Groundwater–Surface Water Exchange and Spatial Distribution of Arsenic in Arid and Semi-Arid Regions: The Case of Aksu River in Xinjiang, Northwestern China
Abstract
:1. Introduction
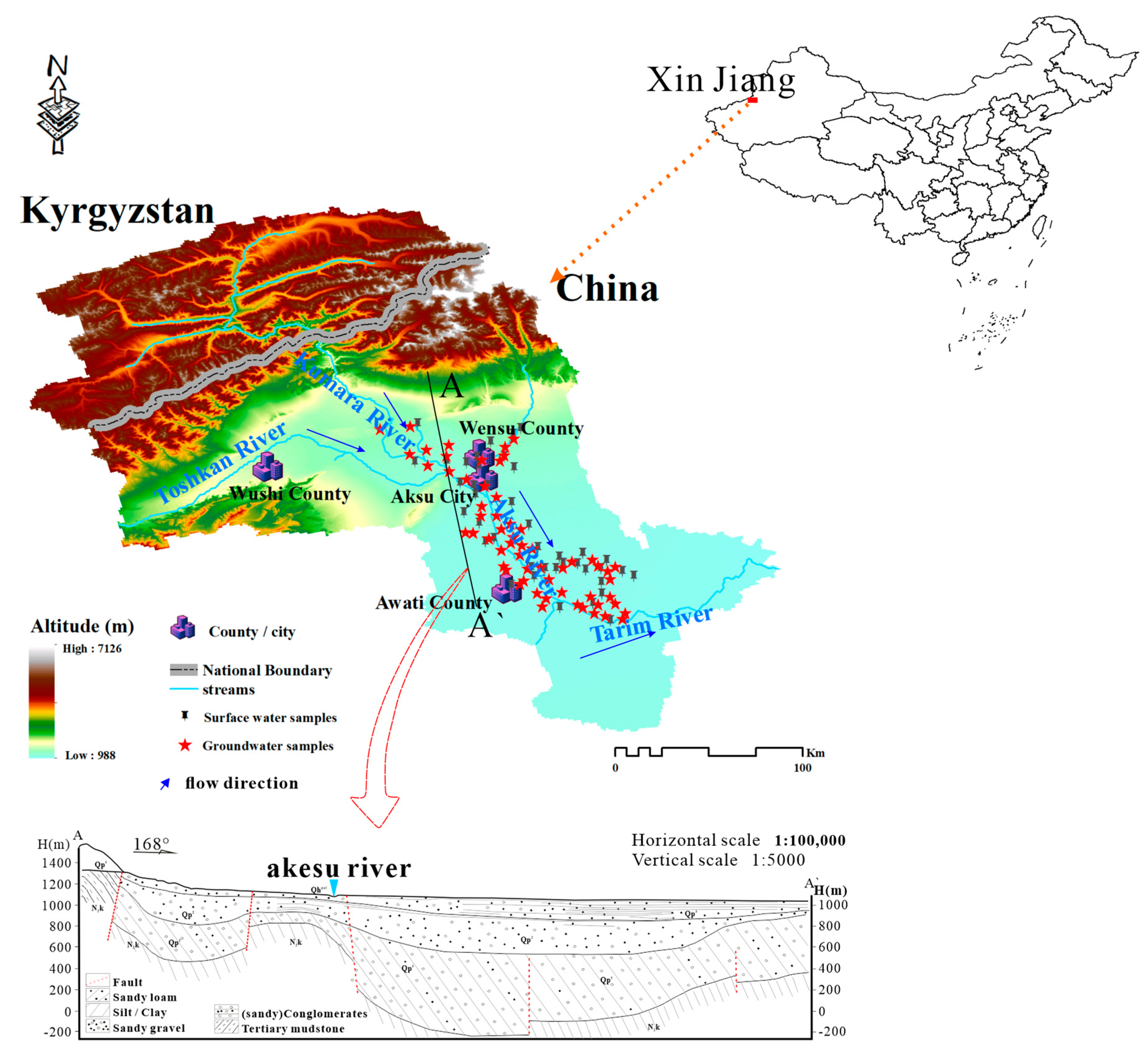
2. Overview of the Study Area
3. Materials and Methods
3.1. Sample Collection and Testing
3.2. Data Handling
4. Results and Discussion
4.1. Hydrochemical Characteristics of Groundwater and Surface Water
4.1.1. Analysis of Anion and Cation Composition
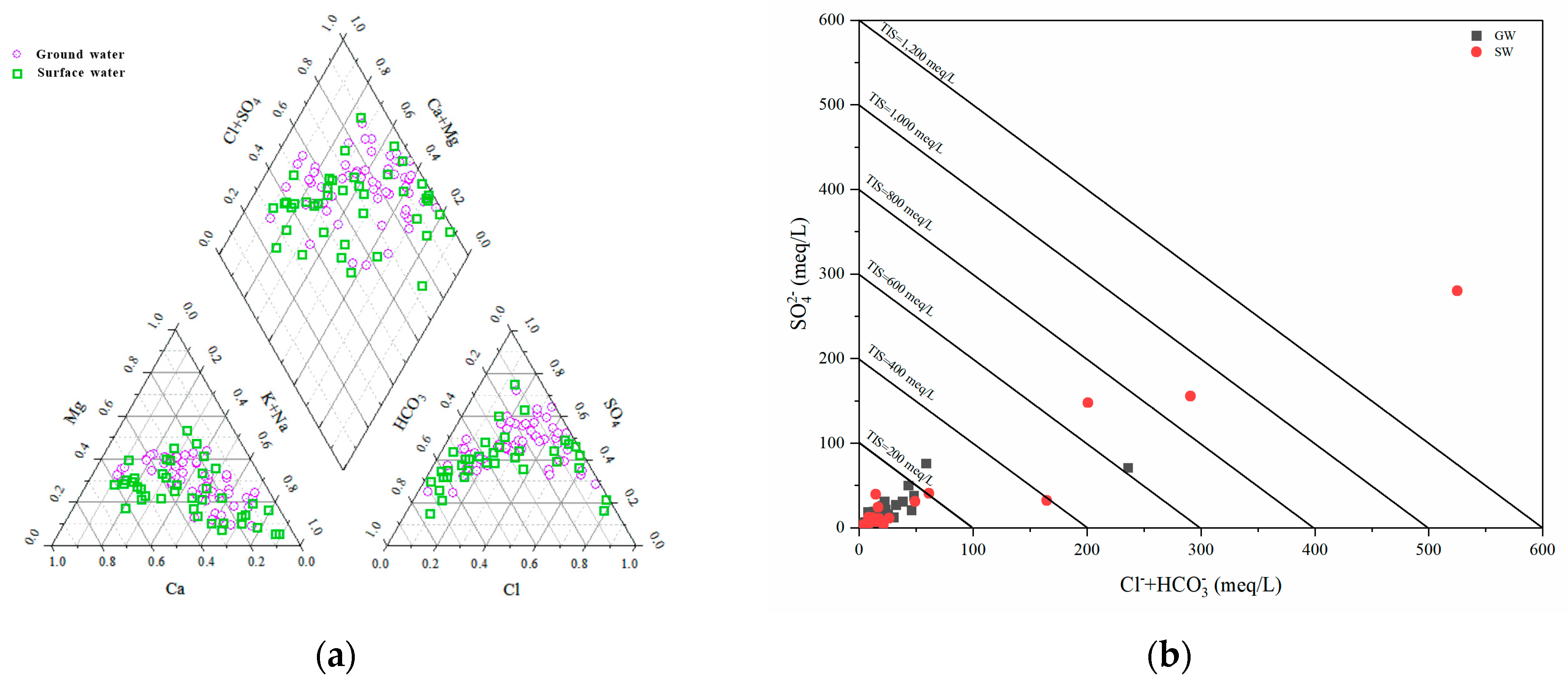
4.1.2. Hydrochemical Type
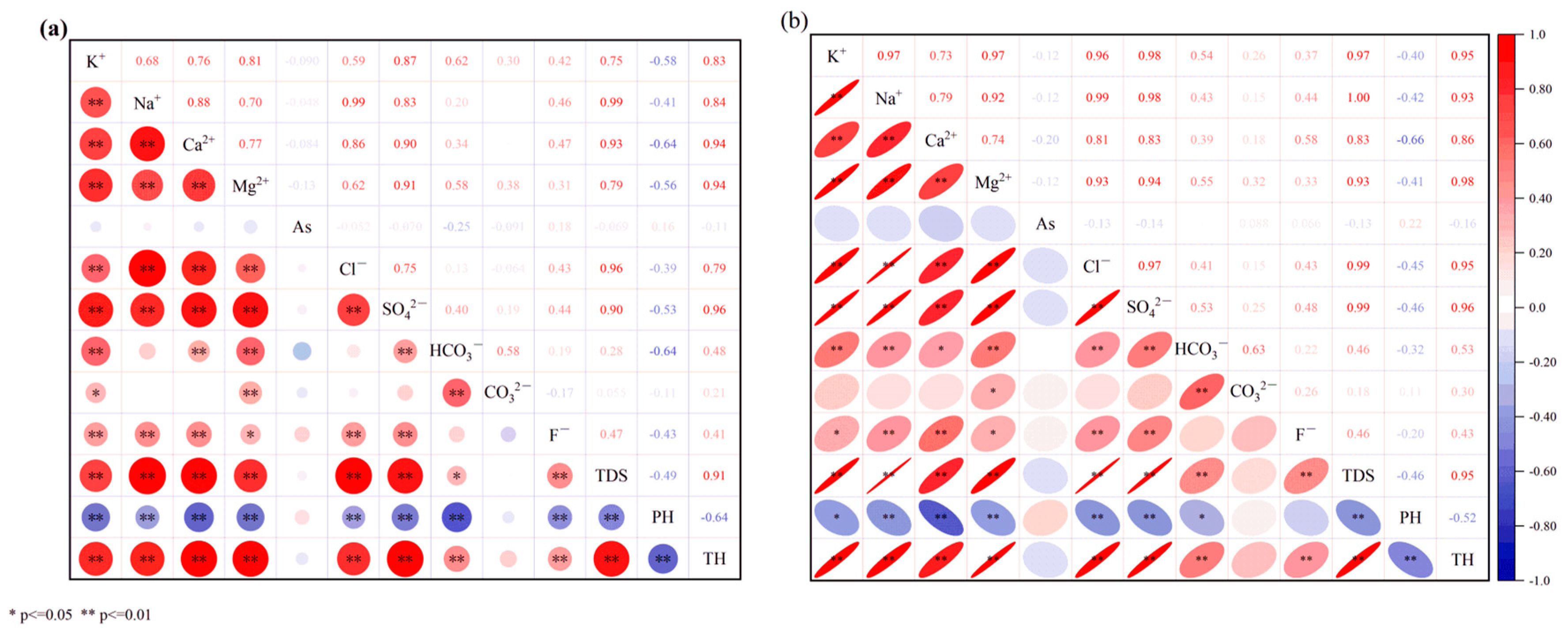
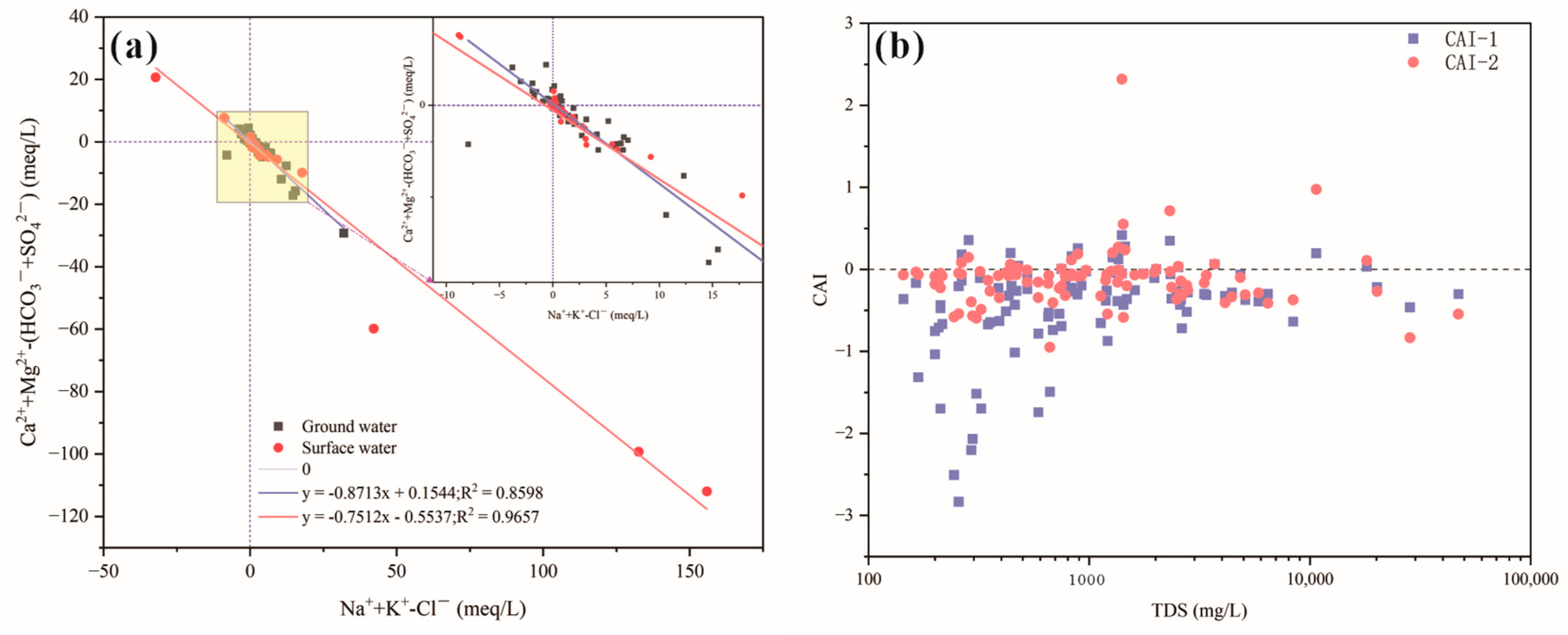
4.1.3. Relationship between Ions in Water
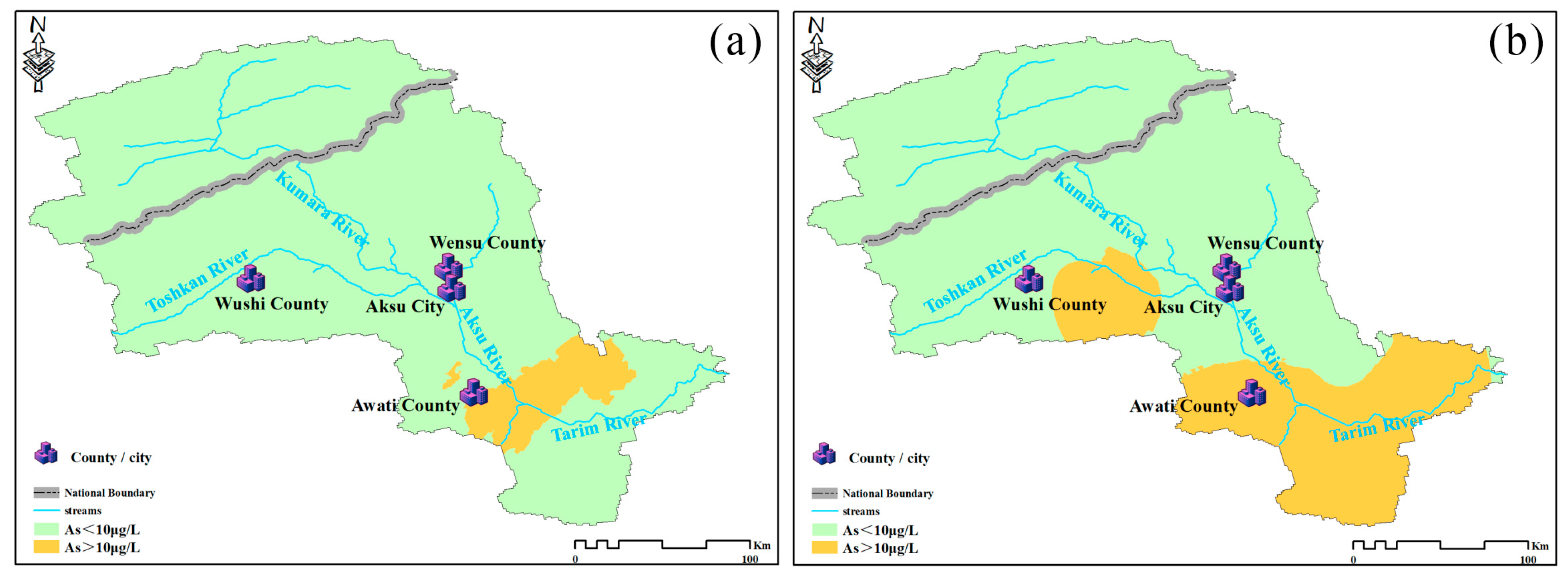
4.2. Spatial Distribution of Arsenic (As)
4.3. Source Appointment by Principal Component Analysis (PCA)
4.4. Major Factors Controlling Hydrogeochemical Processes
4.4.1. Water−Rock Interaction Mechanism
4.4.2. Cation-Exchange Reaction
