Abstract
The effective implementation of in situ thermal treatment (ISTT) technologies requires understanding of gas production and migration in heterogenous media. However, investigations of the effects of high permeability contrast on gas formation, accumulation, and migration, as well as its potential effect on the redistribution of dense non-aqueous phase liquid (DNAPL), are relatively rare. In this study, electrical resistance heating (ERH) experiments were conducted in a thin sand-packed cell to simulate common yet not well-studied scenarios encountered during ISTT applications, such as coarse lenses surrounded by finer material. Two packing configurations were employed: 2 mm glass beads surrounded by 20/30 silica sand and 20/30 silica sand overlaying 40/50 silica sand. Each experiment contained an emplaced pool of trichloroethene (TCE) within the coarse material. If permeable material or pathways were present between the coarse lens and the upper cell boundary, the gas migrated along these pathways, and local DNAPL redistribution was limited to near the top of the pool before it vaporized. In contrast, if the coarse material was surrounded by finer material and contained a sufficient volume of DNAPL, the gas accumulated inside the coarse lens leading to DNAPL displacement from the lens. For five selected DNAPLs, this volume was estimated to be 0.1% to 0.5% of the total pore volume of the coarse material. The conceptual model developed in this study improves our understanding of this common geological scenario, demonstrating the importance of considering both lower- and higher-permeability material and their effects on multiphase flow during co-boiling, as well as the design of gas extraction systems during ISTT applications.
1. Introduction
The remediation of soil and groundwater at sites contaminated by a dense non-aqueous phase liquid (DNAPL) remains a significant challenge. Difficulties in treating DNAPL source zones below the water table are related to their persistence and complex distribution. DNAPL released at or near ground surface migrates downwards through the vadose zone to the capillary fringe and the water table. Because of its density, this downward migration continues below the water table leading to the formation of an immobile DNAPL residual created by snap-off and bypass processes, and higher saturation pools created by differences in the capillary displacement pressure [1]. Increased heterogeneity can also lead to more complex DNAPL distributions within source zones. Figure 1 illustrates how areas of high permeability contrast can result in a tortuous migration pathway for DNAPL. Differences in displacement pressure result in DNAPL pooling on top of capillary barriers (typically lower permeability layers) but can also result in DNAPL pooling within higher permeability layers (Figure 1). Both cases can contribute to the lateral migration of DNAPL, complicating the delineation of DNAPL source zones and their remediation.
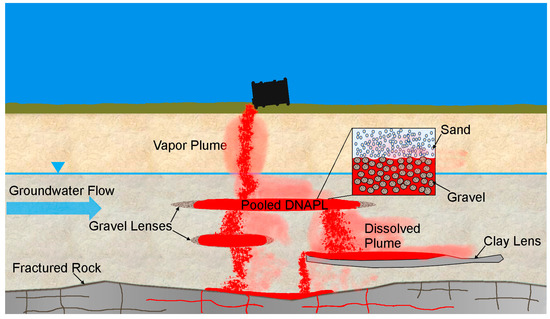
Figure 1.
Schematic representation of a DNAPL source zone showing lower permeability and higher permeability layers and their associated DNAPL pools.
Several in situ remediation technologies have been developed to treat DNAPL source zones. Injection-based remediation technologies, including in situ chemical oxidation (ISCO) and in situ bioremediation (ISB), rely on fluid migration to contact DNAPL or volatile organic compounds (VOCs) dissolved from DNAPL [2,3]. Therefore, the performance of these technologies is strongly influenced by variations in permeability. Because permeability can vary over several orders of magnitude over short distances [4], injection-based remediation technologies may not come into contact with VOCs stored in lower permeability layers. This is particularly important at sites where VOCs have entered lower permeability layers by diffusion, including from DNAPL that has pooled on those layers. The inability to treat VOCs in lower permeability layers can lead to so-called back diffusion, where the release of VOCs from these layers increases dissolved concentrations after treatment (i.e., rebound), and is often cited as the reason why injection-based remediation technologies do not achieve target contaminant concentrations in complex geological settings [5,6,7]. Importantly, higher DNAPL saturations that can form in high permeability layers create zones of lower aqueous relative permeability, which can also limit contact with DNAPL.
Unlike injection-based remediation technologies, some in situ thermal treatment (ISTT) technologies, such as electrical resistance heating (ERH) and thermal conductive heating (TCH), are less sensitive to variations in permeability [8,9]. They are instead controlled by subsurface electrical and/or heat transfer properties, which are often much less variable than permeability. As a result, ERH and TCH can be effective in fine-grained or heterogeneous media (e.g., [7]). Although under some conditions ISTT technologies can be applied to increase VOC reaction rates (e.g., hydrolysis, biodegradation) to promote degradation, subsurface temperatures are often increased to enhance the physical recovery of target constituents by leveraging changes to their thermophysical properties that govern mobility in the subsurface. At sites with VOC DNAPL (e.g., many chlorinated solvents), subsurface temperatures are often targeted to first drive the simultaneous liquid-to-gas phase change of DNAPL and water (i.e., steam distillation or ‘co-boiling’ at the immiscible fluid interfaces). The nucleation of disconnected gas bubbles at higher temperatures is followed by gas expansion, coalescence, and buoyancy-driven migration, which is dictated by the multiphase flow of gas and water. This results in the formation of a gas channel network but can also result in the mobilization of disconnected gas clusters (bubbles) in coarse media, which may coalesce into a connected gas channel network at sufficient rates of vapor generation [10,11,12]. This gas channel network facilitates DNAPL and VOC removal from high permeability regions and can enhance the diffusion of VOCs from neighboring lower permeability zones by increasing the concentration gradient [13].
