Analysis of the Partial Nitrification/Anammox Performance and Microbial Structure of Low C/N Wastewater by A2/O Process
Abstract
1. Introduction
2. Materials and Methods
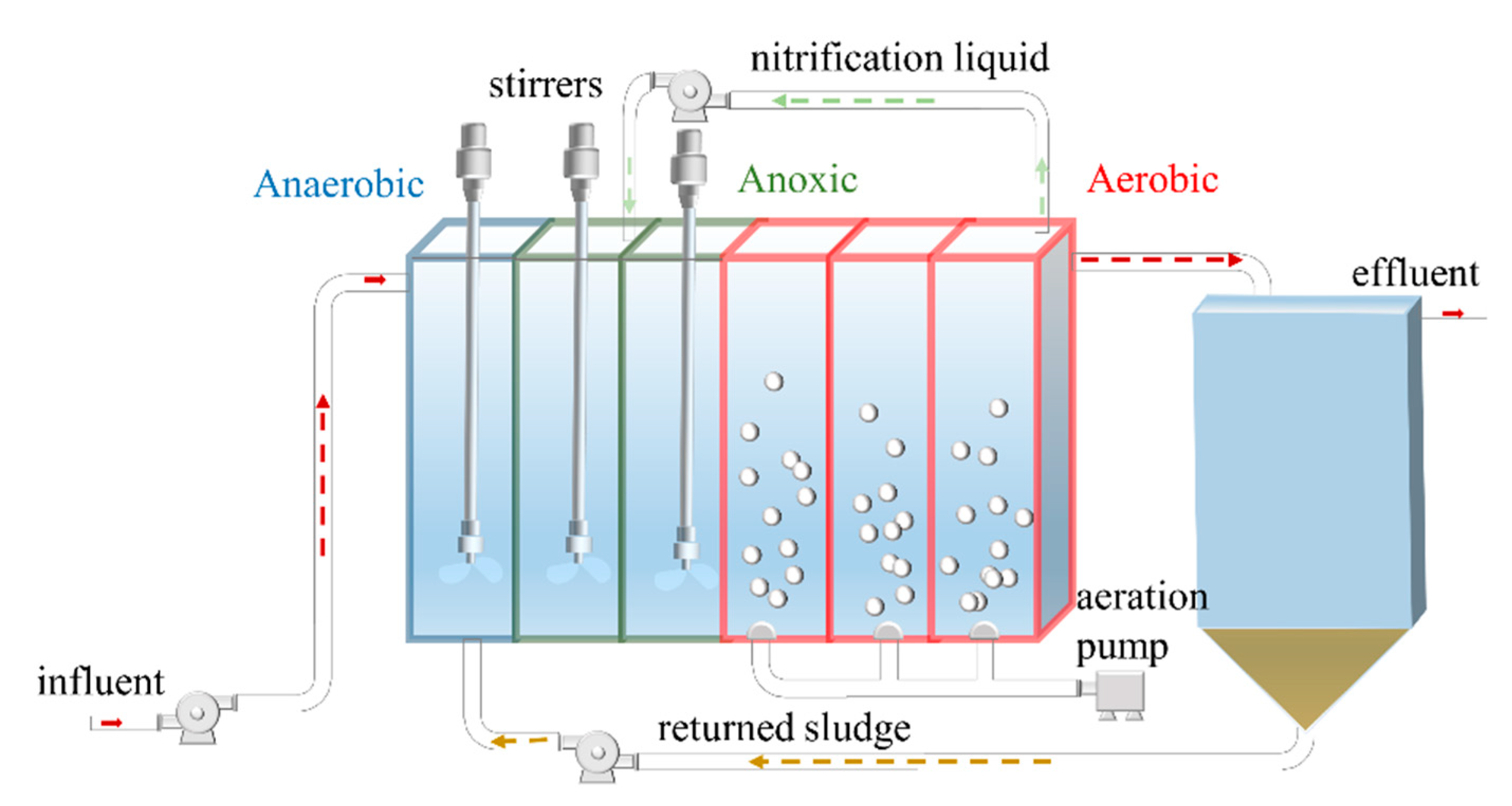
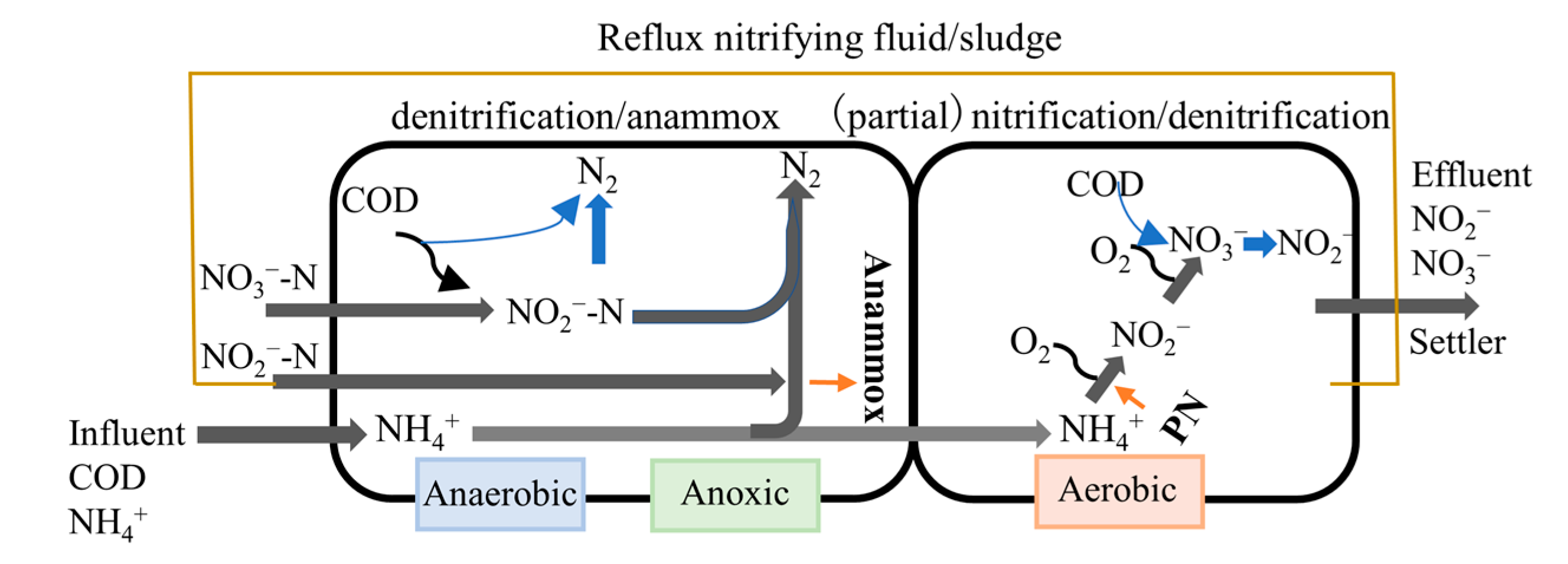
2.1. Operation of the Continuous Process
2.2. Analytical Methods
2.3. Calculations of Experimental Index
2.3.1. Calculation of Nitrite Accumulation Rate
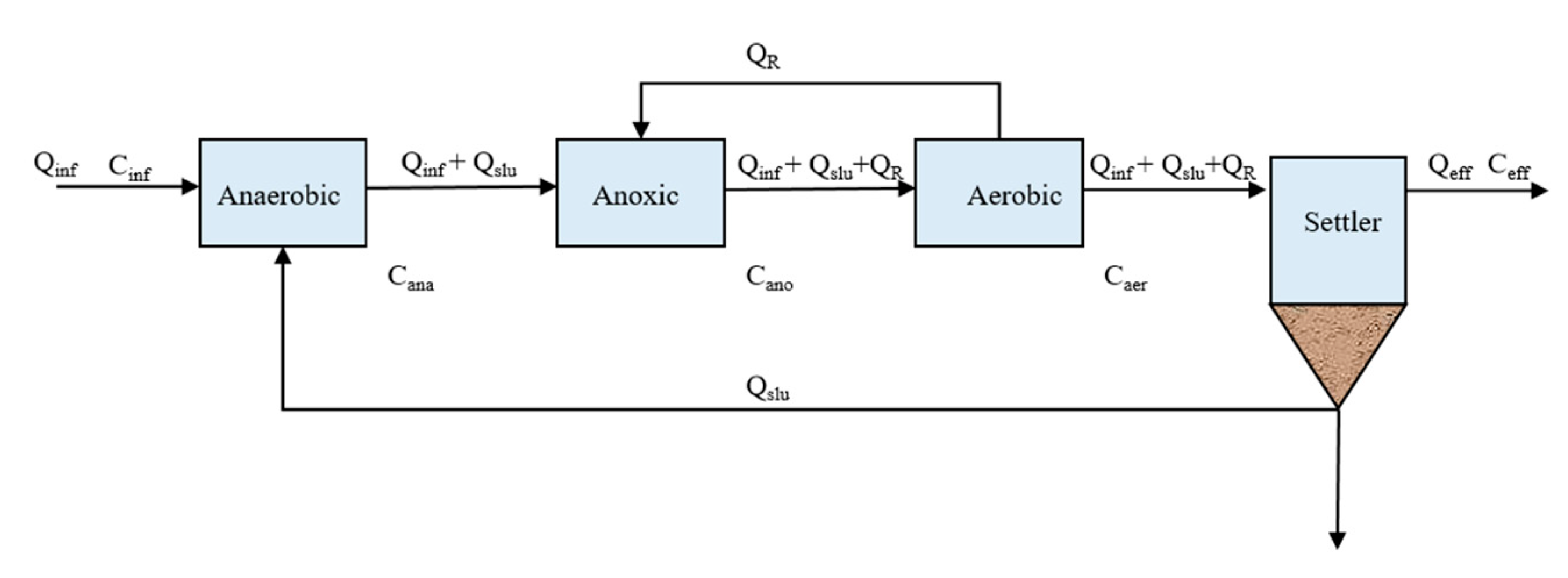
2.3.2. Calculation of Pollutants Mass Balance
2.4. Microbiological Analysis
3. Results and Discussion
3.1. Performance of Nitrogen Removal under Different DO Concentrations
3.2. Performance of Nitrogen Removal under Different Reflux Ratios
3.3. Analysis of Functional Microbial Community for Systemic Nitrogen Transformation
3.3.1. Alpha-Diversity of Microbial Community
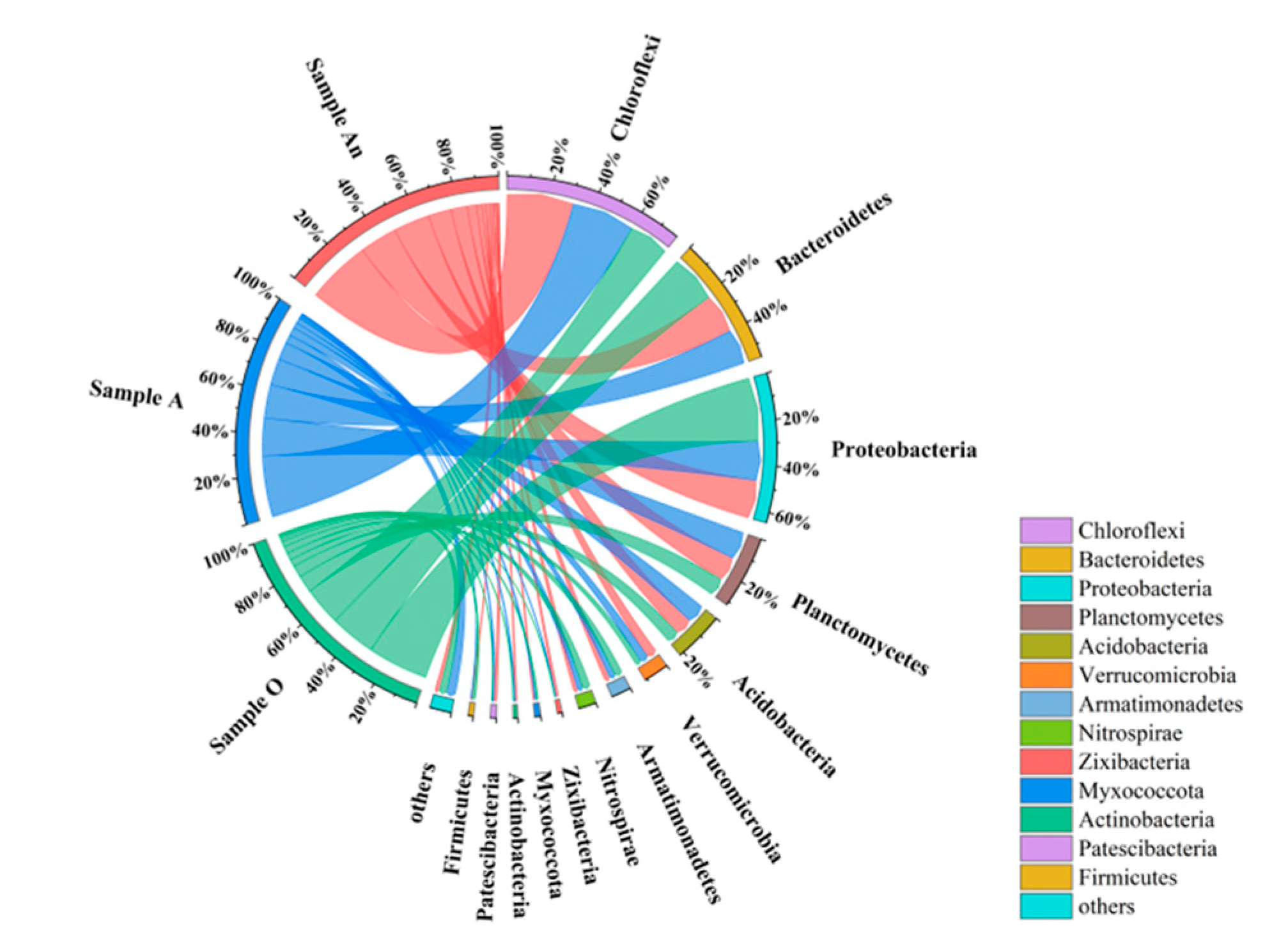
3.3.2. Phylum-Level Microbial Community Composition
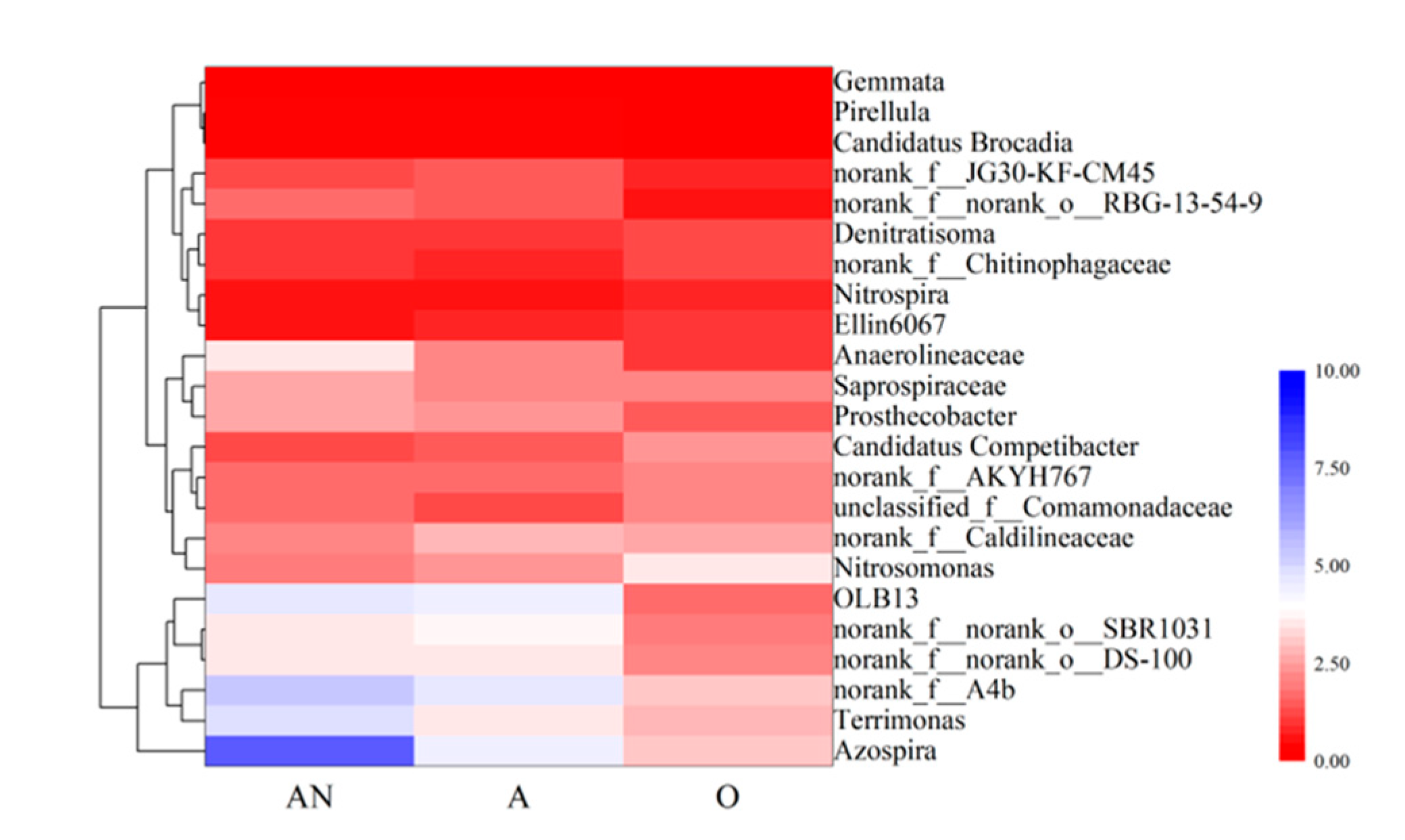
3.3.3. Genus-Level Microbial Community Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Li, Z.; Wang, G.; Hou, Y.; Han, J.; Yi, F. A2O–MBR–BAF–O3 process for treating high organic wastewater with high ammonia nitrogen. Biochem. Eng. J. 2022, 186, 108574. [Google Scholar] [CrossRef]
- Ling, L.; Wenwang, Z.; Ye, Y.; Hui, Z.; Changming, Z. Effects of salinity shock on simultaneous nitrification and denitrification by a membrane bioreactor: Performance, sludge activity, and functional microflora. Sci. Total Environ. 2021, 801, 149748. [Google Scholar]