4.4.3. Main Weathering Processes and Evolution Mechanisms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niazi, N.K.; Bibi, I.; Fatimah, A.; Shahid, M.; Javed, M.T.; Wang, H.; Ok, Y.S.; Bashir, S.; Murtaza, B.; Saqib, Z.A.; et al. Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: Morphological and physiological response. Int. J. Phytoremediation 2017, 19, 670–678. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Ivy, N.; Mukherjee, T.; Bhattacharya, S.; Ghosh, A.; Sharma, P. Arsenic contamination in groundwater and food chain with mitigation options in Bengal delta with special reference to Bangladesh. Environ. Geochem. Health 2023, 45, 1261–1287. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Wen, X.; Wu, J.; Wu, M.; Yu, H.; Zhang, C. Comprehensive Probabilistic Health Risk Assessment for Exposure to Arsenic and Cadmium in Groundwater. Environ. Manag. 2021, 67, 779–792. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, O.; Bakar, C.; Simsek, C.; Baba, A.; Elci, A.; Gurleyuk, H.; Mutlu, M.; Cakir, A. The Health Risk Associated with Chronic Diseases in Villages with High Arsenic Levels in Drinking Water Supplies. Expo. Health 2017, 9, 261–273. [Google Scholar] [CrossRef]
- Rafiq, M.; Shahid, M.; Shamshad, S.; Khalid, S.; Niazi, N.K.; Abbas, G.; Saeed, M.F.; Ali, M.; Murtaza, B. A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. J. Soils Sediments 2018, 18, 2271–2281. [Google Scholar] [CrossRef]
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Castor, J.; Portugal, L.; Ferrer, L.; Guzmán-Mar, J.; Hernández-Ramírez, A.; Cerdà, V.; Hinojosa-Reyes, L. Arsenic fractionation in agricultural soil using an automated three-step sequential extraction method coupled to hydride generation-atomic fluorescence spectrometry. Anal. Chim. Acta 2015, 874, 1–10. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Ji, Y.; Wang, Y.; Su, Z.; Elumalai, V. Groundwater Arsenic and Fluoride and Associated Arsenicosis and Fluorosis in China: Occurrence, Distribution and Management. Expo. Health 2020, 12, 355–368. