The Effect of Nutrient Source and Beneficial Bacteria on Growth of Pythium-Exposed Lettuce at High Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
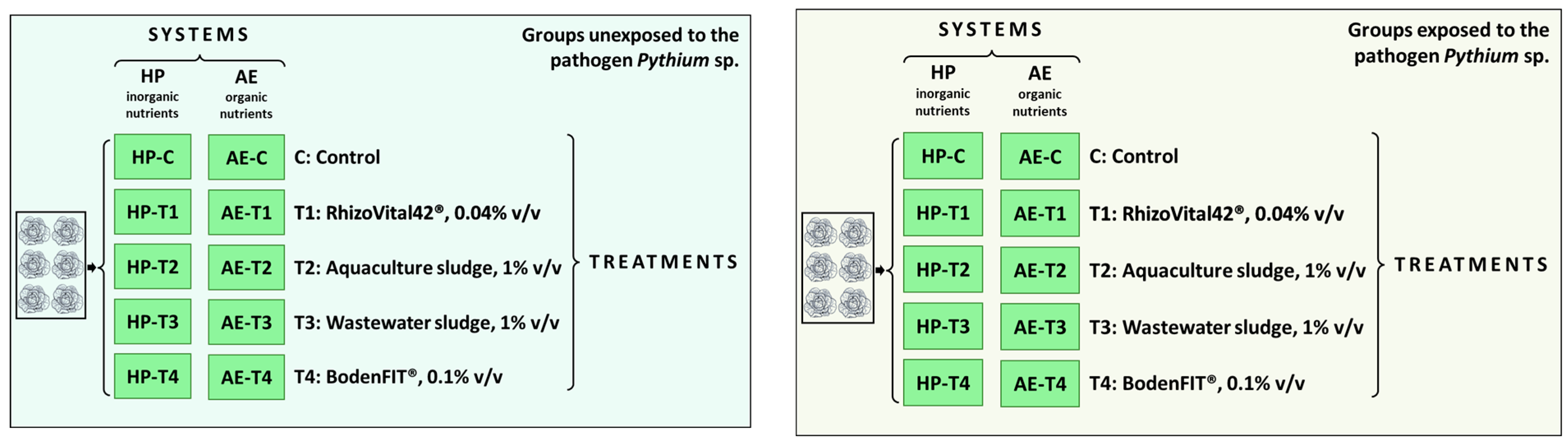
2.1. Experimental Design
- -
- AE: predominantly organic nutrient source functioning as a proxy for decoupled AP consisting of water from an aquaculture farm (Steinibach-Flühli, Switzerland) producing pikeperch (Sander lucioperca) (Table S1). The AE was collected at the beginning of the experiment and stored at +7.5 ± 0.5 °C;
- -
- HP: inorganic nutrient source consisting of an aqueous solution of inorganic fertilisers (Table 1) emulating the nutrient levels of AE with pH adjusted to that of AE with HCl. HP was formulated at the beginning of the experiment and stored at +7.5 ± 0.5 °C;
- -
- T1: RhizoVital42® (Andermatt Biocontrol AG, Grossdietwil, Switzerland), which contains Bacillus velezensis (synonym B. amyloliquefaciens ssp. Plantarum), administered at a concentration of 0.04% v/v;
- -
- T2: sludge from the drum filter of the aquaculture farm (Steinibach-Flühli, Switzerland), administered at a concentration of 1% v/v, collected at the experiment start, and stored at +7.5 ± 0.5 °C. The dry matter content was 0.42%;
- -
- T3: sludge from a municipal wastewater treatment plant (Abwasserreinigungsanlagen, Au, Switzerland), administered at a concentration of 1% v/v, collected at the experiment start, and stored at +7.5 ± 0.5 °C. The dry matter content was 0.35%;
- -
- T4: activated effective microorganism BodenFIT® (EM Schweiz AG, Arni, Switzerland), administered at a concentration of 0.1% v/v.