- Li, X.; Liu, T.; Zhang, Y.; Cai, J.; He, M.; Li, M.; Chen, Z.; Zhang, L. Growth of BiOBr/ZIF-67 Nanocomposites on Carbon Fiber Cloth as Filter-Membrane-Shaped Photocatalyst for Degrading Pollutants in Flowing Wastewater. Adv. Fiber Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, J.; Liang, J.; El-Din, M.G.; Zhao, P.; Xie, W.; Chen, C. Nitrogen removal intensification of aerobic granular sludge through bioaugmentation with “heterotrophic nitrification-aerobic denitrification” consortium during petroleum wastewater treatment. Bioresour. Technol. 2022, 361, 127719. [Google Scholar] [CrossRef]
- Alireza, M.S.; Mohammad, M.; Maryam, K.; Shaliza, I. Effect of air flow rate and C/N ratio on biological nitrogen removal through the CANON process treating reject water. Environ. Technol. 2018, 39, 2891–2899. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Y.; Ren, N.; Liu, Q.; Chen, Y. Improvement of nutrient removal by optimizing the volume ratio of anoxic to aerobic zone in AAO-BAF system. Chemosphere 2013, 93, 2859–2863. [Google Scholar] [CrossRef]
- Sen, Q.; Tian, T.; Xiumei, D.; Jiti, Z.; Yingjun, C. Novel single-stage autotrophic nitrogen removal via co-immobilizing partial nitrifying and anammox biomass. Chem. Eng. J. 2013, 230, 19–26. [Google Scholar]
- Ding, S.; Bao, P.; Wang, B.; Zhang, Q.; Peng, Y. Long-term stable simultaneous partial nitrification, anammox and denitrification (SNAD) process treating real domestic sewage using suspended activated sludge. Chem. Eng. J. 2018, 339, 180–188. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Li, X.; Jia, S.; Peng, Y. Advanced nutrient removal from ammonia and domestic wastewaters by a novel process based on simultaneous partial nitrification-anammox and modified denitrifying phosphorus removal. Chem. Eng. J. 2018, 354, 589–598. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.M.; Jin, R.C. How anammox responds to the emerging contaminants: Status and mechanisms. J. Environ. Manag. 2021, 293, 112906. [Google Scholar] [CrossRef]