The migration of VOC vapors is significantly influenced by variations in permeability [14]. However, most laboratory investigations of ISTT have focused on homogeneous materials, including high permeability sands [12,15,16], silt [17], fractured and unfractured clay [18,19,20], and fractured rock [21]. Few bench-scale studies have incorporated large permeability contrasts despite their common occurrence at sites where ISTT has been implemented [22]. Of these heterogeneous studies, most have focused on lower permeability material surrounded by higher permeability material. For example, Martin and Kueper [23] investigated the removal of DNAPL pools above fine sand capillary barriers during heating by ERH. In a series of studies, Martin et al. [24], Martin et al. [25], and Mumford et al. [13] investigated heating and gas production during ERH of clay and sandy-clay lenses surrounded by sand, but their experiments did not contain DNAPL and only Mumford et al. [13] contained VOCs in the clay. Their results showed that gas was primarily generated at the clay–sand interface (i.e., at the permeability boundary), with continuous gas networks formed in the sand and primarily discontinuous gas formed in the clay.
There have been even fewer laboratory investigations of ISTT under heterogeneous conditions that included higher permeability material surrounded by lower permeability material, despite this being a common scenario to which ISTT is applied. For example, higher permeability surrounded by lower permeability accounted for 36 out of 84 selected ISTT field projects conducted between 2000 and 2007 in a review by Triplett Kingston et al. [22]. One such laboratory study was conducted by Munholland et al. [26], who investigated gas production and migration during the application of ERH for the removal of trichloroethene (TCE) or chloroform (CF) DNAPL in a flow cell containing interbedded fine, medium and coarse sand layers. In their experiments, a DNAPL was located in the medium sand on top of fine sand and below a layer of coarse sand that was below another layer of medium sand. During co-boiling, gas produced at the DNAPL pool surface migrated upwards and accumulated in the coarse sand layer as a gas pool. Further gas production, migration and accumulation led to the expansion and lateral migration of the gas pool within the coarse sand and beneath the medium sand, which acted as a capillary barrier. This lateral migration continued outside of the heated zone resulting in DNAPL condensation. Condensed vapors accounted for 31–56% of the original DNAPL mass emplaced in the pool. These results demonstrate that the presence of coarse material can substantially affect ISTT performance, in this case, by controlling gas migration pathways. This is in addition to the effects of higher groundwater velocity and higher permeability layers that can limit heating [26,27]. Importantly, the study by Munholland et al. [26] did not incorporate DNAPL within the coarse layer; therefore, the multiphase flow of DNAPL and water was separate from the multiphase flow of gas and water. In addition, the coarse layer in their experiments was continuous across their flow cell, which allowed gas to migrate laterally beyond the heated zone. Another such laboratory study was that of Baker and Hiester [28], who investigated the removal of tetrachloroethene (PCE) using TCH and Soil Vapor Extraction (SVE) wells in a stainless-steel flume. The bottom 24 cm of the flume was packed with coarse sand, and the upper 50 cm was packed with a fine sandy and coarse silt material. PCE was injected into a coarse lens packed within the upper fine layer. At the end of the experiment, all PCE was removed from the DNAPL pool, but only 92% was recovered through the SVE wells. This study showed that the complete removal of the DNAPL pool within the coarse lens is possible; however, the effect of material permeability on gas migration was not considered.
There is a need to investigate heating and gas production for DNAPL contained in a coarse lens to improve our conceptual models for this commonly encountered heterogeneous subsurface condition. The overall goal of this study was to investigate the heating of DNAPL in a higher permeability lens surrounded by lower permeability material at a laboratory scale. The specific objectives were to: (i) assess gas production and migration during DNAPL-water co-boiling in a coarse lens, (ii) investigate the associated impact of gas production on DNAPL distribution, and (iii) compare these results to previous experiments where the higher permeability material has not been surrounded. Unlike previous studies, this study included coarse-grained (higher permeability) material surrounded by fine-grained (lower permeability) material (i.e., coarse-grained rather than fine-grained lens). In addition, DNAPL was emplaced in the coarser material to facilitate the investigation of gas production and migration during DNAPL-water co-boiling and any potential associated effects on DNAPL distribution. Light transmission techniques were used to clearly visualize multiphase flow during the experiments.
2. Materials and Methods
2.1. Experimental Setup
Experiments were conducted in a thin flow cell measuring 40 cm high × 20 cm wide × 1 cm thick (Figure 2), which was used previously by Hegele and Mumford [10]. Borosilicate glass walls at the front and back allowed for the visualization of gas phase development during heating. The cell was packed with 20/30 translucent silica sand (d50 = 0.71 mm) and 2 mm glass beads (Figure 2a) and was compared to an experiment packed with 20/30 and 40/50 (d50 = 0.36 mm) silica sand [29] conducted by Hegele and Mumford [10] (Figure 2b). In both cases, sand and glass beads were emplaced using a wet packing procedure in which a slurry was poured continuously into the flow cell [10], and the walls were tapped with a rubber mallet. The porosity of the 20/30 sand was approximately 0.36. To prevent sand grain rearrangement at the top of the packing during the heating stage, the sand was kept in place by a 35 × 35 size polypropylene mesh overlaid by a perforated polytetrafluoroethylene (PTFE) block. The experiment conducted in this study is referred to here as Experiment A, and the experiment by Hegele and Mumford [10] as Experiment B.