2.2. Preparation of the Stock Solution
2.3. Plant Growth Conditions
2.4. Water Quality Monitoring
2.5. Sampling and Chemical Analysis of Plants
2.6. Statistical Analysis
3. Results
3.1. Development of Nutrient Solution Characteristics during the Experiment
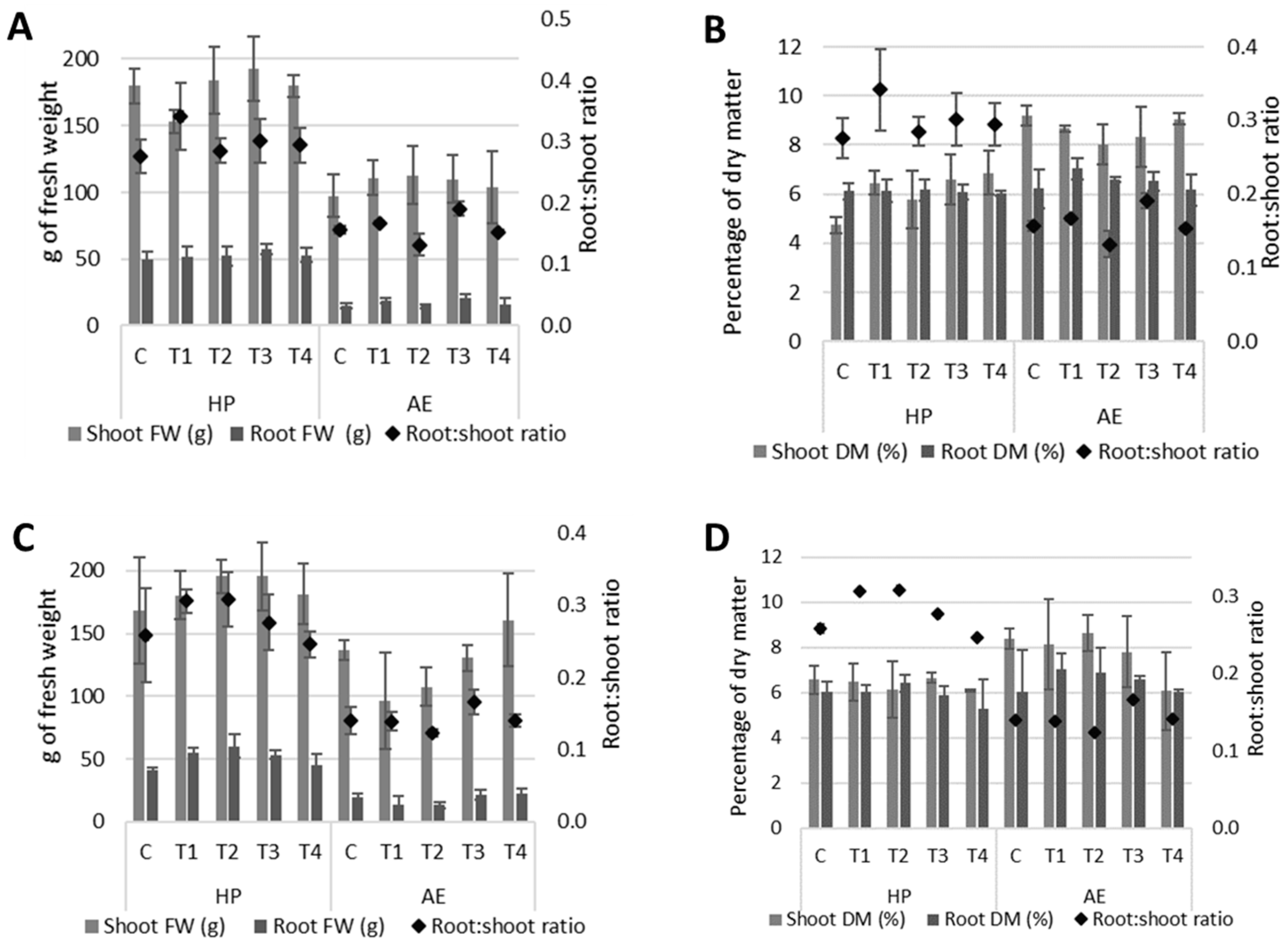
3.2. Plant Growth and Composition
4. Discussion
4.1. Impact of Inorganic or Organic Nutrient Sources on Lettuce Harvest
4.2. Role of Potentially Beneficial Bacteria in the Nutrient Solutions
4.3. Salinity
4.4. System and Treatment Effects on Lettuces Exposed to Pythium sp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food and Agriculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Sathyanarayana, S.R.; Gangadhar, W.V.; Badrinath, M.G.; Ravindra, R.M.; Shriramrao, A.U. Hydroponics: An Intensified Agriculture Practice to Improve Food Production. Rev. Agric. Sci. 2022, 10, 101–114. [Google Scholar] [CrossRef]
- Ilinova, A.; Dmitrieva, D.; Kraslawski, A. Influence of COVID-19 pandemic on fertilizer companies: The role of competitive advantages. Resour. Policy 2021, 71, 102019. [Google Scholar] [CrossRef]
- The World Bank. Fertilizers Price Index for February; The World Bank: Washington, DC, USA, 2023. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020—Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M. Lettuce (Lactuca sativa L. var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms. Hydroponics Water 2016, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baganz, G.F.M.; Junge, R.; Portella, M.C.; Goddek, S.; Keesman, K.J.; Baganz, D.; Staaks, G.; Shaw, C.; Lohrberg, F.; Kloas, W. The aquaponic principle—It is all about coupling. Rev. Aquac. 2022, 14, 252–264. [Google Scholar] [CrossRef]
- Baganz, G.; Baganz, D.; Staaks, G.; Monsees, H.; Kloas, W. Profitability of multi-loop aquaponics: Year-long production data, economic scenarios and a comprehensive model case. Aquac. Res. 2020, 51, 2711–2724. [Google Scholar] [CrossRef] [Green Version]
- Zappernick, N.; Nedunuri, K.V.; Islam, K.R.; Khanal, S.; Worley, T.; Laki, S.L.; Shah, A. Techno-economic analysis of a recirculating tilapia-lettuce aquaponics system. J. Clean. Prod. 2022, 365, 132753. [Google Scholar] [CrossRef]
- Chang, C.; Bowman, J.L.; Meyerowitz, E.M. Field Guide to Plant Model Systems. Cell 2016, 167, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Eck, M.; Szekely, I.; Massart, S.; Jijakli, M.H. Ecological Study of Aquaponics Bacterial Microbiota over the Course of a Lettuce Growth Cycle. Water 2021, 13, 2089. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Beauchamp, W.R.; Pickens, J.M.; Sibley, J.L.; Chappell, J.A.; Martin, N.R.; Newby, A.F. Salt Level in a Simulated Aquaponic System and Effects on Bibb Lettuce. Int. J. Veg. Sci. 2018, 24, 122–136. [Google Scholar] [CrossRef]