- Qi, Z.; Jinli, Z.; Leizhen, Z.; Wenru, L.; Liwei, C.; Tianming, C.; XiaoMing, J. Succession of microbial communities reveals the inevitability of anammox core in the development of anammox processes. Bioresour. Technol. 2023, 371, 128645. [Google Scholar] [CrossRef]
- Yan, F.; Lei, W.; Qiong, Z.; Xiyao, L.; Shuying, W.; Yongzhen, P. Double anammox process in the AOAO process of treating real low C/N sewage: Validation, enhancement, and quantification of the contribution of anammox in the oxic zone. Sci. Total Environ. 2022, 849, 157866. [Google Scholar] [CrossRef]
- Hou, Z.; Jia, Z.; Fu, J.; Shi, X.; Li, Q.; Chen, R. Enhanced nitrogen removal efficiency of partial nitrification/anaerobic ammonia oxidation (PN/A) on the effluent of municipal wastewateranaerobic treatment unit. Chin. J. Environ. Eng. 2022, 16, 806–813. [Google Scholar]
- Wu, L.; Zhang, L.; Shi, X.; Liu, T.; Peng, Y.; Zhang, J. Analysis of the impact of reflux ratio on coupled partial nitrification–anammox for co-treatment of mature landfill leachate and domestic wastewater. Bioresour. Technol. 2015, 198, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Peng, Y.; Zhang, Q.; Liu, Y. Partial nitrification coupled anaerobic ammonia oxidation process to treat low C/N domestic sewage. CIESC J. 2018, 69, 3635–3642. [Google Scholar]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of the People’s Republic of China. Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant; State Environmental Protection Administration of the People’s Republic of China: Beijing, China, 2002. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/200307/t20030701_66529.shtml (accessed on 25 April 2023).