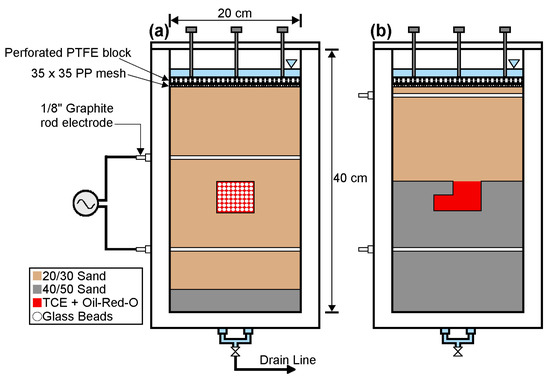
Figure 2.
Experiment cell, packing and heating configurations for (a) Experiment A and (b) Experiment B. Electrical components were the same for both experiments. Adapted from Hegele and Mumford [10].
Trichloroethene (TCE) DNAPL was dyed with Oil-Red-O (100 mg/L) and injected from the top of the cell part-way through the packing procedure using a gastight syringe equipped with a 30 cm long stainless-steel needle (18 gauge). For Experiment A, ~10 mL of TCE was injected into the glass beads surrounded by 20/30 sand and accounted for ~80% of the pore volume in the glass beads. For Experiment B, 4.3 mL of TCE was injected into the 20/30 sand and was allowed to pool on top of the 40/50 sand, which acted as a capillary barrier. The local DNAPL saturation within the pool was estimated to be ~70%, but overall, 4.3 mL of TCE accounted for only ~4% of the pore volume of the entire 20/30 sand pack.
The experiments were heated by ERH using a variable voltage output transformer (0 to 140 V, Staco, 3PN1010B) connected to two 3.2 mm-diameter graphite rod electrodes. ERH is one of the most common in situ thermal technologies applied in the field [22], with the potential to remediate soil and groundwater contaminated with chlorinated solvents and petroleum hydrocarbons. The maximum temperature that can be achieved during ERH applications is constrained by the boiling point of water as resistive heating is the result of an alternating current (AC) between subsurface electrodes and is facilitated by ionic conduction through the pore water [10,30]. In this study, the electrodes were installed horizontally through the entire width of the flow cell above and below the emplaced DNAPL (Figure 2). The same voltage was applied to both experiments, but different heating conditions resulted from different electrode spacing. The electrodes were spaced 16 cm apart in Experiment A, creating a colder region above the target heated zone, and 24 cm apart in Experiment B, which extended the target heated zone to the top of the sand pack. Colder regions can lead to the condensation of gas-phase VOCs, while extended heated zones facilitate the transport of vaporized VOCs to vapor extraction points. To compensate for heat losses from the flow cell that are much higher than in ISTT field applications due to the high surface-area-to-volume ratio of the cell, the effective electrical conductivity and resistive heating power were increased by adding sodium chloride (NaCl) to deionized water, which was used as the pore water for both experiments. Experiment B used 6 g/L NaCl due to larger electrode spacing, whereas only 4 g/L NaCl was used in Experiment A.
2.2. Gas Formation and Maximum DNAPL Volume
Gas production during the heating of DNAPL occurs when the sum of water and DNAPL vapor pressures is equal to the sum of hydrostatic and capillary pressures (e.g., [16]):
where is the vapor pressure of water (Pa), is the vapor pressure of the DNAPL (Pa), is the water pressure (typically assumed to be hydrostatic) (Pa), and is the capillary pressure (Pa). At the onset of gas production, the capillary pressure is defined by a threshold that represents heterogeneous nucleation [31]. Co-boiling occurs at a constant temperature (constant vapor pressure) for a single-component DNAPL as energy is converted exclusively to latent heat but occurs over a range of temperatures for a multi-component DNAPL [16]. The co-boiling temperature is lower than either the water or DNAPL boiling temperatures as the vapor pressures of two immiscible fluids are additive at the DNAPL–water interface, and both contribute to the total gas pressure. For instance, at atmospheric pressure, TCE-water co-boiling occurs at 73 °C [10]. Therefore, gas production during co-boiling is local to the DNAPL–water interface, whereas gas production during water boiling (i.e., Equation (1) with = 0) is distributed throughout the media that has reached the water boiling temperature. The Antoine equation can be used to approximate the vapor pressures of water and DNAPL as a function of temperature:
where represents the vapor pressure of compound i, and , and are compound-specific empirical constants for compound i [32].
The co-boiling of DNAPL and water leads to the production of a significant volume of water vapor along with VOC from vaporized DNAPL. As a result, the volume of gas produced during co-boiling is much more than the volume that would be produced by boiling only DNAPL. The ideal gas law can be used to calculate the total volume of gas produced per volume of DNAPL, assuming the complete vaporization of DNAPL at its co-boiling temperature [26]:
where is the volume of gas produced (L), is the volume of DNAPL co-boiling with water (L), is the DNAPL density (g/L), R is the universal gas constant (L Pa/mol K), is the molecular weight of the DNAPL (g/mol), and is the co-boiling temperature (K) calculated using Equations (1) and (2). Munholland et al. [26] reported the potential for gas volumes ~200× to 800× the DNAPL volume prior to co-boiling for a range of chlorinated solvent DNAPLs at total pressures of 100 to 300 kPa.
To satisfy Equation (1), the water and DNAPL vapor pressure must increase with increasing water depth and capillary pressure, which necessitates a higher co-boiling temperature. The hydrostatic pressure can be expressed as a function of water depth:
where is the unit weight of water (9.81 kN/m3) and (m) is the depth below the water table. The capillary pressure in Equation (1) can be estimated as the displacement pressure () and approximated using the Brooks-Corey model [33]:
where is the water saturation, is the residual water saturation, is the effective water saturation, is the pore size distribution (PSD) index and is the displacement pressure, which is commonly assumed to represent the entry pressure () when fitting data using the Brooks–Corey model.