- Meyer, P.; Förster, N.; Huyskens-Keil, S.; Ulrichs, C.; Geilfus, C. Phenolic compound abundance in Pak choi leaves is controlled by salinity and dependent on pH of the leaf apoplast. Plant-Environ. Interact 2021, 2, 36–44. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Plant Responses to Saline and Sodic Conditions. In Agricultural Salinity Assessment and Management; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 169–205. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Sirakov, I.; Lutz, M.; Graber, A.; Mathis, A.; Staykov, Y.; Smits, T.; Junge, R. Potential for combined biocontrol activity against fungal fish and plant pathogens by bacterial isolates from a model aquaponic system. Water 2016, 8, 518. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, F.A.; Vivian-Rogers, V.R.; Urakawa, H. Tilapia recirculating aquaculture systems as a source of plant growth promoting bacteria. Aquac. Res. 2019, 50, 2054–2065. [Google Scholar] [CrossRef]
- Stouvenakers, G.; Massart, S.; Depireux, P.; Jijakli, M.H. Microbial Origin of Aquaponic Water Suppressiveness against Pythium aphanidermatum Lettuce Root Rot Disease. Microorganisms 2020, 8, 1683. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Gorczyca, A.; Matras, E.; Krawczyk, K.; Mastalerz, J.; Zakrzewski, A. Bacteria Isolated from the Aeration Chamber of Wastewater Treatment Plants Used in the Biocontrol and Promotion of Wheat Growth. Agronomy 2020, 10, 1792. [Google Scholar] [CrossRef]
- Little, K.R.; Gan, H.M.; Surapaneni, A.; Schmidt, J.; Patti, A.F. Characterisation of bacterial diversity in fresh and aged sewage sludge biosolids using next generation sequencing. Detritus 2020, 10, 82–91. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Kitir, N.; Unek, C.; Nikerel, E.; Ozdemir, B.S.; Güneş, A.; Mokhtari, N.E.P. Beneficial Role of Plant Growth-Promoting Bacteria in Vegetable Production Under Abiotic Stress. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 151–166. [Google Scholar]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of Salt Stress by Plant Growth-Promoting Bacteria in Hydroponic Leaf Lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Narsing Rao, M.P.; Wang, H.-F.; Fang, B.-Z.; Liu, Y.-H.; Li, L.; Xiao, M.; Li, W.-J. Transcriptomic analysis of two endophytes involved in enhancing salt stress ability of Arabidopsis thaliana. Sci. Total Environ. 2019, 686, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The Use of PGPB to Promote Plant Hydroponic Growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production-Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper 589; FAO: Rome, Italy, 2014. [Google Scholar]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M.L. Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wendell, R.; Wolff, H. (Eds.) Handbook of Practical X-ray Fluorescence Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 24 April 2023).
