The Influence of DOC on the Migration Forms of Elements and Their Sedimentation from River Waters at an Exploited Diamond Deposit (NW Russia)
Abstract
:1. Introduction
2. Materials and Methods
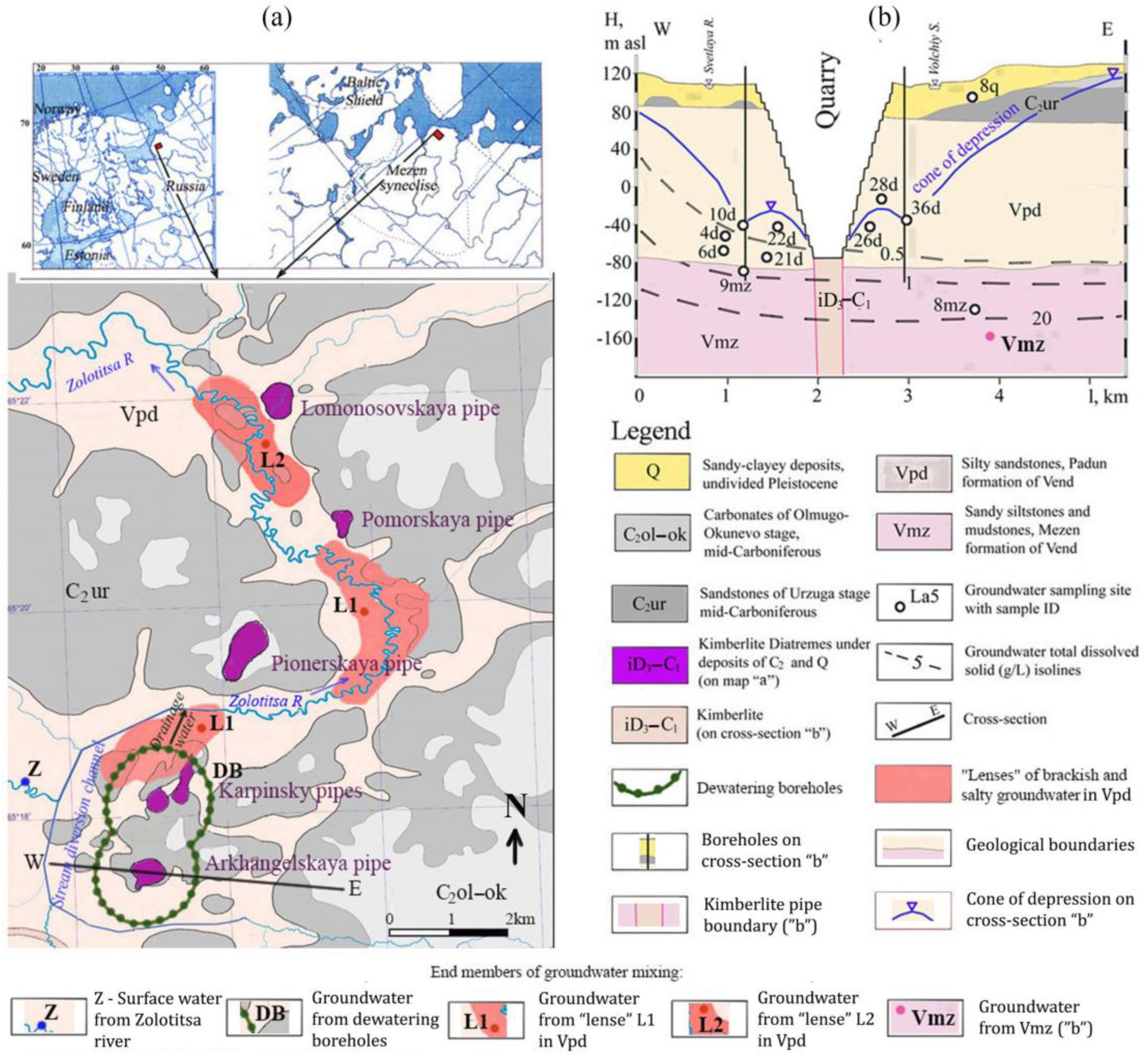
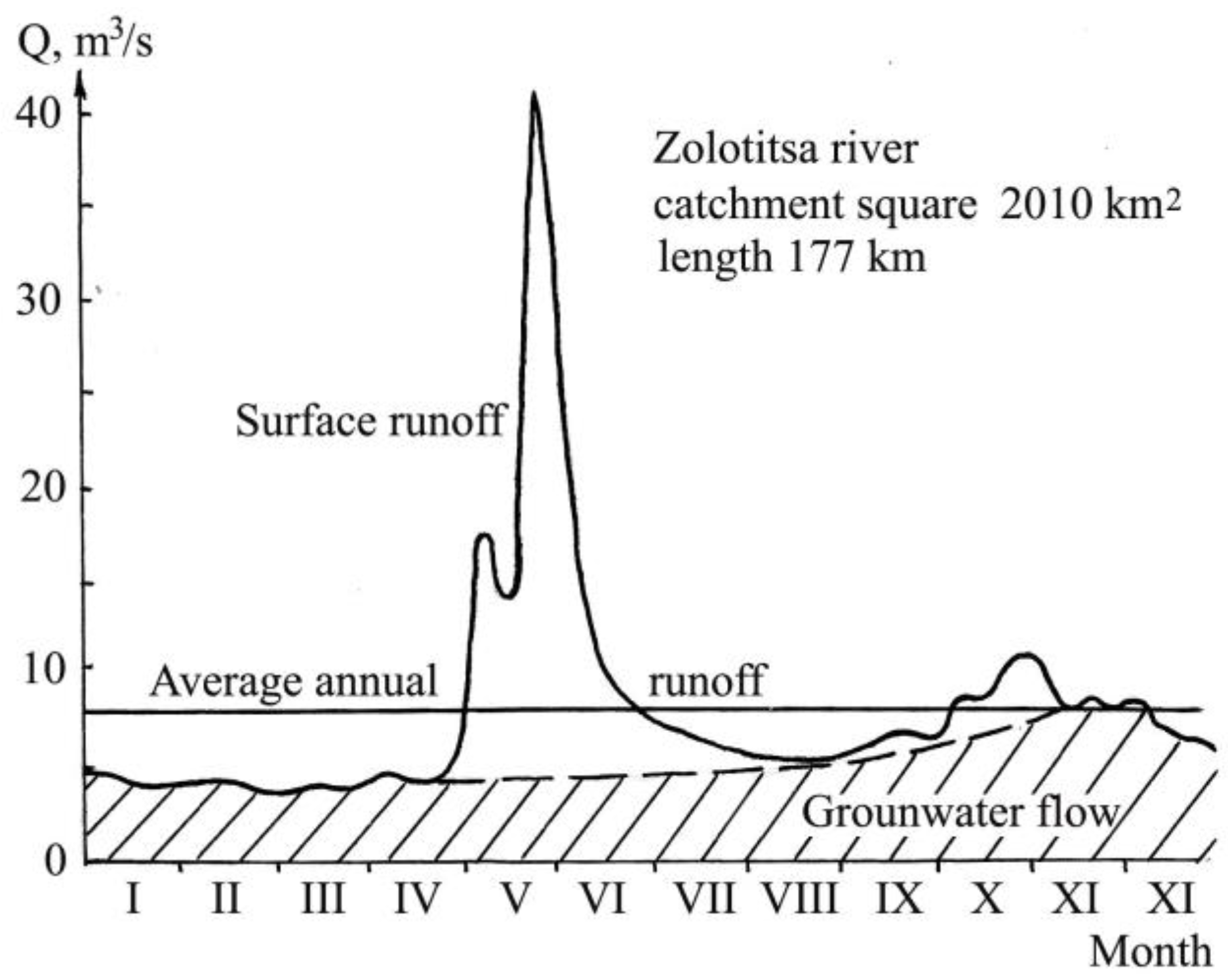
2.1. Natural Conditions of the Study Area
2.2. Methods
3. Results and Discussion
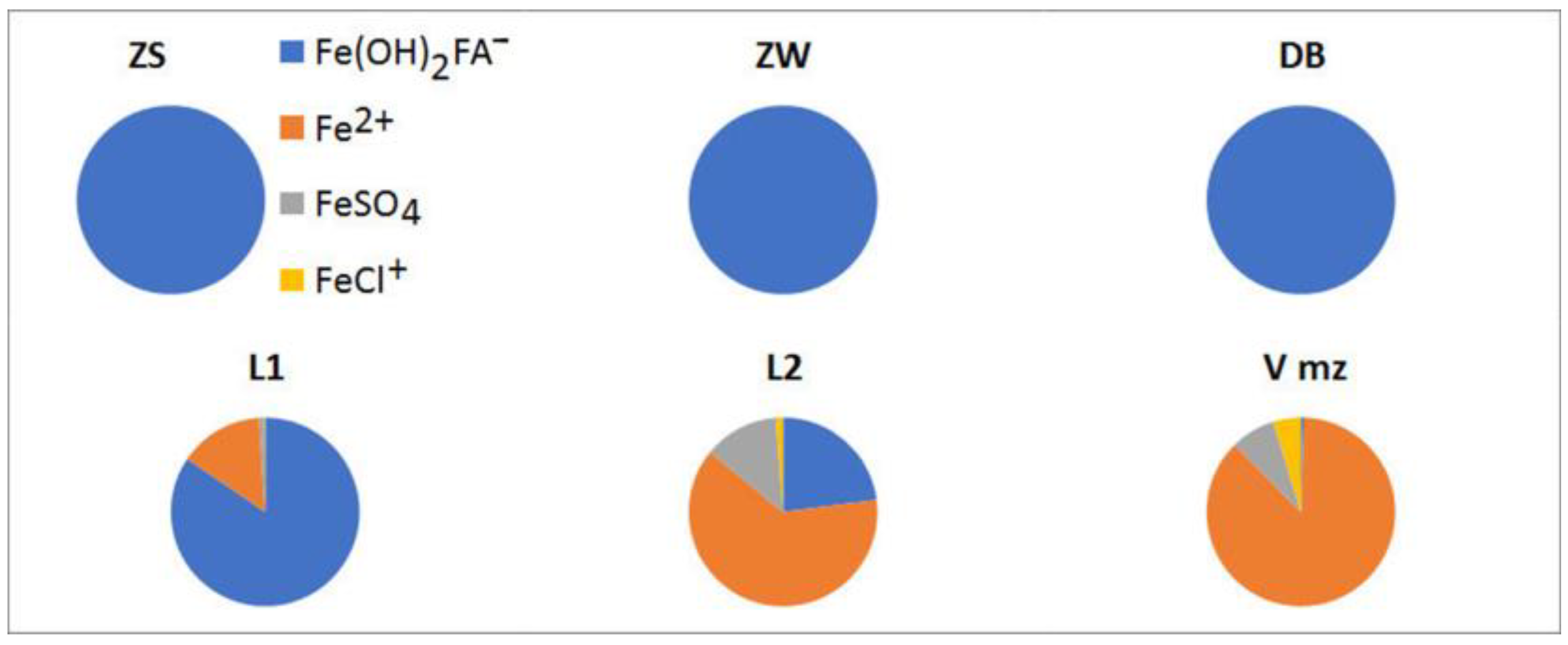
3.1. Organic and Inorganic Aqueous Species of Elements in the Natural Waters of the Study Area
3.1.1. Fulvic Aqueous Species
3.1.2. Humic Aqueous Species
3.1.3. Inorganic Species
3.2. Estimated Aqueous Organic and Inorganic Species in the Zolotitsa River When Draining Groundwater Is Discharged into It from a System of Drainage Wells
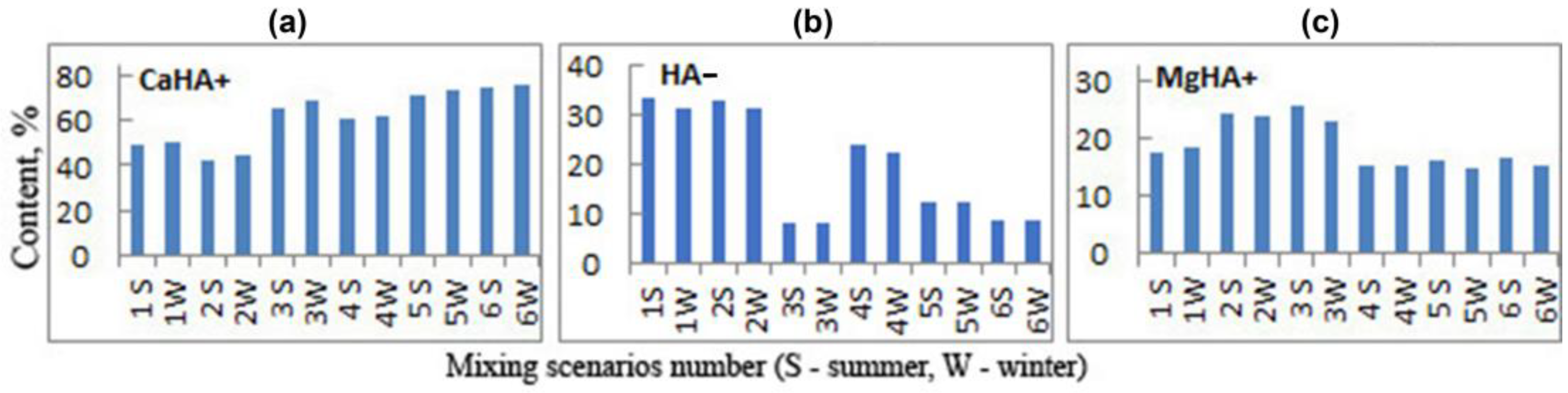
3.2.1. Fulvic and Humic Species
3.2.2. Inorganic Species
3.3. Influence of DOC on the Intensity of Precipitation of Chemical Elements from Mixed Solutions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akimova, T.A.; Kuzmin, A.P.; Khaskin, V.V. Ecology. Nature-Man-Technique; UNITY-DANA: Moscow, Russia, 2001. (In Russian) [Google Scholar]
- Bondarev, L.G. Technogenesis is a new factor in the development of the Earth’s nature. Earth Universe 1996, 1, 30–37. (In Russian) [Google Scholar]
- Gao, P.; Shang, J.; Wu, J.; Mu, Z.; Suo, M.; Fan, J.; Zheng, Y.; Cheng, Y.; Wang, Y. Distribution, Risk Assessment, and Source Identification of Potentially Toxic Elements in the Sediments of the Upper Reaches of Zhanghe River, Haihe Basin. Sustainability 2022, 14, 15885. [Google Scholar] [CrossRef]
- Sidkina, E.S.; Soldatova, E.A.; Cherkasova, E.V.; Konyshev, A.A.; Vorobey, S.S.; Mironenko, M.V. Fate of Heavy Metals in the Surface Water-Dump Rock System of the Mine Lupikko I (Karelia): Field Observations and Geochemical Modeling. Water 2022, 14, 3382. [Google Scholar] [CrossRef]
- Khomenko, M.; Kononenko, I.; Myronova, E. Ecological and technological aspects of iron-ore underground mining. Min. Miner. Depos. 2017, 11, 59–67. [Google Scholar] [CrossRef]
- Samal, P.; Mohanty, A.K.; Khaoash, S.; Mishra, P. Hydrogeochemical Evaluation, Groundwater Quality Appraisal, and Potential Health Risk Assessment in a Coal Mining Region of Eastern India. Water Air Soil Pollut. 2022, 233, 324. [Google Scholar] [CrossRef]
- Krodkiewska, M.; Spyra, A.; Cieplok, A. Assessment of pollution, and ecological status in rivers located in the Vistula and Oder river basins impacted by the mining industry in Central Europe (Poland). Ecol. Indic. 2022, 144, 109505. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Zhang, M.; Liu, B.; Zhang, Y.; Gao, Y.; Ullah, H.; Peng, L.; He, A.; Yu, H. Water quality in a worldwide coal mining city: A scenario in water chemistry and health risks exploration. J. Geochem. Explor. 2020, 213, 106513. [Google Scholar] [CrossRef]
- Gonah, T. Impact of Acid Mine Drainage on Water Resources in South Africa. In Management and Mitigation of Acid Mine Drainage in South Africa: Input for Mineral Beneficiation in Africa; Mujuru, M., Mutanga, S., Eds.; Africa Institute of South Africa: Pretoria, South Africa, 2016; pp. 41–65. [Google Scholar] [CrossRef]
- Jhariya, D.; Khan, R.; Thakur, G.S. Impact of Mining Activity on Water Resources: An Overview Study. In Proceedings of the National Seminar on Recent Practices and Innovations in Mining Industry, Raipur, India, 19–20 February 2016; pp. 271–277. [Google Scholar]
- Environmental Law Alliance Worldwide (ELAW). Guidebook for Evaluating Mining Project EIAs; Environmental Law Alliance Worldwide: Eugene, OR, USA, 2010. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar] [CrossRef]
- Nunoo, S.; Manu, J.; Owusu-Akyaw, F.K.B.; Nyame, F.K. Impact of artisanal small-scale (gold and diamond) mining activities on the Offin, Oda and Pra rivers in Southern Ghana, West Africa: A scientific response to public concern. Heliyon 2022, 8, e12323. [Google Scholar] [CrossRef]
- Galli, N.; Chiarelli, D.D.; D’Angelo, M.; Rulli, M.C. Socio-environmental impacts of diamond mining areas in the Democratic Republic of Congo. Sci. Total Environ. 2022, 810, 152037. [Google Scholar] [CrossRef]
- Wilson, S.A. Measuring the effectiveness of corporate social responsibility initiatives in diamond mining areas of Sierra Leone. Resour. Policy 2022, 77, 102651. [Google Scholar] [CrossRef]