Given that large volumes of gas are created during co-boiling and that in the presence of water, gas can displace DNAPL from larger pores (i.e., the non-wetting fluid displacing the intermediate-wetting fluid), it is useful to consider the maximum volume of DNAPL () that could be present in a coarse lens and generate a sufficient volume of gas to completely fill the pore space () of that coarse lens (Figure 3). Any additional DNAPL above this maximum volume creates a gas that would compete with DNAPL for the pore space in that lens, potentially increasing the gas pressure within the coarse lens and displacing DNAPL to the surrounding finer material.
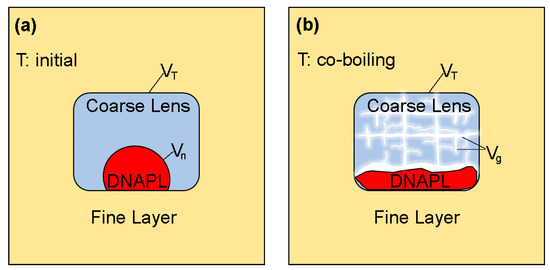
Figure 3.
Schematic representation of (a) Initial conditions before heating and (b) Conditions during DNAPL-water co-boiling.
represents temperature, the bulk volume of the coarse lens, the total gas volume and the DNAPL volume.
This maximum volume can be estimated using Equation (3), where the volume of gas equals the pore volume of the coarse lens (), the residual water saturation is assumed to be negligible, the pore volume is defined by the total volume, and the porosity (), and the DNAPL vapor pressure is the vapor pressure at the co-boiling temperature for a given hydrostatic pressure and displacement pressure of the finer material. This estimate also assumes that the DNAPL residual formed by the three-fluid (water-DNAPL-gas) system [34,35] in the coarse lens is negligible. If three-fluid DNAPL residual cannot be neglected, then the pore volume in Equation (3) represents the effective pore volume (i.e., the pore volume that is not occupied by residual fluids). However, because DNAPL residual in a water-DNAPL-gas system depends on the maximum DNAPL saturation, it is unlikely that high DNAPL residual saturations will be realized throughout the entire coarse lens. This estimate also assumes that the capillary pressure required to drain the coarse lens is negligible compared to the DNAPL-water displacement pressure in the fine material. That is, the permeability contrast between the coarse and fine material is assumed to be sufficiently high such that high gas saturations are achieved in the coarse material at a gas pressure that is lower than what is required for gas to enter the fine material.
3. Results and Discussion
3.1. Visualization of Gas Production and Migration during TCE-Water Co-Boiling
Images of Experiment A and Experiment B are shown in Figure 4. The images span three heating stages: (1) DNAPL-water heating (Figure 4a,f), during which temperatures of the DNAPL and water increase, (2) DNAPL-water co-boiling (Figure 4b–d,g–i) during which gas is produced by co-boiling, and (3) water heating (Figure 4e,j) during which no DNAPL remains and the temperature of the water increases. For a longer heating duration, these stages would be followed by water boiling. Both the DNAPL-water co-boiling stage and the water boiling stage are important because they create the gas phase pathways that are necessary to connect the target treatment zone to gas capture locations (multiphase and/or soil vapor extraction wells) for VOC removal. The gas created after co-boiling is also important to sweep co-boiled vapors towards extraction wells, strip VOCs from the aqueous phase, and promote the desorption and vaporization of VOCs from the solid phase.
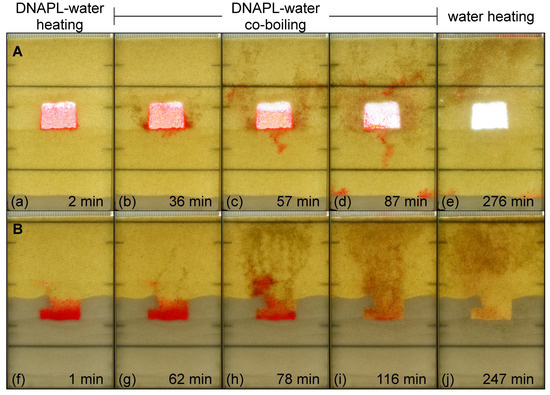
Figure 4.
Images of Experiment A (a–e) and Experiment B (f–j) during electrical resistance heating.
In Experiment A, gas was produced in the coarse lens (glass beads) adjacent to the TCE DNAPL. As gas accumulated within the coarse lens, some local redistribution of DNAPL occurred within the glass beads themselves, and eventually, DNAPL was displaced by the pooled gas both horizontally and downward into the surrounding finer media (Figure 4b–d). This behavior was attributed to gas expansion and pressurization, which caused: (1) gas to invade DNAPL in the glass beads and (2) DNAPL to invade water in the surrounding (finer) sand. Because interfacial tension with water is lower for the DNAPL (~0.038 N/m for TCE-water) than gas (~0.072 N/m for air-water), the DNAPL-water displacement pressure was lower than the gas-water displacement pressure in the sand, resulting in preferential DNAPL invasion. Nonetheless, gas was also observed in the finer media. This gas first appeared as a result of co-boiling once DNAPL had entered the sand; hence, it was produced outside of the coarse lens. For example, early observations show gas migration beginning near the lower corners of the coarse lens rather than from the top of the coarse lens (Figure 4b), which would be the preferential location for gas-water drainage.
It is important to note that the spatial extent of DNAPL migration within the sand following its displacement from the glass beads was limited because of co-boiling within the sand, which highlights the importance of achieving or exceeding co-boiling temperatures around the target treatment zones that contain DNAPL to manage this temporary condition. This includes the establishment of a so-called “hot floor” [36] where temperatures below a target zone are maintained sufficiently high to vaporize any mobilized DNAPL. Provided that DNAPL migration distances following displacement are small, either because of small displaced volumes or the presence of additional nearby capillary barriers that restrict migration, small spatial gradients in temperature during ISTT are likely to favor co-boiling over larger-scale migration.