- Schmautz, Z.; Loeu, F.; Liebisch, F.; Graber, A.; Mathis, A.; Griessler Bulc, T.; Junge, R. Tomato Productivity and Quality in Aquaponics: Comparison of Three Hydroponic Methods. Water 2016, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Maucieri, C.; Nicoletto, C.; Schmautz, Z.; Sambo, P.; Komives, T.; Borin, M.; Junge, R. Vegetable Intercropping in a Small-Scale Aquaponic System. Agronomy 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2006. [Google Scholar]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Buwalda, F.; Warmenhoven, M. Growth-limiting phosphate nutrition suppresses nitrate accumulation in greenhouse lettuce. J. Exp. Bot. 1999, 50, 813–821. [Google Scholar] [CrossRef]
- Waters, B.M. Moving magnesium in plant cells. New Phytol. 2011, 190, 510–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Seawright, D.E.; Stickney, R.R.; Walker, R.B. Nutrient dynamics in integrated aquaculture–hydroponics systems. Aquaculture 1998, 160, 215–237. [Google Scholar] [CrossRef]
- Germano, R.P.; Melito, S.; Cacini, S.; Carmassi, G.; Leoni, F.; Maggini, R.; Montesano, F.F.; Pardossi, A.; Massa, D. Sweet basil can be grown hydroponically at low phosphorus and high sodium chloride concentration: Effect on plant and nutrient solution management. Sci. Hortic. 2022, 304, 111324. [Google Scholar] [CrossRef]
- Shaw, C.; Knopf, K.; Kloas, W. Fish Feeds in Aquaponics and Beyond: A Novel Concept to Evaluate Protein Sources in Diets for Circular Multitrophic Food Production Systems. Sustainability 2022, 14, 4064. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Potassium and micronutrient fertilizer addition in a mock aquaponic system for drug-type Cannabis sativa L. cultivation. Can. J. Plant Sci. 2021, 101, 341–352. [Google Scholar] [CrossRef]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Niazi, A.; Manzoor, S.; Asari, S.; Bejai, S.; Meijer, J.; Bongcam-Rudloff, E. Genome Analysis of Bacillus amyloliquefaciens Subsp. plantarum UCMB5113: A Rhizobacterium That Improves Plant Growth and Stress Management. PLoS ONE 2014, 9, e104651. [Google Scholar] [CrossRef]
- Daly, M.J.; Stewart, D.P.C. Influence of “Effective Microorganisms” (EM) on Vegetable Production and Carbon Mineralization–A Preliminary Investigation. J. Sustain. Agric. 1999, 14, 15–25. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994. [Google Scholar]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on Lettuce Growth and Root Associated Bacterial Community in a Small-Scale Aquaponics System. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef]