- Engwicht, N.; Ankenbrand, C. Natural resource sector reform and human security in post-conflict societies: Insights from diamond mining in Sierra Leone. Extr. Ind. Soc. 2021, 8, 100988. [Google Scholar] [CrossRef]
- Makhetha, E.; Maliehe, S.A. “A concealed economy”: Artisanal diamond mining in Butha-Buthe district, Lesotho. Extr. Ind. Soc. 2020, 7, 975–981. [Google Scholar] [CrossRef]
- Malov, A.I. Transformation of the chemical composition of surface waters in the area of the exploited Lomonosov diamond deposit (NW Russia). Environ. Sci. Pollut. Res. 2018, 25, 33620–33636. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, E.Y.; Malov, A.I.; Druzhinin, S.V.; Zykov, S.B.; Malkov, A.V.; Bedrina, D.D. Heavy metals distribution and environmental risk assessment in river sediments in the area of the Lomonosov diamond deposit (NW Russia). Environ. Sci. Pollut. Res. 2020, 27, 35392–35415. [Google Scholar] [CrossRef] [PubMed]
- Malov, A.I. The role of exogenic groundwaters in kimberlite formations. Dokl. Earth Sci. 2004, 395, 453–455. [Google Scholar]
- Kalyuzhin, S.M. Atlantic Salmon of the White Sea: Problems of Reproduction and Exploitation; PetroPress: Petrozavodsk, Russia, 2003; p. 264. (In Russian) [Google Scholar]
- Bondareva, L.; Fedorova, N. The effect of humic substances on metal migration at the border of sediment and water flow. Environ. Res. 2020, 190, 109985. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Naushad, M.; Ghfar, A.A.; Ahamad, T. Humic acid coated sand as a novel sorbent in permeable reactive barrier for environmental remediation of groundwater polluted with copper and cadmium ions. J. Water Process Eng. 2020, 36, 101373. [Google Scholar] [CrossRef]
- Savichev, O.; Soldatova, E.; Rudmin, M.; Mazurov, A. Geochemical barriers in oligotrophic peat bog (Western Siberia). Appl. Geochem. 2020, 113, 104519. [Google Scholar] [CrossRef]
- Malov, A.I.; Sidkina, E.S.; Mironenko, M.V.; Tyshov, A.S.; Cherkasova, E.V. Modeling Changes in the Composition of River Water with Discharged Wastewater: A Case Study in NW Russia. Water 2022, 14, 165. [Google Scholar] [CrossRef]
- Shvarov, Y.V. Algorithmization of the Numeric Equilibrium Modeling of Dynamic Geochemical Processes. Geochem. Int. 1999, 37, 571–576. [Google Scholar]
- Krainov, S.R. (Ed.) Methods of Geochemical Modeling and Forecasting in Hydrogeology; Nedra: Moscow, Russia, 1988. (In Russian) [Google Scholar]
- Varshal, G.M.; Inzkirveli, I.S.; Sirotkina, I.S.; Kolosov, I.V.; Kosheeva, I.Y. About association of fulvic acids in water solutions. Geokhimiya 1975, 10, 1581–1585. (In Russian) [Google Scholar]
- Varshal, G.M.; Koshcheeva, I.Y.; Sirotkina, I.S. Study of organic substances of surface waters and their interaction with metal ions. Geokhimiya 1979, 4, 598–607. (In Russian) [Google Scholar]
- Varshal, G.M.; Velyukhanova, T.K.; Koshcheeva, I.Y.; Dorofeeva, V.A.; Buachidze, N.S.; Kasimova, O.G.; Makharadze, G.A. Investigation of chemical forms of elements in surface waters. J. Anal. Chem. 1983, 38, 1590–1600. (In Russian) [Google Scholar]
- Varshal, G.M.; Velyukhanova, T.K.; Kosheeva, I.Y.; Kubrakova, I.V.; Baranova, N.N. Complexation of noble metals with fulvic acids of natural waters and geochemical role of these processes. In Analytical Chemistry of Rare Elements; Ermakov, A.N., Ed.; Nedra: Moscow, Russia, 1988; pp. 112–146. (In Russian) [Google Scholar]
- Lipatnikova, O.A.; Grichuk, D.V. Thermodynamic modeling of migration forms of heavy metals in bottom sediments on the example of Ivan’kovskoye reservoir. Mosc. Univ. Geol. Bull. 2011, 2, 5–59. (In Russian) [Google Scholar]
- Mantoura, R.F.C.; Dickson, A.; Riley, S.P. The complexation of metals with humic materials in natural waters. Estuar. Coast. Mar. Sci. 1978, 6, 387–408. [Google Scholar] [CrossRef]
- Krasinzeva, V.V.; Grichuk, D.V.; Romanova, G.I.; Kadukin, A.I. Migration and occurrence forms of chemical elements in pore waters of bottom sediments in Ivankovskoe reservoir. Geokhimiya 1982, 9, 1342–1354. (In Russian) [Google Scholar]
- Yamamoto, Y.; Kita, F.; Isono, N.; Imai, S. Impact of competitive Fe(III) ion on the complexation of humic acid and toxic metal ions. Jpn. Soc. Anal. Chem. 2017, 66, 875–883. [Google Scholar]
- Guy, R.D.; Charabarti, C.L. Studies of metal-organic interactions in model systems pertaining to naturals water. Can. J. Chem. 1976, 54, 2600–2611. [Google Scholar] [CrossRef]