- Liu, G.; Wang, J. Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013, 47, 5109–5117. [Google Scholar] [CrossRef]
- Li, B.; Cao, T.; Wang, L.; Li, Y. A pilot-scale study on nitrogen removal of municipal wastewater by A2/O process under low dissolved oxygen condition. China Environ. Sci. 2019, 39, 134–140. [Google Scholar] [CrossRef]
- Bian, W.; Li, J.; Wang, M.; Hou, A.; Yue, Y. Effects of Dissolved Oxygen on Partial Nitrification under Different Temperatures and Related Research. China Water Wastewater 2017, 33, 84–88. [Google Scholar] [CrossRef]
- Chi, Y.; Shi, X.; Jin, P.; Wang, X.C.; Ren, T.; Ren, B.; Jin, X. Enhanced nitrogen removal by partial nitrification-anammox process with a novel high-frequency micro-aeration (HFMA) mode: Metabolic interactions among functional bacteria. Bioresour. Technol. 2021, 342, 125917. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, M.; Meng, J.; Hu, Z.; Ni, G.; Guerrero Calderon, A.; Li, H.; De Clippeleir, H.; Al-Omari, A.; Hu, S.; et al. Robust Nitritation Sustained by Acid-Tolerant Ammonia-Oxidizing Bacteria. Environ. Sci. Technol. 2021, 55, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Peng, Y.Z.; Wang, J.H.; Zhang, L.C. Effect of Internal Recycle Ratio on Nitrogen and Phosphorus Removal Characteristics in A2/O-BAF Process. Environ. Sci. 2011, 32, 193–198. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2007, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Shuying, W.; Rulong, Z.; Lei, M.; Yongzhen, P. Advanced nitrogen removal from landfill leachate without addition of external carbon using a novel system coupling ASBR and modified SBR. Bioresour. Technol. 2013, 134, 212–218. [Google Scholar] [CrossRef]
