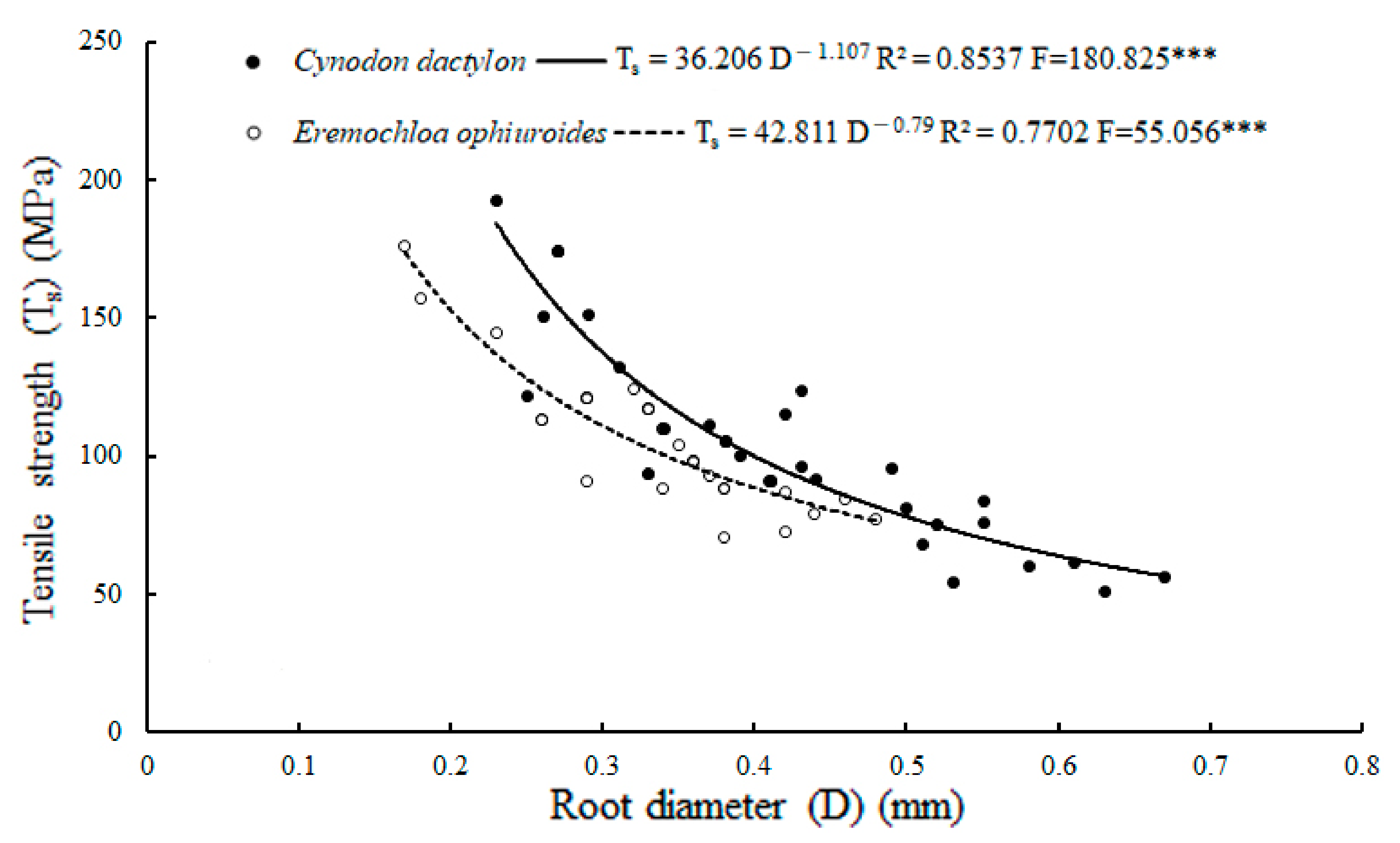
| Root diameters (mm) | 0.42 ± 0.02 a | 0.34 ± 0.01 b | 0.001 *** |
| Tensile resistance force (N) | 13.21 ± 0.65 a | 9.0 ± 0.46 b | 0.000 *** |
| Tensile strength (MPa) | 104.705 ± 6.56 a | 104.54 ± 4.24 a | 0.978 ns |
| Slope (°) | Soil Loss Amount (g min−1) | ANOVA (p) | ||
|---|---|---|---|---|
| C. Dactylon | E. Ophiuroides | Bare Soil | ||
| 15 | 44.74 ± 6.62 c | 72.8 ± 9.81 b | 411.11 ± 30.13 a | 0.000 *** |
| Slope (°) | Relative Soil Detachment Rate (%) | p | |
|---|---|---|---|
| C. Dactylon | E. Ophiuroides | ||
| 15 | 16.15 ± 2.26 b | 27.36 ± 3.82 a | 0.031 * |
| Root Functional Traits | Species | Regression Equation | R2 | F |
|---|---|---|---|---|
| RD (kg m−3) | C. dactylon | RSD = −80.14RD + 32.805 | 0.573 * | 6.452 |
| E. ophiuroides | RSD = −219.95RD + 47.063 | 0.607 * | 7.713 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-T.; Lin, Y.-S.; Shih, C.-Y.; Lee, M.-J. Root Functional Traits and Water Erosion-Reducing Potential of Two Indigenous C4 Grass Species for Erosion Control of Mudstone Badlands in Taiwan. Water 2022, 14, 1342. https://doi.org/10.3390/w14091342
Lee J-T, Lin Y-S, Shih C-Y, Lee M-J. Root Functional Traits and Water Erosion-Reducing Potential of Two Indigenous C4 Grass Species for Erosion Control of Mudstone Badlands in Taiwan. Water. 2022; 14(9):1342. https://doi.org/10.3390/w14091342
Chicago/Turabian StyleLee, Jung-Tai, Yu-Syuan Lin, Cheng-Ying Shih, and Ming-Jen Lee. 2022. "Root Functional Traits and Water Erosion-Reducing Potential of Two Indigenous C4 Grass Species for Erosion Control of Mudstone Badlands in Taiwan" Water 14, no. 9: 1342. https://doi.org/10.3390/w14091342
APA StyleLee, J.-T., Lin, Y.-S., Shih, C.-Y., & Lee, M.-J. (2022). Root Functional Traits and Water Erosion-Reducing Potential of Two Indigenous C4 Grass Species for Erosion Control of Mudstone Badlands in Taiwan. Water, 14(9), 1342. https://doi.org/10.3390/w14091342