- Iriti, M.; Scarafoni, A.; Pierce, S.; Castorina, G.; Vitalini, S. Soil Application of Effective Microorganisms (EM) Maintains Leaf Photosynthetic Efficiency, Increases Seed Yield and Quality Traits of Bean (Phaseolus vulgaris L.) Plants Grown on Different Substrates. Int. J. Mol. Sci. 2019, 20, 2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-D.; He, L.-Y.; Huang, Z.; Sheng, X.-F. Myroides xuanwuensis sp. nov., a mineral-weathering bacterium isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, K.E.; Evans, D.E.; Hodson, M.J. Aluminium/silicon interactions in barley (Hordeum vulgare L.) seedlings. Plant Soil 1995, 173, 89–95. [Google Scholar] [CrossRef]
- Kidd, P.S.; Llugany, M.; Poschenrieder, C.; Gunsé, B.; Barceló, J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J. Exp. Bot. 2001, 52, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Ekinci, M.; Yildirim, E.; Kotan, R.; Dursun, A.; Turan, M.; Güneş, A. Effect of plant growth promoting rhizobacteria on growth, nutrient, organic acid, amino acid and hormone content of cauliflower (Brassica oleracea L. var. botrytis) transplants. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–85. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press, Institute of Medicine: Washington, DC, USA, 2005. [Google Scholar]
- Pérez-López, U.; Miranda-Apodaca, J.; Mena-Petite, A.; Muñoz-Rueda, A. Responses of nutrient dynamics in barley seedlings to the interaction of salinity and carbon dioxide enrichment. Environ. Exp. Bot. 2014, 99, 86–99. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Dodd, I.C.; Perez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yep, B.; Gale, N.V.; Zheng, Y. Aquaponic and Hydroponic Solutions Modulate NaCl-Induced Stress in Drug-Type Cannabis sativa L. Front. Plant Sci. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kaloterakis, N.; van Delden, S.H.; Hartley, S.; De Deyn, G.B. Silicon application and plant growth promoting rhizobacteria consisting of six pure Bacillus species alleviate salinity stress in cucumber (Cucumis sativus L). Sci. Hortic. 2021, 288, 110383. [Google Scholar] [CrossRef]
- Panova, G.G.; Grote, D.; Kläring, H.-P. Population dynamics of Pythium aphanidermatum and response of tomato plants as affected by root-zone temperature. J. Plant Dis. Prot. 2004, 111, 52–63. [Google Scholar] [CrossRef]
- Martin, F.N.; Loper, J.E. Soilborne Plant Diseases Caused by Pythium spp. Ecology, Epidemiology, and Prospects for Biological Control. Crit. Rev. Plant Sci. 1999, 18, 111–181. [Google Scholar] [CrossRef]
| Nutrient Supplementation | Supplied Nutrient (Concentration in the Stock Nutrient) | Target Concentration in the Final Solution (mg/L Final Solution) | Company |
|---|---|---|---|
| Calcinit | CaNO3 | 175.8 | Yara UK Limited, Grimsby, United Kingdom |
| Phoskalin | 52% P2O5 34% K2O | 56.1 | Hauert HBG Dünger AG, Grossaffoltern, Switzerland |
| Calcium hydroxide | Ca(OH)2 | 35.3 | Kalkfabrik Netstal AG, Netstal, Switzerland |
| Magnesium sulphate | MgSO4(H2O)7 | 81.8 | K + S Kali GmbH, Kassel, Germany |
| Plantspeed Iron EDTA | Fe EDTA | 60.0 | Ökohum GmbH, Herrenhof, Switzerland |