- Hongyu, M.; Li, X.; Xi, L.; Zi, Y. Characteristics of anaerobic ammonium oxidation initiated by anaerobic granular sludge. EnEng 2020, 38, 93–98. [Google Scholar] [CrossRef]
- Bae, H.; Chung, Y.C.; Jung, J.Y. Microbial community structure and occurrence of diverse autotrophic ammonium oxidizing microorganisms in the anammox process. Water Sci. Technol. 2010, 61, 2723–2732. [Google Scholar] [CrossRef]
- Ya, T.; Huang, Y.; Wang, K.; Wang, J.; Liu, J.; Hai, R.; Zhang, T.; Wang, X. Functional stability correlates with dynamic microbial networks in anammox process. Bioresour. Technol. 2022, 370, 128557. [Google Scholar] [CrossRef]
- Tomonori, K.; Shota, Y.; Noriatsu, O.; Akiyoshi, O. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor. Water Sci. Technol. 2012, 66, 2556–2561. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Jiang, B.; Feng, Y.; Zhu, T.; Tao, H.; Tang, X.; Liu, S. Genome-Centered Metagenomics Analysis Reveals the Symbiotic Organisms Possessing Ability to Cross-Feed with Anammox Bacteria in Anammox Consortia. Environ. Sci. Technol. 2018, 52, 11285–11296. [Google Scholar] [CrossRef]
- Song, Y.P.; Gong, H.; Yuan, Q.; Chang, F. Nitrogen removal performance through different AnAOB processes treating wastewater pretreated by magnetic separation and microbial community structure shifting analysis. Acta Sci. Circumstantiae 2021, 41, 70–82. [Google Scholar] [CrossRef]