- Stevenson, F.J. Stability constants of Cu2+, Pb2+ and Cd2+ complexes with humic acids. Soil Sci. Soc. Am. J. 1976, 40, 665–672. [Google Scholar] [CrossRef]
- Samadfam, M.; Niitsu, Y.; Sato, S.; Ohashi, H. Complexation thermodynamics of Sr (II) and humic acid. Radiochim. Acta 1996, 73, 211–216. [Google Scholar] [CrossRef]
- Catrouillet, C.; Davranche, M.; Dia, A.; Bouhnik-Le Coz, M.; Marsac, R.; Pourret, O.; Gruau, G. Geochemical modeling of Fe(II) binding to humic and fulvic acids. Chem. Geol. 2014, 372, 109–118. [Google Scholar] [CrossRef]
- Lepokurova, O.E.; Trifonov, N.S.; Domrocheva, E.V. Migration forms of basic ions in groundwater of coal-bearing sediments of Kuzbass with a focus on compounds with humic acids (by simulation results). Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2022, 332, 76–89. [Google Scholar]
- Konyshev, A.A.; Sidkina, E.S.; Cherkasova, E.V.; Mironenko, M.V.; Gridasov, A.G.; Zhilkina, A.V.; Bugaev, I.A. Migration forms of heavy metals and chemical composition of surface waters in the “Arsenic” shaft area (Pitkäranta Ore District, South Karelia). Geochem. Int. 2020, 58, 1068–1074. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Dinu, M.I.; Gashkina, N.A.; Kremleva, T.A. Occurrence forms of metals in natural waters depending on water chemistry. Water Resour. 2013, 40, 407–416. [Google Scholar] [CrossRef]
- Krainov, S.R.; Ryzhenko, B.N.; Shvets, V.M. Geochemistry of Groundwater. Fundamental, Applied and Environmental Aspects; CentrLitNefteGaz: Moskow, Russia, 2012. (In Russian) [Google Scholar]
- Malov, A.I.; Sidkina, E.S.; Ryzhenko, B.N. Model of the Lomonosov diamond deposit as a water–rock system: Migration Species, Groundwater Saturation with Rock-Forming and Ore Minerals, and Ecological Assessment of Water Quality. Geochem. Int. 2017, 55, 1118–1130. [Google Scholar] [CrossRef]




| H2O | CdCl3− | Fe2+ | MnO0 | PbCO30 | UO22+ | Cu(OH)2FA2− |
|---|---|---|---|---|---|---|
| H+ | CdCl42− | FeOH+ | HMnO2− | PbHCO3+ | UO2OH+ | ZnFA0 |
| OH− | Cd(HSO4)20 | FeO0 | MnO22− | SO42− | U2O4(OH)22− | PbFA0 |
| H2 (aq) | CdHCO3+ | HFeO2− | MnCl+ | HSO4− | UO2(OH)20 | HA− |
| O2 (aq) | Cl− | FeCl+ | MnCl20 | Sr2+ | UO2SO40 | HHA0 |
| Al3+ | HCl0 | FeCl20 | MnSO40 | SrOH+ | UO2(SO4)22− | CaHA+ |
| AlOH2+ | Cr2+ | FeSO40 | MnHCO3+ | SrCl+ | UO2CO30 | MgHA+ |
| AlO+ | Cr3+ | FeCO30 | Mn3+ | SrCl20 | UO2(CO3)22− | FeHA30 |
| AlOOH0 | CrO+ | Fe3+ | MnO4− | SrSO40 | UO30 | CuHA20 |
| AlO2− | CrOH2+ | FeOH2+ | MnO42− | SrCO30 | UO42− | CuHA+ |
| HAsO20 | HCrO20 | FeO+ | MoO42− | SrHCO3+ | HUO4− | ZnHA+ |
| AsO2− | CrO2− | HFeO20 | HMoO4− | U3+ | Zn2+ | PbHA20 |
| HAsO32− | CrO42− | FeO2− | Na+ | UOH2+ | ZnOH+ | PbHA+ |
| AsO43− | HCrO4− | FeCl2+ | NaOH0 | UO+ | ZnO0 | H2FA0 |
| HAsO42− | Cr2O72− | FeCl2+ | NaCl0 | HUO20 | HZnO2− | MnFA0 |
| H2AsO4− | Cu+ | FeCl30 | NaSO4− | U4+ | ZnO22− | CdFA0 |
| H3AsO40 | CuOH0 | FeSO4+ | NaCO3− | UOH3+ | ZnCl+ | MnHA+ |
| CO32− | CuCl0 | FeHSO42+ | NaHCO30 | UO2+ | ZnCl20 | CdHA20 |
| HCO3− | CuCl2− | K+ | Ni2+ | HUO2+ | ZnCl3− | MoO2HA+ |
| CO2 (aq) | CuCl32− | KOH0 | NiOH+ | UO20 | ZnCl42− | MoO2HA20 |
| Ca2+ | CuHCO30 | KCl0 | NiO0 | HUO3− | ZnSO40 | UO2FA0 |
| CaOH+ | Cu2+ | KSO4− | HNiO2− | UCl3+ | ZnHSO4+ | UO2FA22− |
| CaCl+ | CuOH+ | KHSO40 | NiO22− | UCl22+ | ZnCO30 | UO2HA+ |
| CaCl20 | CuO0 | KCO3− | NiCl+ | USO42+ | ZnHCO3+ | UO2HA20 |
| CaSO40 | HCuO2− | KHCO30 | Pb2+ | UHSO43+ | FA2− | SrFA0 |
| CaCO30 | CuO22− | Mg2+ | PbOH+ | UCO32+ | HFA− | SrHu+ |
| CaHCO3+ | CuCl+ | MgOH+ | PbO0 | UHCO33+ | CaFA0 | Sr(HA)20 |
| Cd2+ | CuCl20 | MgCl+ | HPbO2− | UO2+ | MgFA0 | CrFA+ |
| CdOH+ | CuCl3− | MgCl20 | PbCl+ | UO2OH0 | FeFA0 | CrHu+2 |
| CdO0 | CuCl42− | MgSO40 | PbCl20 | UO3− | FeFA+ | Cr(OH)FA0 |
| HCdO2− | CuSO40 | MgCO30 | PbCl3− | UO2Cl0 | FeOHFA0 | NiFA0 |
| CdO22− | CuHSO4+ | MgHCO3+ | PbCl42− | UO2Cl2− | Fe(OH)2FA− | NiHA+ |
| CdCl+ | CuCO30 | Mn2+ | PbSO40 | UO2HCO30 | AlFA+ | |
| CdCl20 | CuHCO3+ | MnOH+ | PbHSO4+ | UO2(HCO3)2− | CuFA0 |
| ZS 1 | ZW 1 | DBs | L1 | L2 | Vmz | |
|---|---|---|---|---|---|---|
| T °C | 14 | 0.6 | 4.5 | 4.9 | 5.7 | 6.9 |
| pH | 7.5 | 7.3 | 8.6 | 7.9 | 7.7 | 8.3 |
| Eh, mV | 281 | 120 | 65 | −22 | −38 | −80 |
| mg/kg H2O | ||||||
| Fulvic acids (FA) | 28.2 | 16.1 | 3.03 | 7.55 | 2.92 | 2.56 |
| Humic acids (HA) | 1.57 | 0.9 | 0.17 | 0.42 | 0.16 | 0.14 |
| O2 | 10.3 | 6.1 | 1.7 | 0 | 1.2 | 0 |
| Na | 13.3 | 13.3 | 101.3 | 792 | 1960 | 5374 |