Although much of the DNAPL displaced from the glass beads in Experiment A was removed by co-boiling, some DNAPL was mobilized upwards ahead of the co-boiled vapors (Figure 4c) and into the colder zone above the upper electrode (Figure 4d). Some DNAPL was also mobilized downward once displaced from the glass beads. This began as a DNAPL finger in the center of the lens’ lower edge (Figure 4c) and continued as a lateral migration around the lower electrode and down along the edge of the flow cell, where it accumulated on a layer of 40/50 sand (Figure 4d). This further underscores the importance of maintaining higher temperatures around target treatment zones.
The gas and DNAPL behavior in Experiment B [10] was different than that of Experiment A. The initial conditions of Experiment B differed from those of Experiment A in two important aspects. First, the emplaced DNAPL occupied a substantially lower fraction of the higher permeability layer pore space (4% of the 20/30 sand compared to 80% of the glass beads). Therefore, there was less potential for DNAPL to be displaced by gas. Second, this higher permeability layer was connected to an extraction location (i.e., passive extraction through the unsealed top of the flow cell). Therefore, gas produced at the DNAPL–water interface during co-boiling formed discontinuous gas cluster pipelines above the DNAPL pool rather than accumulating in the immediate vicinity of the DNAPL. DNAPL displacement occurred but only upward and further into the higher permeability sand rather than downward into the lower permeability (higher displacement pressure) sand. Similar results were reported by Martin and Kueper [23], where gas produced during the co-boiling of a DNAPL pool in higher permeability sand between the two layers of lower permeability sand was able to exit their experiment through wells installed into the top layer. As a result, a gas pool was formed at the top of the higher permeability sand but did not occupy that entire layer. A comparison of Experiments A and B suggests that providing a means for passive venting below the water table (e.g., screening vertical extraction points across coarse layers) will minimize the potential for DNAPL to be displaced by pooled or trapped gas.
The different geologic scenarios represented in Experiment A and Experiment B are common at contaminated sites and will influence gas migration pathways. Gas dynamics can be affected by small changes in site geology, even at scales on the order of centimeters. Therefore, laboratory experiments capable of visually assessing gas formation, movement and extraction are essential to developing conceptual models of the complex processes taking place during ISTT. Such conceptual models could inform the design of heating and extraction systems to accommodate the mechanisms observed in these experiments.
3.2. Estimates of Relative DNAPL Volume
The initial condition of Experiment A represented an extreme case of high DNAPL volume in a small lens, with relatively large temperature differences over short-length scales. However, because a substantial gas volume was generated during co-boiling, if there were no means for venting trapped or pooled gas, hypothetically, smaller volumes of DNAPL in larger coarse lenses could be temporarily displaced into finer material before co-boiling or migrating further. It is not yet known whether this experimentally observed NAPL displacement mechanism could be of potential importance at the field scale, given that temperature differences and DNAPL migration pathways occur over much larger length scales, where the degree of heterogeneity and vertical anisotropy is often higher, and energy delivery points are often oriented vertically.
As a preliminary evaluation, the maximum DNAPL volume, expressed as a fraction of the pore volume of a coarse lens in which the DNAPL resides and as a function of the entry pressure of the surrounding finer material, is shown in Figure 5. These estimates do not include residual DNAPL formed by the drainage of water and DNAPL by gas within the coarse layer. It is unclear whether the DNAPL that remained in the coarse lens partway through heating (e.g., Figure 4d) was present as a residual that was not displaced or was simply DNAPL that had yet to be displaced. At the end of heating, no DNAPL remained in the coarse lens. The estimated maximum volume was affected by the water pressure (i.e., depth below the water table). Lower water pressure resulted in lower values because the gas pressure required to meet the capillary displacement pressure decreased, which requires less DNAPL to be vaporized (Figure 5a). The maximum volume was also affected by the DNAPL vapor pressure. Figure 5b shows the effect of DNAPL vapor pressure on for five VOCs: carbon tetrachloride (CT), dichloromethane (DCM), TCE, 1,2-dichloroethane (1,2-DCA), and tetrachloroethene (PCE), at a constant hydrostatic pressure of 20 m of H2O. Similar to the effect of displacement and water pressure, less volatile DNAPL (PCE compared to CT) resulted in more DNAPL remaining when DNAPL-water drainage conditions were met, and the corresponding values were lower. Note that the co-boiling temperature also depends on water pressure and DNAPL vapor pressure. Therefore, the onset of DNAPL displacement for DNAPL volumes greater than will occur at different temperatures across the conditions depicted in Figure 5 [26].
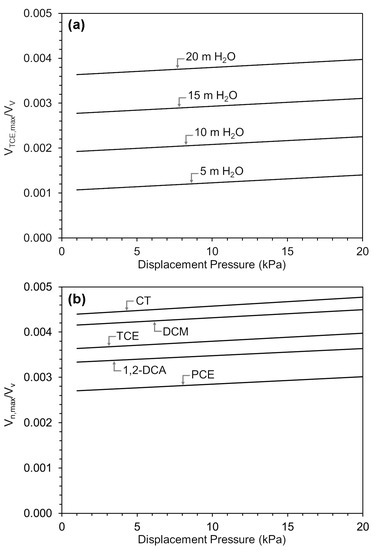
Figure 5.
Relative maximum DNAPL volumes at (a) Different water depths for TCE, and (b) Various DNAPLs at a water depth of 20 m.