| Plantspeed Micromix | Micronutrients | 11.5 | Ökohum GmbH, Herrenhof, Switzerland |
| Calcium sulphate | CaSO4(H2O)2 | 13.3 | Carl Roth GmbH, Karlsruhe, Germany |
| Reosal | NaCl | 1985.9 | Schweizer Salinen AG, Switzerland |
| Unit | HP Solution | AE Solution | |
|---|---|---|---|
| pH | - | 7.3 | 6.9 |
| temperature | °C | 7.1 | 7.5 |
| EC | mS/cm | 4.5 | 4.2 |
| water hardness | °dH | 18.7 | 12.1 |
| NO3−-N | mg/L | 32.1 | 21.9 |
| PO42-P | mg/L | 19.1 | 13.0 |
| SO42− | mg/L | 68.9 | 25.0 |
| Na+ | mg/L | 808.0 | 782.2 |
| K+ | mg/L | 69.5 | 68.2 |
| Ca2+ | mg/L | 121.4 | 89.6 |
| Mg2+ | mg/L | 58.4 | 39.9 |
| Cl− | mg/L | 1300.5 | 830.4 |
| Parameter | Where | Sample Preparation | Laboratory Equipment | Company |
|---|---|---|---|---|
| Dissolved oxygen [mg/L], temperature [°C] | Directly at the sampling point | - | Probe LDO10101, PHC10103, CDC40103 respectively and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| pH [-] | Directly at the sampling point | - | Probe LDO10101, PHC10103, CDC40103 respectively and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| Electrical conductivity [µS/cm] | Directly at the sampling point | - | Probe LDO10101, PHC10103, CDC40103 respectively and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| Nutrient solution use [L] | Directly at the sampling point | - | Beaker and graduation lines on the boxes | - |
| NO3−-N, PO42−-P, SO42−, Cl− [mg/L] | Stored at −20 °C in 15 mL tube, laboratory | Filtered 0.45 μm, adding 1 drop 2M HNO3 per 15 mL | 930 Compact IC flex, Column Metrosep A Supp 5–250/4.0 | Metrohm Schweiz AG, Zofingen, Switzerland |
| Na+, K+, Ca2+, Mg2+ [mg/L] | Stored at −20 °C in 15 mL tube, laboratory | Filtered 0.45 μm | 930 Compact IC flex, Column Metrohm C 6–150/4.0 | Metrohm Schweiz AG, Zofingen, Switzerland |
| System | HP | AE | ANOVA | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | T1 | T2 | T3 | T4 | C | T1 | T2 | T3 | T4 | ||
| Shoot DM, % | 4.7 e | 6.4 cde | 5.8 de | 6.6 cde | 6.9 bcde | 9.2 a | 8.7 abc | 8.0 abcd | 8.3 abc | 9.0 ab | S: *** S × T: 0.053 | 0.3 |
| Shoot FW, g | 179.8 | 152.9 | 184.0 | 192.5 | 180.0 | 97.5 | 110.8 | 112.9 | 110.0 | 104.0 | S: *** | 7.4 |
| Root DM, % | 6.1 | 6.1 | 6.2 | 6.1 | 6.0 | 6.2 | 7.0 | 6.6 | 6.5 | 6.2 | S: * | 0.1 |
| Root:shoot ratio | 0.28 | 0.34 | 0.29 | 0.30 | 0.29 | 0.15 | 0.17 | 0.13 | 0.19 | 0.15 | S: *** T: * | 0.01 |
| Chlorophyll | 22.99 cd | 22.71 cd | 23.84 bcd | 18.98 d | 23.24 cd | 27.58 abc | 25.26 abcd | 30.43 abc | 32.15 a | 31.43 ab | S: 0.058 S × T: * | 0.88 |
| Flavonoids | 0.113 | 0.128 | 0.048 | 0.002 | 0.092 | 0.200 | 0.269 | 0.168 | 0.154 | 0.124 | ns | 0.023 |
| NBI | 217.0 | 463.2 | 286.5 | <LOD | 232.7 | 227.4 | 163.1 | 295.4 | 269.4 | 250.7 | ns | 36.0 |
| System | HP | AE | ANOVA | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | T1 | T2 | T3 | T4 | C | T1 | T2 | T3 | T4 | ||