| Mg | 3.12 | 3.12 | 9.86 | 48.4 | 298 | 484 |
| K | 0.8 | 0.8 | 4.24 | 6.88 | 33.6 | 52.8 |
| Ca | 6.06 | 6.06 | 17.6 | 49.6 | 495 | 1804 |
| Cl | 8.22 | 8.22 | 73 | 1009 | 3034 | 11,502 |
| HCO3− | 48.8 | 48.8 | 211 | 325 | 255 | 19.8 |
| SO42− | 4.2 | 4.2 | 33.6 | 292 | 2323 | 2326 |
| TDS | 87.1 | 87.1 | 455 | 2528 | 8418 | 21,664 |
| µg/kg H2O | ||||||
| Al | 57.2 | 57.2 | 7 | 25.2 | 34 | 187 |
| Cr | 0.37 | 0.37 | 0.63 | 0.99 | 1.54 | 18.5 |
| Mn | 13.4 | 13.4 | 12.5 | 78.6 | 814 | 3153 |
| Fe | 339 | 339 | 35.1 | 1341 | 1872 | 6564 |
| Ni | 0.3 | 0.3 | 0.11 | 0.82 | 1.86 | 2.31 |
| Cu | 0.25 | 0.25 | 0.16 | 1.2 | 1.84 | 0.29 |
| Zn | 5.13 | 5.13 | 2.17 | 14.8 | 23.8 | 49.2 |
| As | 0.5 | 0.5 | 0.6 | 0.39 | 0.36 | 1.22 |
| Sr | 64.3 | 64.3 | 168 | 671 | 12,301 | 38,594 |
| Mo | 0.37 | 0.37 | 2.7 | 18.1 | 3.46 | 9.3 |
| Cd | 0.0028 | 0.0028 | 0.0044 | 0.04 | 0.04 | 0.035 |
| Pb | 0.106 | 0.106 | 0.045 | 1.47 | 2.02 | 0.06 |
| U | 0.59 | 0.59 | 6.78 | 1.57 | 15.2 | 0.15 |
| Aqueous Species | ZS | ZW | DBs | L1 | L2 | Vmz | Aqueous Species | ZS | ZW | DBs | L1 | L2 | Vmz |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | CuCl2− | 0 | 0 | 0 | 80.86 | 57.38 | 24.38 | ||||||
| Fe(OH)2 FA− | 100 | 100 | 100 | 100 | 100 | 100 | CuCl32− | 0 | 0 | 0 | 17.60 | 42.26 | 75.58 |
| HA | Cu2+ | 4.77 | 10.17 | 0.77 | 0 | 0 | 0 | ||||||
| HA− | 40.67 | 40.67 | 34.49 | 12.91 | 2.27 | 0.86 | CuOH+ | 1.99 | 2.82 | 0.55 | 0 | 0 | 0 |
| HHA0 | 0.01 | 0.01 | 0 | 0.01 | 0 | 0 | CuO0 | 12.86 | 11.97 | 7.34 | 0 | 0 | 0 |
| MgHA+ | 15.02 | 15.02 | 14.72 | 32.27 | 25.33 | 14.49 | CuCl+ | 0 | 0.01 | 0 | 0 | 0 | 0 |
| CaHA+ | 43.94 | 43.94 | 50.74 | 54.64 | 72.29 | 84.37 | CuSO40 | 0 | 0.06 | 0.02 | 0 | 0 | 0 |
| MnHA+ | 0 | 0 | 0 | 0.12 | 0.08 | 0.22 | CuCO30 | 80.19 | 74.63 | 91.31 | 0 | 0 | 0 |
| CuHA+ | 0.01 | 0.01 | 0 | 0 | 0 | 0.02 | CuHCO3+ | 0.02 | 0.03 | 0.01 | 0 | 0 | 0 |
| ZnHA+ | 0.33 | 0.33 | 0.05 | 0.04 | 0.02 | 0 | Cu(OH)2 FA2− | 0 | 0 | 0 | 0 | 0 | 0 |
| SrHA+ | 0.01 | 0.01 | 0 | 0.01 | 0.01 | 0.04 | CuHA+ | 0.14 | 0.31 | 0 | 0 | 0 | 0 |
| PbHA+ | 0 | 0 | 0 | 0 | 0 | 0 | Zn | ||||||
| Mg | Zn2+ | 70.70 | 80.12 | 44.46 | 76.64 | 73.22 | 83.25 | ||||||
| Mg2+ | 98.49 | 98.82 | 93.92 | 86.50 | 71.95 | 78.88 | ZnOH+ | 19.02 | 14.32 | 16.01 | 2.70 | 0.82 | 0.65 |
| MgOH+ | 0.01 | 0 | 0.01 | 0 | 0 | 0 | ZnO0 | 0.07 | 0.03 | 0.11 | 0 | 0 | 0 |
| MgCl+ | 0.01 | 0.01 | 0.11 | 1.00 | 1.8 | 5.87 | HZnO2− | 0 | 0 | 0 | 0 | 0 | 3.62 |
| MgCl20 | 0 | 0 | 0 | 0.05 | 0.23 | 2.49 | ZnCl+ | 0.01 | 0.01 | 0.03 | 0.52 | 1.07 | 0.85 |
| MgSO40 | 0.55 | 0.59 | 2.89 | 9.32 | 25.05 | 12.7 | ZnCl20 | 0 | 0 | 0 | 0.02 | 0.08 | 0.21 |
| MgCO30 | 0.18 | 0.08 | 1.04 | 0.17 | 0.02 | 0 | ZnCl3− | 0 | 0 | 0 | 0.01 | 0.05 | |
| Mg HCO3+ | 0.76 | 0.5 | 2.04 | 2.96 | 0.95 | 0.06 | ZnHS+ | 0 | 0 | 0 | 0.02 | 0.01 | 0.01 |
| MgHA+ | 0 | 0 | 0 | 0 | 0 | 0 | ZnSO40 | 0.41 | 0.49 | 1.38 | 7.97 | 23.06 | 11.27 |
| Ca | ZnCO30 | 9.33 | 4.61 | 37.52 | 10.86 | 1.29 | 0.06 | ||||||
| Ca2+ | 98.49 | 98.93 | 94.05 | 89.96 | 81.24 | 88.71 | ZnHCO3+ | 0.30 | 0.23 | 0.48 | 1.26 | 0.44 | 0.03 |
| CaCl+ | 0.01 | 0.01 | 0.06 | 0.57 | 1.05 | 3.19 | ZnHA+ | 0.16 | 0.19 | 0.01 | 0.01 | 0 | 0 |
| CaCl20 | 0 | 0 | 0 | 0.01 | 0.03 | 0.3 | As | ||||||
| CaSO40 | 0.36 | 0.38 | 1.93 | 6.15 | 16.68 | 7.74 | AsO43− | 0.02 | 0.01 | 0.06 | 0 | 0 | 0 |
| CaCO30 | 0.35 | 0.15 | 1.87 | 0.30 | 0.03 | 0 | HAsO42− | 94.87 | 92.22 | 98.16 | 0 | 0 | 0 |
| CaHCO3+ | 0.78 | 0.52 | 2.09 | 3.01 | 0.97 | 0.06 | H2AsO4− | 5.11 | 7.77 | 1.78 | 0 | 0 | 0 |
| CaHA+ | 0.01 | 0.01 | 0 | 0 | 0 | 0 | HAsO20 | 0 | 0 | 0 | 98.92 | 99.55 | 99.62 |
| Al | AsO2− | 0 | 0 | 0 | 1.08 | 0.45 | 0.38 | ||||||
| Al3+ | 0 | 0 | 0 | 0 | 0.02 | 0.07 | Sr | ||||||
| AlOH2+ | 0 | 0 | 0 | 0.02 | 0.38 | 0.9 | Sr2+ | 98.19 | 98.44 | 92.86 | 83.30 | 66.76 | 81.37 |
| AlO+ | 0.03 | 0.07 | 0.01 | 0.38 | 2.13 | 3.14 | SrCl+ | 0.01 | 0.01 | 0.07 | 0.55 | 0.87 | 2.8 |
| AlOOH0 | 5.49 | 8.22 | 2.29 | 14.60 | 28.71 | 32.13 | SrCl20 | 0 | 0 | 0 | 0 | 0.01 | 0.15 |
| AlO2− | 94.48 | 91.71 | 97.7 | 85.00 | 68.76 | 63.76 | SrSO40 | 0.90 | 0.97 | 4.71 | 13.71 | 31.68 | 15.64 |
| Cr | SrCO30 | 0.11 | 0.05 | 0.59 | 0.09 | 0.01 | 0 | ||||||
| Cr3+ | 0 | 0 | 0 | 0.02 | 0.15 | 0.26 | SrHCO3+ | 0.79 | 0.53 | 1.77 | 2.35 | 0.67 | 0.04 |
| CrO+ | 0 | 0 | 0 | 87.73 | 70.35 | 60.12 | Mo | ||||||