In general, the volume ratios shown in Figure 5 are small; they represent approximately 0.1% to 0.5% of the total pore volume of the coarse material. They also do not depend strongly on displacement pressure, where lower displacement pressures are expected for sands and gravels, and higher displacement pressures are expected for silts and clays. For example, for a 30 m × 30 m coarse lens that is 0.1 m thick with a porosity of 0.25, surrounded by finer material with an entry pressure of 5 kPa and a depth of 20 m below the water table, the maximum volume of CT DNAPL that could be co-boiled without displacing DNAPL from the coarse lens is 100.7 L. It is important to note that the relative volume shown in Figure 5 should not be interpreted as local DNAPL saturations, which greatly exceed 0.5% in DNAPL pools. Instead, these values represent a macroscopic relative volume at the scale of the entire coarse lens. Nevertheless, the DNAPL volume present in a lens that exceeds could potentially be displaced by gas during co-boiling (Figure 6) if no gas extraction occurs from that coarse lens. The further migration of displaced DNAPL will be limited by temperatures in the surrounding finer material at or above the co-boiling temperature, the presence of nearby capillary barriers, or displaced DNAPL volumes that do not exceed residual (immobile) saturations in the finer material.
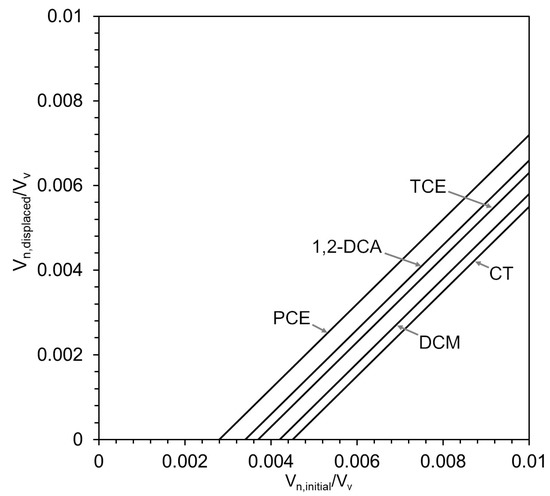
Figure 6.
Ratios of displaced DNAPL volume to the initial DNAPL volume for various VOCs at a water depth of 20 m and a displacement pressure of 5 kPa in the surrounding finer material.
3.3. Conceptual Model and ISTT Implications
The mechanisms that control the mass transfer of VOCs from DNAPL in higher permeability (coarse) lenses surrounded by lower permeability (fine) material change over the course of heating during an ISTT application. Prior to heating, mass transfer from a DNAPL pool to the surrounding groundwater occurs by dissolution and is affected by advection, dispersion and diffusion in the coarse lens (Figure 7a). At the onset of co-boiling, gas is produced at DNAPL–water interfaces, leading to gas migration in the coarse material and potential gas accumulation underneath fine material that acts as a capillary barrier. This gas lowers the aqueous relative permeability of the coarse lens, limiting advection through the lens and lowering the overall mass transfer to the aqueous phase (Figure 7b). If sufficient DNAPL is present and gas is not extracted from the coarse lens, continued DNAPL-water co-boiling produces gas that fills the pore space of the coarse lens, further lowering aqueous relative permeability and limiting advection. This continued gas production can also lead to local DNAPL displacement inside the coarse lens as well as DNAPL displacement into the surrounding finer material (Figure 7c). If co-boiling temperatures are met or exceeded outside of the coarse lens, any displaced DNAPL is vaporized from its new location.
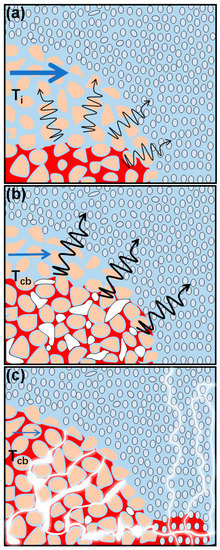
Figure 7.
Conceptual model of gas production during co-boiling within a coarse lens (higher permeability surrounded by lower permeability) without gas extraction from the lens, showing conditions (a) Prior to co-boiling, (b) During co-boiling as gas is first produced, and (c) Later during co-boiling as additional gas is produced to create a connected gas network, potentially leading to DNAPL displacement in the finer material followed by its co-boiling at that location. Black arrows represent diffusion from the DNAPL. Blue arrows represent groundwater flow and a decrease in arrow size from (a–c) emphasizes the decrease in pore-water velocity because of lower aqueous relative permeability in the presence of gas.
The implications of these results for ISTT practitioners may include the use of high-resolution site characterization tools (e.g., vertical aquifer profiling, dye-enhanced laser-induced fluorescence) in combination with three-dimensional geostatistical modeling to better identify coarser lenses containing pooled DNAPL. Energy delivery strategies could be designed to ensure that any DNAPL temporarily displaced out of the coarse lenses is heated to co-boiling temperatures before it migrates to colder zones. This may include implementing a hot floor in cases where sufficient quantities of DNAPL are present in coarse layers and vertical pathways for migration can be identified. It is also a common industry practice to screen multiphase extraction wells across the entire heated domain or to install filter packs around energy delivery points on a horizontal grid spacing that is tight enough to intercept gas and provide passive venting within coarse lenses [37].