- Li, X.R.; Du, B.; Fu, H.X.; Wang, R.F.; Shi, J.H.; Wang, Y.; Jetten, M.S.; Quan, Z.X. The bacterial diversity in an anaerobic ammonium-oxidizing (anammox) reactor community. Syst. Appl. Microbiol. 2009, 32, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.; Sliekers, A.O.; Lavik, G.; Schmid, M.; Jørgensen, B.B.; Kuenen, J.G.; Sinninghe Damsté, J.S.; Strous, M.; Jetten, M.S. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 2003, 422, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, G.; Wang, Y.; Zhang, Y.; Wang, H.; Qi, L.; Wang, H. Analysis of key microbial community during the start-up of anaerobic ammonium oxidation process with paddy soil as inoculated sludge. J. Environ. Sci. 2018, 64, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, B.; Mu, D.; Li, X.; Peng, Y. High-efficient nitrogen removal from municipal wastewater via two-stage nitritation/anammox process: Long-term stability assessment and mechanism analysis. Bioresour. Technol. 2018, 271, 150–158. [Google Scholar] [CrossRef]
- Molinuevo, B.; García, M.C.; Karakashev, D.; Angelidaki, I. Anammox for ammonia removal from pig manure effluents: Effect of organic matter content on process performance. Bioresour. Technol. 2008, 100, 2171–2175. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, R.; Sui, Q.; Yu, D.; Ren, J. Engineering Application of Anammox in Treatment of Anaerobic Digestion Liquor in Swine Farm. China Water Wastewater 2022, 38, 3. [Google Scholar] [CrossRef]