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Ma, T.; Xie, X.; Deng, Y.; Gan, Y. Genesis of geogenic contaminated groundwater: As, F and I. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2895–2933. [Google Scholar] [CrossRef]
- Rodríguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Xu, J.-M.; Gomez, M.A.; Shi, Z.-L.; Li, S.-F.; Zang, S.-Y. Arsenic concentration, speciation, and risk assessment in sediments of the Xijiang River basin, China. Environ. Monit. Assess. 2019, 191, 663. [Google Scholar] [CrossRef]
- Li, M.; Ma, Z.; Niu, G.-Y. Modeling spatial and temporal variations in soil moisture in China. Chin. Sci. Bull. 2011, 56, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tu, Z.; Zhou, J.; Han, S.; Sun, Y.; Liu, X.; Liu, J.; Liu, J. Distribution, dynamic and influence factors of groundwater arsenic in the Manas River Basin in Xinjiang, P.R.China. Appl. Geochem. 2022, 146, 105441. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Y.; Zhou, J.; Guo, H.; Li, Q.; Jia, R.; Chen, Y.; Zhao, J. Distribution of groundwater arsenic in Xinjiang, P.R. China. Appl. Geochem. 2017, 77, 116–125. [Google Scholar] [CrossRef]
- Yang, P.; Xia, J.; Zhan, C.; Mo, X.; Chen, X.; Hu, S.; Chen, J. Estimation of water consumption for ecosystems based on Vegetation Interfaces Processes Model: A case study of the Aksu River Basin, Northwest China. Sci. Total. Environ. 2018, 613–614, 186–195. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, K.; Fendorf, S.; Deng, Y.; Xie, X. Sedimentogenesis and hydrobiogeochemistry of high arsenic Late Pleistocene-Holocene aquifer systems. Earth-Science Rev. 2019, 189, 79–98. [Google Scholar] [CrossRef]
- Benner, S.G.; Fendorf, S. Arsenic in South Asia Groundwater. Geogr. Compass 2010, 4, 1532–1552. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Wang, Y.; Li, P.; Guo, Q.; Jiang, Z.; Liu, M. Environmental biogeochemistry of high arsenic geothermal fluids. Appl. Geochem. 2018, 97, 81–92. [Google Scholar] [CrossRef]