4. Conclusions
Laboratory heating experiments using ERH were conducted in a thin flow cell to study the removal of pooled TCE DNAPL from a coarse lens surrounded by finer material. Photos taken during the co-boiling stage showed that vaporization first occurred at DNAPL–water interfaces at the top of the DNAPL pool. In the experiment with a discontinuous coarse lens, substantial initial DNAPL saturations and no gas extraction from the lens, gas formation resulted in gas accumulation in the coarse lens followed by the lateral and downward displacement of DNAPL. Conversely, in the experiment where the DNAPL pool was initially placed in coarse material on a capillary barrier and this coarse material extended to the top of the cell to allow the passive release of gas, gas did not accumulate within the coarse material and instead formed a stable network of continuous gas channels upward from the top of the DNAPL pool. Importantly, as heating continued and the heated zone expanded to cover the entire treatment area, displaced DNAPL was removed by co-boiling. Estimates based on the volume of gas produced during co-boiling demonstrated that the maximum DNAPL volume for which displacement did not occur was less than 0.5% of the pore volume of the entire coarse lens. The conceptual model developed in this study highlighted the importance of characterizing coarse and fine lenses at ISTT sites, which could have important implications for the design of energy delivery systems and the installation of vapor extraction wells. Maintaining elevated temperatures near DNAPL sources, as well as providing pathways for gas capture that reduces gas accumulation near these sources, could help manage any temporary DNAPL displacement.
Author Contributions
Conceptualization, P.R.H. and K.G.M.; methodology, P.R.H. and K.G.M.; validation, A.N.G., P.R.H. and K.G.M.; formal analysis, A.N.G., P.W. and K.G.M.; investigation, P.R.H.; resources, K.G.M.; writing—original draft preparation, A.N.G.; writing—review and editing, A.N.G., P.R.H. and K.G.M.; visualization, A.N.G. and P.W.; supervision, K.G.M.; project administration, P.R.H. and K.G.M.; funding acquisition, K.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Project Grant (STPGP 396730-10).
Data Availability Statement
The data presented in this study is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kueper, B.H.; Stroo, H.F.; Vogel, C.M.; Ward, C.H. (Eds.) Source Zone Remediation: The State of the Practice. In Chlorinated Solvent Source Zone Remediation; Springer: New York, NY, USA, 2014; pp. 1–27. [Google Scholar]
- Head, N.A.; Gerhard, J.I.; Inglis, A.M.; Nunez Garcia, A.; Chowdhury, A.I.A.; Reynolds, D.A.; de Boer, C.V.; Sidebottom, A.; Austrins, L.M.; Eimers, J.; et al. Field test of electrokinetically-delivered thermally activated persulfate for remediation of chlorinated solvents in clay. Water Res. 2020, 183, 116061. [Google Scholar] [CrossRef]
- Inglis, A.M.; Head, N.A.; Chowdhury, A.I.A.; Nunez Garcia, A.; Reynolds, D.A.; Hogberg, D.; Edwards, E.; Lomheim, L.; Weber, K.; Wallace, S.J.; et al. Electrokinetically-enhanced emplacement of lactate in a chlorinated solvent contaminated clay site to promote bioremediation. Water Res. 2021, 201, 117305. [Google Scholar] [CrossRef]
- Saenton, S.; Illangasekare, T.H.; Soga, K.; Saba, T.A. Effects of source zone heterogeneity on surfactant-enhanced NAPL dissolution and resulting remediation end-points. J. Contam. Hydrol. 2002, 59, 27–44. [Google Scholar] [CrossRef]
- Siegrist, R.L.; Crimi, M.; Simpkin, T.J. In Situ Chemical Oxidation for Groundwater Remediation; Springer Science & Business Media: New York, NY, USA, 2011; Volume 3. [Google Scholar]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbaek, H.; Siegrist, R.L.; Bjerg, P.L. In situ chemical oxidation of contaminated soil and groundwater using persulfate: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Triplett Kingston, J.L.T.; Johnson, P.C.; Kueper, B.H.; Mumford, K.G. In Situ Thermal Treatment of Chlorinated Solvent Source Zones. In Chlorinated Solvent Source Zone Remediation; Kueper, B.H., Stroo, H.F., Vogel, C.M., Ward, C.H., Eds.; Springer: New York, NY, USA, 2014; pp. 509–557. [Google Scholar]
- Ding, D.; Song, X.; Wei, C.; LaChance, J. A review on the sustainability of thermal treatment for contaminated soils. Environ. Pollut. 2019, 253, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qin, X.; Yang, X.; Zhao, Y. Study on the heat transfer in different aquifer media with different groundwater velocities during thermal conductive heating. Environ. Sci. Pollut. Res. 2020, 27, 36316–36329. [Google Scholar] [CrossRef]
- Hegele, P.R.; Mumford, K.G. Gas production and transport during bench-scale electrical resistance heating of water and trichloroethene. J. Contam. Hydrol. 2014, 165, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Dahmani, A.; Ahlfeld, D.P.; Lin, J.D.; Hill, E., III. Laboratory Study of Air Sparging: Air Flow Visualization. Groundw. Monit. Remediat. 1993, 13, 115–126. [Google Scholar] [CrossRef]
- Krol, M.M.; Mumford, K.G.; Johnson, R.L.; Sleep, B.E. Modeling discrete gas bubble formation and mobilization during subsurface heating of contaminated zones. Adv. Water Resour. 2011, 34, 537–549. [Google Scholar] [CrossRef]