- Bartosch, S.; Wolgast, I.; Spieck, E.; Bock, E. Identification of nitrite-oxidizing bacteria with monoclonal antibodies recognizing the nitrite oxidoreductase. Appl. Environ. Microbiol. 1999, 65, 4126–4133. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Tan, B.; Li, M.; Peng, H.; He, J.; Zhang, Y.; Su, J. Microbial community and metabolic characteristics evaluation in start-up stage of electro-enhanced SBR for aniline wastewater treatment. J. Water Process Eng. 2022, 45, 102489. [Google Scholar] [CrossRef]
- Roy, D.; Lemay, J.F.; Drogui, P.; Tyagi, R.; Landry, D.; Rahni, M. Identifying the link between MBRs’ key operating parameters and bacterial community: A step towards optimized leachate treatment. Water Res. 2020, 172, 115509. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, B.; Gong, X.; Liu, X.; Peng, Y. Anammox bacteria enrich naturally in suspended sludge system during partial nitrification of domestic sewage and contribute to nitrogen removal. Sci. Total Environ. 2021, 787, 147658. [Google Scholar] [CrossRef]
- Song, Z.Z.; Lü, S.; Liu, Z.; Shi, X.D.; Pan, A.; Zhang, Z. Start-up of Simultaneous ANAMMOX and Denitrification Process and Changes in Microbial Community Characteristics. Environ. Sci. 2019, 40, 5057–5065. [Google Scholar] [CrossRef]
- Xin, X.I.N.; Guan, L.; Yao, Y.D.; Yang, Y.J.; Guo, J.Y.; Cheng, Q.F. Long-term Performance and Bacterial Community Composition Analysis of AGS-SBR Treating the Low COD/N Sewage at Low DO Concentration Condition. Environ. Sci. 2016, 37, 2259–2265. [Google Scholar] [CrossRef]
- Lin, J.; Meng, Y.; Shi, Y.; Lin, X. Complete Genome Sequences of Colwellia sp. Arc7-635, a Denitrifying Bacterium Isolated from Arctic Seawater. Curr. Microbiol. 2019, 76, 1061–1065. [Google Scholar] [CrossRef] [PubMed]



| Items | Phase I | Phase II | Phase III | Phase IV | Phase V |
|---|---|---|---|---|---|