| CrOH2+ | 0 | 0 | 0 | 10.59 | 29.02 | 39.3 | MoO42− | 99.98 | 99.97 | 99.99 | 99.96 | 99.92 | 99.92 |
| HCrO20 | 0 | 0 | 0 | 1.63 | 0.47 | 0.32 | HMoO4− | 0.02 | 0.03 | 0.01 | 0.04 | 0.08 | 0.08 |
| CrO2− | 0 | 0 | 0 | 0.01 | 0 | 0 | Cd | ||||||
| CrO42− | 0 | 95.88 | 99.13 | 0 | 0 | 0 | Cd2+ | 97.36 | 97.49 | 83.85 | 35.39 | 19.95 | 5.7 |
| HCrO4− | 0 | 4.12 | 0.87 | 0 | 0 | 0 | CdOH+ | 0.24 | 0.16 | 0.32 | 0.01 | ||
| CrHA2+ | 0 | 0 | 0 | 0.02 | 0.01 | 0 | CdCl+ | 2.10 | 2.15 | 15.02 | 57.58 | 62.44 | 46.01 |
| Mn | CdCl20 | 0 | 0 | 0.15 | 6.42 | 16.15 | 36.38 | ||||||
| Mn2+ | 96.83 | 98.49 | 83.8 | 92.23 | 87.51 | 91.92 | CdCl3− | 0 | 0 | 0 | 0.19 | 1.33 | 10.39 |
| MnOH+ | 0.06 | 0.04 | 0.08 | 0.01 | CdCl42− | 0 | 0 | 0 | 0 | 0.05 | 1.52 | ||
| MnCl+ | 0.01 | 0.01 | 0.06 | 0.59 | 1.16 | 3.48 | CdHCO3+ | 0.30 | 0.2 | 0.66 | 0.41 | 0.08 | 0 |
| MnSO40 | 0.20 | 0.22 | 0.95 | 3.51 | 10.06 | 4.53 | Pb | ||||||
| Mn HCO3+ | 1.01 | 0.68 | 2.2 | 3.66 | 1.27 | 0.07 | Pb2+ | 1.03 | 1.74 | 0.25 | 1.87 | 9.59 | 16.25 |
| MnO4- | 1.87 | 0.54 | 12.9 | 0 | 0 | 0 | PbOH+ | 64.95 | 73 | 40.07 | 26.53 | 36.86 | 34.8 |
| MnO4-- | 0 | 0.01 | 0 | 0 | 0 | PbO0 | 0.01 | 0.01 | 0.01 | ||||
| MnHA+ | 0.02 | 0.02 | 0 | 0 | 0 | 0 | PbCl+ | 0.01 | 0.01 | 0.01 | 0.64 | 6.27 | 27.01 |
| Fe | PbCl20 | 0 | 0 | 0 | 0.04 | 0.99 | 13.39 | ||||||
| Fe2+ | 0 | 0 | 0 | 14.11 | 62.3 | 86.98 | PbCl3− | 0 | 0 | 0 | 0 | 0.04 | 1.88 |
| FeOH+ | 0 | 0 | 0 | 0.03 | 0.05 | 0.04 | PbCl42− | 0 | 0 | 0 | 0 | 0 | 0.78 |
| FeCl+ | 0 | 0 | 0 | 0.14 | 1.23 | 4.75 | PbHS+ | 0 | 0 | 0 | 0.01 | 0.02 | 0.01 |
| FeSO40 | 0 | 0 | 0 | 0.95 | 12.66 | 7.63 | PbSO40 | 0.01 | 0.02 | 0.02 | 0.36 | 5.18 | 3.44 |
| FeCO30 | 0 | 0 | 0 | 1.92 | 1.05 | 0.06 | PbCO30 | 33.84 | 24.97 | 59.64 | 70.52 | 41.02 | 2.44 |
| Fe(OH)2 FA− | 100 | 100 | 100 | 82.85 | 22.71 | 0.54 | PbHCO3+ | 0 | 0 | 0 | 0.01 | 0.03 | 0 |
| Ni | PbHA20 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Ni2+ | 99.96 | 99.96 | 99.92 | 99.86 | 99.74 | 99.27 | PbHA+ | 0.15 | 0.25 | 0 | 0.02 | 0 | 0 |
| NiOH+ | 0.04 | 0.04 | 0.07 | 0.01 | U | ||||||||
| NiCl+ | 0 | 0 | 0.01 | 0.13 | 0.26 | 0.73 | UO2OH+ | 0.02 | 0.06 | 0 | 99.99 | 99.96 | 100 |
| NiHA+ | 0 | 0 | 0 | 0 | 0 | 0 | UO2CO30 | 1.84 | 5.11 | 11.52 | 0 | 0 | 0 |
| Cu | UO2 (CO3)22− | 55.66 | 57.18 | 0 | 0 | 0 | 0 | ||||||
| Cu+ | 0 | 0 | 0 | 0.19 | 0.02 | 0 | UO2 (CO3)34− | 26.98 | 12.16 | 88.19 | 0 | 0.01 | 0 |
| CuOH0 | 0 | 0 | 0 | 0.04 | 0 | 0 | UO30 | 14.32 | 24.48 | 0.20 | 0.01 | 0.03 | 0 |
| CuCl0 | 0 | 0 | 0 | 1.31 | 0.34 | 0.04 | HUO4− | 1.18 | 1.01 | 0.04 | 0 | 0 | 0 |
| ZS | ZW | DBs | L1 | L2 | Vmz | |
|---|---|---|---|---|---|---|
| Minerals, mol/kg H2O | ||||||
| Ankerite CaFe(CO3)2 | 0 | 0 | 0 | 2.22 × 10−5 | 3.09 × 10−5 | 0 |
| Chalcocite Cu2S | 0 | 0 | 0 | 9.44 × 10−9 | 1.45 × 10−8 | 2.27 × 10−9 |
| Chromite FeCr2O4 | 0 | 0 | 0 | 9.52 × 10−9 | 1.48 × 10−8 | 1.78 × 10−7 |
| Dolomite CaMg(CO3)2 | 0 | 0 | 0.000196 | 1.75 × 10−4 | 0 | 0 |
| Gibbsite Al(OH)3 | 2.11 × 10−6 | 2.11 × 10−6 | 2.42 × 10−7 | 9.31 × 10−7 | 1.26 × 10−6 | 6.93 × 10−6 |
| Goethite FeO(OH) | 4.29 × 10−7 | 2.85 × 10−6 | 2.25 × 10−8 | 0 | 0 | 0 |
| Pyrolusite MnO2 | 2.44 × 10−7 | 2.44 × 10−7 | 2.28 × 10−7 | 0 | 0 | 0 |
| Siderite FeCO3 | 0 | 0 | 0 | 0 | 0 | 2.22 × 10−5 |
| Sphalerite ZnS | 0 | 0 | 0 | 1.75 × 10−7 | 5.86 × 10−8 | 8.26 × 10−8 |
| UO2(cr) | 0 | 0 | 0 | 6.16 × 10−9 | 6.34 × 10−8 | 1.97 × 10−10 |
| 2.22Galena PbS | 0 | 0 | 0 | 5.20 × 10−9 | 0 | 0 |
| (Ca, Sr, Zn, Pb, Mn)CO3, mol/kg H2O (solid solution) | ||||||
| Ca | 0 | 0 | 6.01 × 10−6 | 5.42 × 10−6 | 3.21 × 10−4 | 0 |
| Sr | 0 | 0 | 1.65 × 10−6 | 4.13 × 10−6 | 1.17 × 10−4 | 0 |
| Zn | 0 | 0 | 6.67 × 10−9 | 4.41 × 10−9 | 1.03 × 10−7 | 0 |
| Pb | 0 | 0 | 1.87 × 10−10 | 1.71 × 10−9 | 9.71 × 10−9 | 0 |
| Mn | 0 | 0 | 8.18 × 10−17 | 2.75 × 10−7 | 8.29 × 10−6 | 0 |
| Aqueous Species | Scenarios of Mixing of River Waters with Drainage Groundwater | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1S | 1W | 2S | 2W | 3S | 3W | 4S | 4W | 5S | 5W | 6S | 6W | |
| % of Total Content | ||||||||||||
| FA | ||||||||||||
| Fe(OH)2 FA− | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| HA | ||||||||||||
| HA− | 33.03 | 30.96 | 32.82 | 30.93 | 8.27 | 8.20 | 23.58 | 22.19 | 12.42 | 12.07 | 8.6 | 8.47 |
| CaHA+ | 49.14 | 50.57 | 42.43 | 44.93 | 65.67 | 68.55 | 60.92 | 62.38 | 71.5 | 73.02 | 74.75 | 76.2 |
| MgHA+ | 17.74 | 18.38 | 24.61 | 24.00 | 26.01 | 23.21 | 15.46 | 15.38 | 16.06 | 14.89 | 16.64 | 15.32 |