- Mumford, K.G.; Martin, E.J.; Kueper, B.H. Removal of trichloroethene from thin clay lenses by electrical resistance heating: Laboratory experiments and the effects of gas saturation. J. Contam. Hydrol. 2021, 243, 103892. [Google Scholar] [CrossRef]
- Xie, Q.; Mumford, K.G.; Kueper, B.H. Simulating field-scale thermal conductive heating with the potential for the migration and condensation of vapors. J. Hazard. Mater. 2023, 453, 131439. [Google Scholar] [CrossRef] [PubMed]
- Hicknell, B.N.; Mumford, K.G.; Kueper, B.H. Laboratory study of creosote removal from sand at elevated temperatures. J. Contam. Hydrol. 2018, 219, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Mumford, K.G.; Kueper, B.H. Laboratory study of non-aqueous phase liquid and water co-boiling during thermal treatment. J. Contam. Hydrol. 2014, 164, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Heron, G.; Van Zutphen, M.; Christensen, T.H.; Enfield, C.G. Soil Heating for Enhanced Remediation of Chlorinated Solvents: A Laboratory Study on Resistive Heating and Vapor Extraction in a Silty, Low-Permeable Soil Contaminated with Trichloroethylene. Environ. Sci. Technol. 1998, 32, 1474–1481. [Google Scholar] [CrossRef]
- Heron, G.; Lachance, J.; Baker, R. Removal of PCE DNAPL from Tight Clays Using In Situ Thermal Desorption. Groundw. Monit. Remediat. 2013, 33, 31–43. [Google Scholar] [CrossRef]
- Liu, X.; Murdoch, L.C.; Falta, R.W.; Tan, T. Experimental characterization of CVOC removal from fractured clay during boiling. Int. J. Heat Mass Transf. 2014, 70, 764–778. [Google Scholar] [CrossRef]
- Liu, X.; Tan, T.; Falta, R.W.; Murdoch, L.C. Experimental method for characterizing CVOC removal from fractured clays during boiling. J. Contam. Hydrol. 2013, 152, 44–59. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Falta, R.W.; Murdoch, L.C. Experimental Demonstration of Contaminant Removal from Fractured Rock by Boiling. Environ. Sci. Technol. 2010, 44, 6437–6442. [Google Scholar] [CrossRef]
- Triplett Kingston, J.L.; Dahlen, P.R.; Johnson, P.C. State-of-the-Practice Review of In Situ Thermal Technologies. Groundw. Monit. Remediat. 2010, 30, 64–72. [Google Scholar] [CrossRef]
- Martin, E.J.; Kueper, B.H. Observation of trapped gas during electrical resistance heating of trichloroethylene under passive venting conditions. J. Contam. Hydrol. 2011, 126, 291–300. [Google Scholar] [CrossRef]
- Martin, E.J.; Mumford, K.G.; Kueper, B.H. Electrical Resistance Heating of Clay Layers in Water-Saturated Sand. Groundw. Monit. Remediat. 2016, 36, 54–61. [Google Scholar] [CrossRef]
- Martin, E.J.; Mumford, K.G.; Kueper, B.H.; Siemens, G.A. Gas formation in sand and clay during electrical resistance heating. Int. J. Heat Mass Transf. 2017, 110, 855–862. [Google Scholar] [CrossRef]
- Munholland, J.L.; Mumford, K.G.; Kueper, B.H. Factors affecting gas migration and contaminant redistribution in heterogeneous porous media subject to electrical resistance heating. J. Contam. Hydrol. 2016, 184, 14–24. [Google Scholar] [CrossRef]
- Hegele, P.R.; McGee, B.C.W. Managing the negative impacts of groundwater flow on electrothermal remediation. Remediat. J. 2017, 27, 29–38. [Google Scholar] [CrossRef]
- Baker, R.S.; Hiester, U. Large-Scale Physical Models of Thermal Remediation of DNAPL Source Zones in Aquitards; SERDP # ER-1423; Terratherm Inc.: Fitchburg, MA, USA, 2009. [Google Scholar]
- Schroth, M.H.; Ahearn, S.J.; Selker, J.S.; Istok, J.D. Characterization of Miller-similar silica sands for laboratory hydrologic studies. Soil Sci. Soc. Am. J. 1996, 60, 1331–1339. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, B.; Li, G.; Zhang, F. Enhancing DNAPL removal from low permeability zone using electrical resistance heating with pulsed direct current. J. Hazard. Mater. 2021, 413, 125455. [Google Scholar] [CrossRef]
- Jones, S.F.; Evans, G.M.; Galvin, K.P. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Yaws, C.L.; Narasimhan, P.K.; Gabbula, C. Yaws’ Handbook of Antoine Coefficients for Vapor Pressure; Knovel: Norwich, NY, USA, 2005. [Google Scholar]
- Brooks, R.H.; Corey, A.T. Properties of Porous Media Affecting Fluid Flow. J. Irrig. Drain. Div. 1966, 92, 61–88. [Google Scholar] [CrossRef]
- Van Geel, P.J.; Roy, S.D. A proposed model to include a residual NAPL saturation in a hysteretic capillary pressure–saturation relationship. J. Contam. Hydrol. 2002, 58, 79–110. [Google Scholar] [CrossRef]
- Lenhard, R.J.; Oostrom, M.; Dane, J.H. A constitutive model for air–NAPL–water flow in the vadose zone accounting for immobile, non-occluded (residual) NAPL in strongly water-wet porous media. J. Contam. Hydrol. 2004, 71, 261–282. [Google Scholar] [CrossRef]
- Heron, G.; Baker, R.; Bierschenk, J.; LaChance, J. Use of Thermal Conduction Heating for the Remediation of DNAPL in Fractured Bedrock. In Proceedings of the Remediation of Chlorinated and Recalcitrant Compounds: Proceedings of the Sixth International Conference, Columbus, OH, USA, 19–22 May 2008. [Google Scholar]
- Horst, J.; Munholland, J.; Hegele, P.; Klemmer, M.; Gattenby, J. In Situ Thermal Remediation for Source Areas: Technology Advances and a Review of the Market From 1988–2020. Groundw. Monit. Remediat. 2021, 41, 17–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).