| Days/d | 1–30 | 31–60 | 61–90 | 91–111 | 112–132 |
| DO/mg·L−1 | 2.0–3.0 | 1.0–1.5 | 0.5–0.8 | 0.5–0.8 | 0.5–0.8 |
| Nitrification reflux ratio (R1)/% | 200 | 200 | 200 | 250 | 300 |
| Sludge reflux Ratio (R2)/% | 100 | 100 | 100 | 100 | 100 |
| Inf.COD/mg·L−1 | |||||
| Inf.NH4+-N/mg·L−1 | |||||
| Inf.NO2−-N/mg·L−1 | |||||
| Inf.NO3−-N/mg·L−1 | |||||
| Inf.pH |
| Sample | Diversity Index | Richness Index | Coverage Index/% | ||
|---|---|---|---|---|---|
| Shannon | Simpson | Ace | Chao | ||
| An | 5.68 | 0.010 | 1742.13 | 1733.28 | 99.93 |
| A | 5.55 | 0.011 | 1573.91 | 1622.82 | 98.83 |
| O | 5.48 | 0.014 | 1523.88 | 1581.04 | 99.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Zhou, Y.; Tang, L.; Chen, S.; Zhao, X. Analysis of the Partial Nitrification/Anammox Performance and Microbial Structure of Low C/N Wastewater by A2/O Process. Water 2023, 15, 2300. https://doi.org/10.3390/w15122300
Ye L, Zhou Y, Tang L, Chen S, Zhao X. Analysis of the Partial Nitrification/Anammox Performance and Microbial Structure of Low C/N Wastewater by A2/O Process. Water. 2023; 15(12):2300. https://doi.org/10.3390/w15122300
Chicago/Turabian StyleYe, Lei, Yanhao Zhou, Liangdong Tang, Sixing Chen, and Xianguang Zhao. 2023. "Analysis of the Partial Nitrification/Anammox Performance and Microbial Structure of Low C/N Wastewater by A2/O Process" Water 15, no. 12: 2300. https://doi.org/10.3390/w15122300
APA StyleYe, L., Zhou, Y., Tang, L., Chen, S., & Zhao, X. (2023). Analysis of the Partial Nitrification/Anammox Performance and Microbial Structure of Low C/N Wastewater by A2/O Process. Water, 15(12), 2300. https://doi.org/10.3390/w15122300