| SrHA+ | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnHA+ | 0.08 | 0.08 | 0.13 | 0.13 | 0.04 | 0.04 | 0.04 | 0.05 | 0.02 | 0.02 | 0.01 | 0.01 |
| Al | ||||||||||||
| AlO+ | 0 | 0.01 | 0.01 | 0.01 | 0.03 | 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| AlOOH | 2.22 | 2.54 | 2.26 | 2.56 | 4.7 | 5.06 | 2.64 | 2.97 | 3.64 | 3.98 | 4.35 | 4.71 |
| AlO2− | 97.78 | 97.45 | 97.73 | 97.43 | 95.27 | 94.90 | 97.35 | 97.02 | 96.35 | 96 | 95.63 | 95.26 |
| As | ||||||||||||
| AsO43− | 0.06 | 0.06 | 0.07 | 0.06 | 0.04 | 0.04 | 0.06 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 |
| HAsO42− | 97.99 | 97.90 | 98.07 | 98.01 | 96.47 | 96.52 | 97.7 | 97.65 | 97.03 | 97.04 | 96.63 | 96.65 |
| H2AsO4− | 1.95 | 2.04 | 1.86 | 1.93 | 3.49 | 3.44 | 2.24 | 2.3 | 2.92 | 2.92 | 3.33 | 3.31 |
| Ca | ||||||||||||
| Ca2+ | 94.88 | 95.02 | 93.67 | 93.75 | 88.4 | 88.42 | 95.17 | 95.19 | 95.01 | 94.96 | 94.6 | 94.57 |
| CaCl+ | 0.05 | 0.05 | 0.17 | 0.16 | 0.33 | 0.32 | 0.11 | 0.11 | 0.26 | 0.25 | 0.38 | 0.37 |
| CaSO40 | 1.49 | 1.46 | 3.18 | 3.12 | 10.5 | 10.40 | 2.14 | 2.1 | 3.34 | 3.3 | 4.03 | 3.98 |
| CaCO30 | 1.86 | 1.57 | 1.49 | 1.29 | 0.23 | 0.22 | 1.21 | 1.06 | 0.52 | 0.48 | 0.32 | 0.3 |
| CaHCO3+ | 1.72 | 1.90 | 1.49 | 1.67 | 0.54 | 0.64 | 1.37 | 1.54 | 0.87 | 1.01 | 0.67 | 0.78 |
| Cd | ||||||||||||
| Cd2+ | 88.3 | 87.70 | 69.08 | 67.42 | 51.92 | 49.73 | 77.63 | 76.34 | 59.45 | 57.41 | 49.66 | 47.52 |
| CdOH+ | 0.49 | 0.32 | 0.33 | 0.22 | 0.09 | 0.06 | 0.34 | 0.22 | 0.16 | 0.11 | 0.1 | 0.07 |
| CdCl+ | 10.53 | 11.28 | 29.45 | 31.09 | 45.3 | 47.13 | 21.27 | 22.62 | 38.67 | 40.52 | 47.28 | 49.02 |
| CdCl20 | 0.07 | 0.08 | 0.72 | 0.84 | 2.54 | 2.91 | 0.33 | 0.38 | 1.5 | 1.73 | 2.79 | 3.2 |
| CdCl3− | 0 | 0 | 0 | 0.01 | 0.03 | 0.05 | 0 | 0 | 0.01 | 0.02 | 0.04 | 0.05 |
| CdHCO3+ | 0.62 | 0.62 | 0.42 | 0.42 | 0.12 | 0.12 | 0.43 | 0.44 | 0.21 | 0.21 | 0.13 | 0.14 |
| Cr | ||||||||||||
| CrO42− | 99 | 99 | 9905 | 99.05 | 98.21 | 98.31 | 98.85 | 98.87 | 98.5 | 98.56 | 98.29 | 98.37 |
| HCrO4− | 1 | 1 | 0.95 | 0.95 | 1.79 | 1.69 | 1.15 | 1.13 | 1.5 | 1.44 | 1.71 | 1.63 |
| Cu | ||||||||||||
| Cu2+ | 0.92 | 0.94 | 1.12 | 1.13 | 6.42 | 5.87 | 1.4 | 1.39 | 3.21 | 3.01 | 5.06 | 4.68 |
| CuOH+ | 0.87 | 0.64 | 0.94 | 0.68 | 2.06 | 1.43 | 1.06 | 0.76 | 1.54 | 1.08 | 1.88 | 1.31 |
| CuO0 | 13.57 | 7.76 | 13.52 | 7.74 | 12.47 | 7.22 | 13.5 | 7.74 | 13.12 | 7.56 | 12.78 | 7.38 |
| CuCl+ | 0 | 0 | 0.01 | 0.01 | 0.13 | 0.13 | 0.01 | 0.01 | 0.05 | 0.05 | 0.11 | 0.11 |
| CuSO40 | 0.02 | 0.02 | 0.06 | 0.05 | 1.16 | 1 | 0.05 | 0.04 | 0.17 | 0.15 | 0.33 | 0.29 |
| CuCO30 | 84.6 | 90.62 | 84.33 | 90.37 | 77.73 | 84.32 | 83.96 | 90.04 | 81.88 | 88.12 | 79.81 | 86.2 |
| CuHCO3+ | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| CuHA+ | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| CuHA20 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fe | ||||||||||||
| Fe(OH)2 FA− | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Mg | ||||||||||||
| Mg2+ | 94.93 | 95 | 92.54 | 92.69 | 82.93 | 83.44 | 94.53 | 94.58 | 93.23 | 93.38 | 92.23 | 92.41 |
| MgOH+ | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0 |
| MgCl+ | 0.08 | 0.08 | 0.28 | 0.28 | 0.53 | 0.55 | 0.18 | 0.18 | 0.43 | 0.44 | 0.63 | 0.65 |
| MgCl20 | 0 | 0 | 0 | 0 | 0.02 | 0.02 | 0 | 0 | 0.01 | 0.01 | 0.02 | 0.02 |
| MgSO40 | 2.32 | 2.19 | 4.94 | 4.68 | 15.89 | 15.26 | 3.32 | 3.15 | 5.2 | 4.93 | 6.29 | 5.99 |
| MgCO30 | 0.98 | 0.87 | 0.78 | 0.71 | 0.12 | 0.12 | 0.63 | 0.58 | 0.27 | 0.26 | 0.17 | 0.17 |
| MgHCO3+ | 1.68 | 1.85 | 1.45 | 1.62 | 0.51 | 0.61 | 1.33 | 1.5 | 0.85 | 0.98 | 0.65 | 0.76 |
| Mn | ||||||||||||
| Mn2+ | 76.48 | 86.59 | 79.28 | 87.52 | 91.11 | 91.98 | 84.54 | 90.65 | 92.47 | 94.49 | 94.14 | 95.25 |
| MnOH+ | 0.11 | 0.08 | 0.1 | 0.07 | 0.04 | 0.03 | 0.09 | 0.07 | 0.07 | 0.04 | 0.05 | 0.03 |
| MnCl+ | 0.04 | 0.04 | 0.16 | 0.16 | 0.38 | 0.34 | 0.11 | 0.1 | 0.28 | 0.26 | 0.42 | 0.38 |
| MnSO40 | 0.69 | 0.74 | 1.55 | 1.63 | 6.24 | 6.04 | 1.09 | 1.12 | 1.88 | 1.83 | 2.32 | 2.23 |
| MnHCO3+ | 1.82 | 2.06 | 1.65 | 1.85 | 0.73 | 0.79 | 1.6 | 1.76 | 1.11 | 1.2 | 0.87 | 0.94 |
| MnO4− | 20.83 | 10.48 | 17.23 | 8.76 | 1.5 | 0.82 | 12.56 | 6.3 | 4.19 | 2.18 | 2.19 | 1.17 |
| MnO42− | 0.02 | 0.1 | 0.02 | 0.01 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 |
| MnHA+ | 0.01 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mo | ||||||||||||