| Shoot DM, % | 6.6 | 6.5 | 6.1 | 6.7 | 6.1 | 8.4 | 8.1 | 8.6 | 7.8 | 6.1 | S: ** | 0.2 |
| Shoot FW, g | 168.0 | 180.6 | 195.6 | 195.5 | 181.5 | 137.0 | 96.8 | 107.6 | 130.6 | 160.8 | S: *** | 7.6 |
| Root DM, % | 6.0 | 6.1 | 6.4 | 5.9 | 5.3 | 6.1 | 7.0 | 6.9 | 6.6 | 6.0 | S: * | 0.2 |
| Root:shoot ratio | 0.26 | 0.30 | 0.31 | 0.28 | 0.24 | 0.14 | 0.14 | 0.13 | 0.17 | 0.14 | S: *** | 0.01 |
| Chlorophyll | 24.38 | 25.21 | 23.26 | 23.15 | 23.82 | 30.96 | 25.88 | 28.90 | 29.17 | 26.48 | S: ** | 0.073 |
| Flavonoids | 0.159 | 0.268 | 0.107 | 0.228 | 0.160 | 0.291 | 0.558 | 0.255 | 0.101 | 0.308 | S: * | 0.030 |
| NBI | 282.56 | 150.73 | 116.71 | 151.65 | 147.98 | 120.06 | 50.50 | 176.71 | 322.20 | 103.09 | ns | 20.90 |
| System | HP | AE | ANOVA | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | T1 | T2 | T3 | T4 | C | T1 | T2 | T3 | T4 | ||
| C, mg/g | 384 | 387 | 386 | 385 | 389 | 396 | 397 | 391 | 395 | 394 | S: *** | 1.0 |
| N, mg/g | 32 | 28 | 30 | 28 | 28 | 28 | 27 | 32 | 32 | 29 | ns | 0.5 |
| P, mg/g | 9.77 a | 6.95 bcd | 8.75 ab | 7.72 bc | 7.69 bc | 5.39 d | 5.52 d | 6.20 cd | 6.10 cd | 5.54 cd | S: ***; S × T: * | 0.29 |
| C/N | 11.85 | 13.86 | 13.2 | 13.96 | 13.98 | 14.35 | 14.84 | 12.4 | 12.6 | 13.75 | ns | 0.28 |
| C/P | 39.41 e | 55.85 bcd | 44.85 de | 50.63 cde | 51.02 cde | 73.53 a | 71.94 a | 63.50 abc | 65.00 abc | 71.10 ab | S: *** S × T: * | 2.30 |
| K, mg/g | 32.80 a | 28.84 ab | 28.22 ab | 24.89 b | 27.30 ab | 27.83 ab | 28.45 ab | 30.48 ab | 31.84 ab | 28.27 ab | S: *; S × T: ** | 0.56 |
| Ca, mg/g | 14.06 a | 11.56 ab | 12.29 ab | 10.69 b | 10.75 b | 7.06 c | 7.54 c | 7.49 c | 7.51c | 6.69 c | S: ***; S × T: * | 0.49 |
| Mg, mg/g | 5.42 | 4.15 | 5.15 | 5.14 | 4.09 | 2.86 | 2.79 | 3.38 | 3.14 | 2.76 | S: ***; T: ** | 0.20 |
| S, mg/g | 2.91 a | 2.18 b | 2.52 ab | 2.34 b | 2.23 b | 2.18 b | 2.02 b | 2.52 ab | 2.45 ab | 2.12 b | S: ***; T: *; S × T: ** | 0.05 |
| Na, mg/g | 51.92 | 39.97 | 47.33 | 46.26 | 41.92 | 32.85 | 33.99 | 36.59 | 34.20 | 31.19 | S: *** | 1.36 |
| Cl, mg/g | 32.79 a | 27.87 abc | 29.86 ab | 27.80 abc | 26.48 abcd | 19.15 e | 24.18 bcde | 21.85 cde | 21.21 cde | 19.77 de | S: ***; S × T: * | 0.89 |
| Al, µg/g | 407.6 | 428.5 | 477.7 | 412.6 | 378.6 | 422.8 | 460.8 | 619.4 | 365.0 | 400.8 | ns | 19.83 |
| Mn, µg/g | 174.1 a | 149.7 ab | 115.3 b | 66.7 c | 180.6 a | 19.4 d | 36.0 cd | 36.0 cd | 38.7 cd | 26.6 cd | S: ***; S × T: *** | 11.74 |
| Fe, µg/g | 80.8 | 101.4 | 103.4 | 99.1 | 73.6 | 113.6 | 88.6 | 161.8 | 117.9 | 119.5 | ns | 7.56 |
| Cu, µg/g | 11.2 a | 8.8 abc | 11.1 a | 8.6 abc | 8.9 abc | 7.1 bc | 6.2 c | 7.9 bc | 9.3 ab | 8.3 abc | S: ***; S × T: ** | 0.33 |
| Zn, µg/g | 50.0 | 41.6 | 45.7 | 52.1 | 38.7 | 36.1 | 32.3 | 41.9 | 45.7 | 33.0 | S: ***; T: *** | 1.40 |