| MoO42− | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 | 99.99 |
| HMoO4− | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Ni | ||||||||||||
| Ni2+ | 99.89 | 99.92 | 99.87 | 99.9 | 99.89 | 99.91 | 99.9 | 99.93 | 99.89 | 99.91 | 99.88 | 99.89 |
| NiOH+ | 0.1 | 0.07 | 0.09 | 0.06 | 0.03 | 0.02 | 0.08 | 0.05 | 0.05 | 0.04 | 0.04 | 0.03 |
| NiCl+ | 0.01 | 0.01 | 0.04 | 0.04 | 0.08 | 0.07 | 0.02 | 0.02 | 0.06 | 0.05 | 0.08 | 0.08 |
| Pb | ||||||||||||
| Pb2+ | 0.31 | 0.30 | 0.37 | 0.35 | 1.41 | 1.25 | 0.43 | 0.4 | 0.82 | 0.74 | 1.17 | 1.05 |
| PbOH+ | 44.18 | 42.78 | 46.05 | 44.36 | 65.71 | 63.21 | 49.15 | 47.2 | 58.72 | 56.2 | 63.5 | 60.99 |
| PbO0 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 |
| PbCl+ | 0.01 | 0.01 | 0.04 | 0.03 | 0.28 | 0.25 | 0.03 | 0.03 | 0.12 | 0.11 | 0.26 | 0.23 |
| PbCl20 | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0.01 | 0 |
| PbSO40 | 0.01 | 0.01 | 0.04 | 0.03 | 0.49 | 0.42 | 0.03 | 0.03 | 0.09 | 0.07 | 0.15 | 0.13 |
| PbCO30 | 55.46 | 56.88 | 53.47 | 55.20 | 32.08 | 34.84 | 50.32 | 52.2 | 40.22 | 42.86 | 34.88 | 37.58 |
| PbHA+ | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Sr | ||||||||||||
| Sr2+ | 93.88 | 94.21 | 90.19 | 90.60 | 76.53 | 77.09 | 92.84 | 93.19 | 90.69 | 90.99 | 3.62 | 89.66 |
| SrCl+ | 0.05 | 0.05 | 0.17 | 0.17 | 0.3 | 0.30 | 0.12 | 0.11 | 0.27 | 0.26 | 0.02 | 0.38 |
| SrSO40 | 3.75 | 3.59 | 7.74 | 7.44 | 22.5 | 22.07 | 5.31 | 5.06 | 8.05 | 7.77 | 89.56 | 9.23 |
| SrCO30 | 0.6 | 0.50 | 0.46 | 0.39 | 0.06 | 0.06 | 0.38 | 0.33 | 0.16 | 0.14 | 0.9 | 0.09 |
| SrHCO3+ | 1.72 | 1.65 | 1.44 | 1.40 | 0.46 | 0.48 | 1.35 | 1.31 | 0.83 | 0.84 | 5.9 | 0.64 |
| U | ||||||||||||
| UO2OH+ | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 |
| UO2CO30 | 0.09 | 0.07 | 0.07 | 0.05 | 0.56 | 0.39 | 0.14 | 0.11 | 0.34 | 0.24 | 0.50 | 0.35 |
| UO2 (CO3)22− | 18.9 | 15.32 | 15.02 | 12.03 | 29.96 | 23.95 | 21.0 | 17.3 | 28.1 | 22.42 | 30.27 | 24.9 |
| UO2 (CO3)34− | 80.38 | 84.18 | 84.26 | 87.60 | 64.65 | 73.47 | 76.75 | 81.51 | 68.70 | 75.96 | 64.92 | 73.17 |
| UO30 | 0.69 | 0.36 | 0.54 | 0.27 | 4.40 | 2.01 | 1.11 | 0.56 | 2.62 | 1.24 | 3.89 | 1.82 |
| HUO4− | 0.15 | 0.07 | 0.11 | 0.05 | 0.42 | 0.18 | 0.20 | 0.09 | 0.33 | 0.14 | 0.41 | 0.17 |
| Zn | ||||||||||||
| Zn2+ | 42.01 | 48.14 | 45.5 | 51.33 | 66.6 | 70.64 | 49.88 | 55.76 | 63.37 | 68.71 | 69.36 | 74.09 |
| ZnOH+ | 25.76 | 16.72 | 24.49 | 15.84 | 13.76 | 8.83 | 24.26 | 15.71 | 19.67 | 12.62 | 16.59 | 10.64 |
| ZnO0 | 0.23 | 0.11 | 0.2 | 0.10 | 0.05 | 0.02 | 0.17 | 0.09 | 0.09 | 0.05 | 0.06 | 0.03 |
| HZnO2− | 0.01 | 0 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnCl+ | 0.03 | 0.03 | 0.12 | 0.10 | 0.35 | 0.28 | 0.08 | 0.07 | 0.25 | 0.2 | 0.4 | 0.32 |
| ZnCl20 | 0 | 0 | 0 | 0 | 0.01 | 0.01 | 0 | 0 | 0 | 0 | 0.01 | 0.01 |
| ZnSO40 | 1.06 | 1.13 | 2.48 | 2.60 | 12.67 | 12.66 | 1.79 | 1.88 | 3.58 | 3.62 | 4.73 | 4.76 |
| ZnCO30 | 30.46 | 33.37 | 26.78 | 29.58 | 6.33 | 7.30 | 23.42 | 26.02 | 12.72 | 14.43 | 8.58 | 9.84 |
| ZnHCO3+ | 0.41 | 0.48 | 0.39 | 0.45 | 0.22 | 0.25 | 0.38 | 0.45 | 0.31 | 0.36 | 0.26 | 0.3 |
| ZnHA+ | 0.03 | 0.02 | 0.03 | 0.00 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 |
| Scenarios of Mixing of River Waters with Drainage Groundwater | ||||||
|---|---|---|---|---|---|---|
| 1S | 1W | 2S | 2W | 3S | 3W | |
| mg/kg H2O | H2O | |||||
| FA | 11.4 | 7.4 | 12.2 | 815 | 11.4 | 7.35 |
| HA | 0.64 | 0.412 | 0.68 | 0.45 | 0.64 | 0.41 |
| Na | 72 | 72 | 187 | 187 | 382 | 382 |
| Mg | 5.25 | 6.76 | 8.34 | 9.97 | 46.4 | 47.9 |
| S | 7.95 | 7.95 | 22.3 | 22.3 | 135 | 135 |
| Cl | 51.4 | 51.4 | 207 | 207 | 532 | 532 |
| K | 3.09 | 3.09 | 3.53 | 3.53 | 7.98 | 7.98 |
| Ca | 9.74 | 10.2 | 9.58 | 10.3 | 76.2 | 76.6 |
| HCO3− | 133 | 142 | 131 | 142 | 63 | 72.1 |
| SO42− | 23.9 | 23.9 | 66.9 | 66.9 | 405 | 405 |
| TDS | 310 | 318 | 625 | 635 | 1525 | 1532 |
| µg/kg H2O | ||||||
| Al | 0.90 | 0.5 | 0.89 | 0.5 | 0.43 | 0.25 |
| Cr | 0.54 | 0.54 | 0.6 | 0.6 | 0.7 | 0.7 |
| Mn | 6.81 × 10−9 | 5.38 × 10−9 | 8.08 × 10−9 | 6.37 × 10−9 | 4.36 × 10−9 | 3.4 × 10−9 |
| Fe | 127 | 82.6 | 136 | 91 | 127 | 82.1 |
| Ni | 0.17 | 0.17 | 0.29 | 0.29 | 0.46 | 0.46 |
| Cu | 0.19 | 0.19 | 0.36 | 0.36 | 0.47 | 0.47 |
| Zn | 2.8 | 2.85 | 4.77 | 4.75 | 4.75 | 4.49 |
| As | 0.57 | 0.57 | 0.53 | 0.53 | 0.53 | 0.53 |
| Sr | 35.1 | 38.2 | 72.4 | 67.8 | 380 | 293 |
| Mo | 1.92 | 1.92 | 4.49 | 4.49 | 2.05 | 2.05 |
| Cd | 0.0039 | 0.0039 | 0.0098 | 0.0098 | 0.0098 | 0.0098 |
| Pb | 0.017 | 0.017 | 0.086 | 0.076 | 0.014 | 0.011 |
| U | 4.71 | 4.71 | 3.86 | 3.86 | 6.12 | 6.12 |
| 4S | 4W | 5S | 5W | 6S | 6W | |
| mg/kg H2O | ||||||
| FA | 11.4 | 7.4 | 11.4 | 7.35 | 11.4 | 7.35 |
| HA | 0.64 | 0.41 | 0.64 | 0.41 | 0.64 | 0.41 |
| Na | 107 | 107 | 207 | 207 | 301 | 301 |
| Mg | 6.78 | 8.26 | 15 | 16.6 | 24.3 | 26.2 |
| S | 13 | 13 | 27.6 | 27.6 | 41.4 | 41.4 |
| Cl | 125 | 125 | 338 | 338 | 539 | 539 |
| K | 3.41 | 3.41 | 4.34 | 4.34 | 5.2 | 5.2 |
| Ca | 17.8 | 18.5 | 44.5 | 44.9 | 72.5 | 72.1 |
| HCO3− | 112 | 121 | 80.8 | 90.4 | 68.6 | 76.7 |
| SO42− | 39 | 39 | 82.8 | 82.8 | 124 | 124 |
| TDS | 423 | 431 | 785 | 791 | 1147 | 1152 |
| µg/kg H2O | ||||||
| Al | 0.76 | 0.43 | 0.55 | 0.32 | 0.46 | 0.27 |
| Cr | 0.66 | 0.66 | 1 | 1 | 1.32 | 1.32 |
| Mn | 9.45 × 10−9 | 7.64 × 10−9 | 2.03 × 10−8 | 1.62 × 10−8 | 3.24 × 10−8 | 2.56 × 10−8 |
| Fe | 127 | 82.6 | 127 | 82.1 | 127 | 82.1 |
| Ni | 0.19 | 0.19 | 0.23 | 0.23 | 0.27 | 0.27 |
| Cu | 0.19 | 0.19 | 0.19 | 0.19 | 0.2 | 0.2 |
| Zn | 1.94 | 2.1 | 1.29 | 1.23 | 1.4 | 1.24 |
| As | 0.57 | 0.57 | 0.58 | 0.58 | 0.59 | 0.59 |
| Sr | 25.1 | 28.2 | 32.8 | 27.6 | 51.6 | 39.2 |
| Mo | 1.97 | 1.97 | 2.9 | 2.09 | 2.21 | 2.21 |
| Cd | 0.0041 | 0.0041 | 0.0046 | 0.0046 | 0.0052 | 0.0052 |
| Pb | 0.003 | 0.0032 | 0.00071 | 0.00057 | 0.00048 | 0.00036 |
| U | 4.67 | 4.67 | 4.55 | 4.55 | 4.43 | 4.43 |
| Scenarios of Mixing of River Waters with Drainage Groundwater | ||||||
|---|---|---|---|---|---|---|
| 1S | 1W | 2S | 2W | 3S | 3W | |
| Phase, mol/kg H2O | ||||||
| Dolomite | 9.74 × 10−5 | 3.57 × 10−5 | 2.35 × 10−4 | 1.67 × 10−4 | 3.80 × 10−4 | 3.16 × 10−4 |
| Gibbsite | 8.49 × 10−7 | 8.63 × 10−7 | 9.62 × 10−7 | 9.76 × 10−7 | 1.03 × 10−6 | 1.04 × 10−6 |
| Goethite | 1.58 × 10−7 | 9.66 × 10−7 | 3.90 × 10−6 | 4.71 × 10−6 | 5.64 × 10−6 | 6.45 × 10−6 |
| Pyrolusite | 2.33 × 10−7 | 2.33 × 10−7 | 4.34 × 10−7 | 4.34 × 10−7 | 2.66 × 10−6 | 2.66 × 10−6 |
| (Ca, Sr, Zn, Pb, Mn)CO3, mol/kg H2O (solid solution) | ||||||
| Ca | 3.19 × 10−6 | 2.58 × 10−6 | 2.32 × 10−6 | 2.37 × 10−6 | 4.86 × 10−5 | 5.72 × 10−5 |
| Sr | 1.12 × 10−6 | 1.09 × 10−6 | 1.65 × 10−6 | 1.71 × 10−6 | 2.03 × 10−5 | 2.13 × 10−5 |
| Zn | 5.46 × 10−9 | 4.71 × 10−9 | 7.54 × 10−9 | 7.75 × 10−9 | 3.07 × 10−8 | 3.47 × 10−8 |
| Pb | 2.33 × 10−10 | 2.31 × 10−10 | 1.05 × 10−9 | 1.09 × 10−9 | 1.83 × 10−9 | 1.85 × 10−9 |
| Mn | 5.65 × 10−17 | 3.99 × 10−17 | 5.21 × 10−17 | 4.44 × 10−17 | 9.01 × 10−16 | 8.54 × 10−16 |
| 4S | 4W | 5S | 5W | 6S | 6W | |
| Phase, mol/kg H2O | ||||||
| Dolomite | 1.63 × 10−4 | 1.02 × 10−4 | 1.96 × 10−4 | 1.28 × 10−4 | 1.64 × 10−4 | 8.29 × 10−5 |
| Gibbsite | 8.98 × 10−7 | 9.09 × 10−7 | 1.03 × 10−6 | 1.04 × 10−6 | 1.16 × 10−6 | 1.16 × 10−6 |
| Goethite | 9.30 × 10−7 | 1.74 × 10−6 | 3.16 × 10−6 | 3.97 × 10−6 | 5.26 × 10−6 | 6.07 × 10−6 |
| Pyrolusite | 6.10 × 10−7 | 6.10 × 10−7 | 1.70 × 10−6 | 1.70 × 10−6 | 2.73 × 10−6 | 2.73 × 10−6 |
| (Ca, Sr, Zn, Pb, Mn)CO3, mol/kg H2O (solid solution) | ||||||
| Ca | 3.06 × 10−5 | 2.42 × 10−5 | 1.81 × 10−4 | 1.88 × 10−4 | 3.09 × 10−4 | 3.51 × 10−4 |
| Sr | 2.34 × 10−8 | 4.10 × 10−6 | 1.24 × 10−5 | 1.25 × 10−5 | 2.01 × 10−5 | 2.02 × 10−5 |
| Zn | 4.13 × 10−6 | 2.09 × 10−8 | 4.70 × 10−8 | 4.80 × 10−8 | 5.83 × 10−8 | 6.07 × 10−8 |
| Pb | 3.01 × 10−10 | 3.00 × 10−10 | 3.14 × 10−10 | 3.14 × 10−10 | 3.16 × 10−10 | 3.17 × 10−10 |
| Mn | 4.54 × 10−16 | 3.08 × 10−16 | 2.53 × 10−15 | 2.18 × 10−15 | 4.30 × 10−15 | 4.04 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malov, A.I.; Sidkina, E.S.; Cherkasova, E.V. The Influence of DOC on the Migration Forms of Elements and Their Sedimentation from River Waters at an Exploited Diamond Deposit (NW Russia). Water 2023, 15, 2160. https://doi.org/10.3390/w15122160
Malov AI, Sidkina ES, Cherkasova EV. The Influence of DOC on the Migration Forms of Elements and Their Sedimentation from River Waters at an Exploited Diamond Deposit (NW Russia). Water. 2023; 15(12):2160. https://doi.org/10.3390/w15122160
Chicago/Turabian StyleMalov, Alexander I., Evgeniya S. Sidkina, and Elena V. Cherkasova. 2023. "The Influence of DOC on the Migration Forms of Elements and Their Sedimentation from River Waters at an Exploited Diamond Deposit (NW Russia)" Water 15, no. 12: 2160. https://doi.org/10.3390/w15122160