| System | HP | AE | ANOVA | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | T1 | T2 | T3 | T4 | C | T1 | T2 | T3 | T4 | ||
| C, mg/g | 385 | 385 | 387 | 387 | 384 | 396 | 390 | 390 | 393 | 390 | S: *** | 1.0 |
| N, mg/g | 29 | 27 | 28 | 26 | 31 | 32 | 30 | 29 | 31 | 33 | S: ** | 0.6 |
| P, mg/g | 7.35 | 7.23 | 8.10 | 7.53 | 8.13 | 5.25 | 6.32 | 5.80 | 6.08 | 5.89 | S: *** | 0.24 |
| C/N | 13.54 | 14.07 | 13.99 | 14.69 | 12.47 | 12.26 | 13.43 | 13.29 | 12.71 | 11.85 | S: 0.057 | 0.26 |
| C/P | 52.63 | 53.63 | 48.97 | 51.52 | 47.33 | 67.88 | 64.15 | 67.68 | 65.89 | 56.97 | S: *** | 1.78 |
| K, mg/g | 27.81 | 25.76 | 25.97 | 24.93 | 29.76 | 27.80 | 31.93 | 29.02 | 30.07 | 33.24 | S: * | 0.75 |
| Ca, mg/g | 9.94 | 11.51 | 12.62 | 10.59 | 10.64 | 7.10 | 7.94 | 7.59 | 8.18 | 9.05 | S: *** | 0.37 |
| Mg, mg/g | 4.26 | 4.21 | 4.86 | 4.73 | 4.33 | 2.72 | 2.98 | 2.96 | 3.23 | 3.28 | S: *** | 0.16 |
| S, mg/g | 2.30 | 2.17 | 2.52 | 2.31 | 2.45 | 2.19 | 2.42 | 2.40 | 2.57 | 2.50 | ns | 0.07 |
| Na, mg/g | 42.59 | 44.44 | 46.96 | 40.45 | 43.90 | 33.85 | 35.46 | 34.32 | 37.88 | 39.51 | S: *** | 1.09 |
| Cl, mg/g | 27.79 | 29.41 | 29.43 | 25.87 | 28.53 | 19.64 | 23.94 | 18.94 | 22.15 | 22.77 | S: *** | 0.81 |
| Al, µg/g | 414.5 | 549.1 | 413.4 | 324.2 | 450.7 | 464.0 | 442.1 | 423.2 | 568.2 | 510.9 | ns | 21.8 |
| Mn, µg/g | 231.9a | 149.0 b | 84.6 c | 71.6 cd | 163.6 b | 17.2 e | 22.6 e | 26.8 e | 39.3 de | 29.6 e | S: ***; S × T: *** | 13.1 |
| Fe, µg/g | 69.2 bc | 82.5 abc | 66.1 bc | 69.3 bc | 62.9c | 96.1 abc | 86.3 abc | 137.9 abc | 152.5 ab | 167.7 a | S × T: * | 8.2 |
| Cu, µg/g | 9.0 | 10.0 | 10.6 | 11.1 | 9.6 | 7.8 | 7.9 | 7.3 | 10.2 | 7.3 | S: ***; T: * | 0.3 |
| Zn, µg/g | 42.6 abc | 59.7 a | 54.5 a | 56.1 a | 45 abc | 35.7 bc | 32.9 c | 44.0 abc | 51.7 ab | 45.1 abc | T: *; S × T: * | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruni, L.; Junge, R.; Gartmann, F.; Parisi, G.; Schmautz, Z. The Effect of Nutrient Source and Beneficial Bacteria on Growth of Pythium-Exposed Lettuce at High Salt Stress. Water 2023, 15, 2109. https://doi.org/10.3390/w15112109
Bruni L, Junge R, Gartmann F, Parisi G, Schmautz Z. The Effect of Nutrient Source and Beneficial Bacteria on Growth of Pythium-Exposed Lettuce at High Salt Stress. Water. 2023; 15(11):2109. https://doi.org/10.3390/w15112109
Chicago/Turabian StyleBruni, Leonardo, Ranka Junge, Florentina Gartmann, Giuliana Parisi, and Zala Schmautz. 2023. "The Effect of Nutrient Source and Beneficial Bacteria on Growth of Pythium-Exposed Lettuce at High Salt Stress" Water 15, no. 11: 2109. https://doi.org/10.3390/w15112109
APA StyleBruni, L., Junge, R., Gartmann, F., Parisi, G., & Schmautz, Z. (2023). The Effect of Nutrient Source and Beneficial Bacteria on Growth of Pythium-Exposed Lettuce at High Salt Stress. Water, 15(11), 2109. https://doi.org/10.3390/w15112109