- Apollaro, C.; Tripodi, V.; Vespasiano, G.; De Rosa, R.; Dotsika, E.; Fuoco, I.; Critelli, S.; Muto, F. Chemical, isotopic and geotectonic relations of the warm and cold waters of the Galatro and Antonimina thermal areas, southern Calabria, Italy. Mar. Pet. Geol. 2019, 109, 469–483. [Google Scholar] [CrossRef]
- Apollaro, C.; Marini, L.; De Rosa, R. Use of reaction path modeling to predict the chemistry of stream water and groundwater: A case study from the Fiume Grande valley (Calabria, Italy). Environ. Geol. 2007, 51, 1133–1145. [Google Scholar] [CrossRef]
- Liu, T.; Shao, F.; Zhang, Z.; Li, T. Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution. Water 2023, 15, 926. [Google Scholar] [CrossRef]
- Ke, T.; Zhang, D.; Guo, H.; Xiu, W.; Zhao, Y. Geogenic arsenic and arsenotrophic microbiome in groundwater from the Hetao Basin. Sci. Total. Environ. 2022, 852, 158549. [Google Scholar] [CrossRef]
- Qiao, W.; Guo, H.; He, C.; Shi, Q.; Xing, S.; Gao, Z. Identification of processes mobilizing organic molecules and arsenic in geothermal confined groundwater from Pliocene aquifers. Water Res. 2021, 198, 117140. [Google Scholar] [CrossRef]
- Li, D.; Gao, X.; Wang, Y.; Luo, W. Diverse mechanisms drive fluoride enrichment in groundwater in two neighboring sites in northern China. Environ. Pollut. 2018, 237, 430–441. [Google Scholar] [CrossRef] [PubMed]

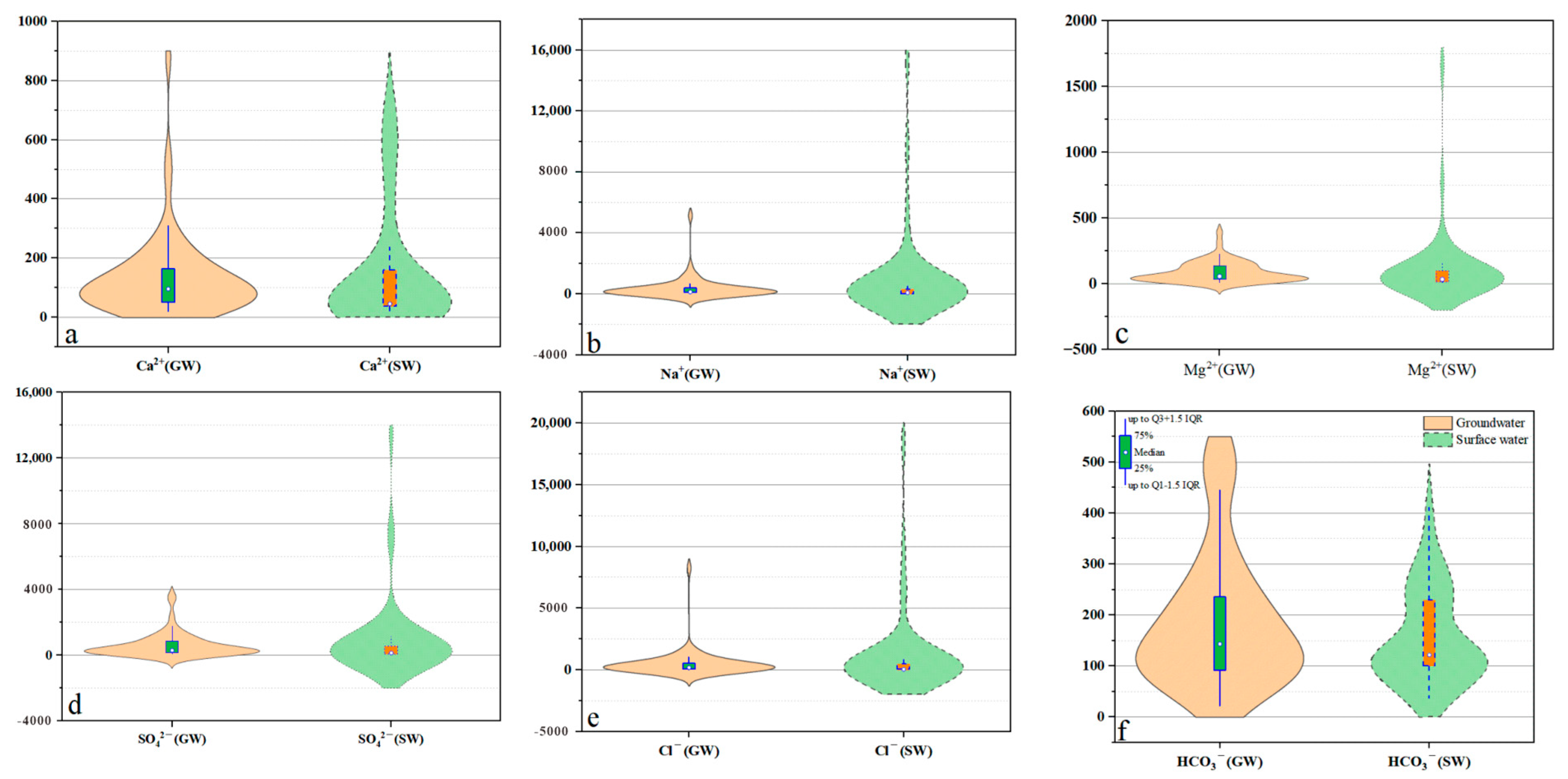



| Groundwater (n = 59) | Surface Water (n = 41) | WHO 2022 [23] Standard | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Ave ± s.e | s.d. | Min | Max | Ave ± s.e | s.d. | |||
| K+ | mg/L | 1 | 86 | 10.9 ± 1.86 | 14.3 | 1.30 | 251 | 16.3 ± 6.76 | 43.3 | 12 |
| Na+ | mg/L | 17.4 | 5143 | 375 ± 95.0 | 730 | 5.98 | 15,371 | 989 ± 450 | 2880 | 200 |
| Ca2+ | mg/L | 20.7 | 879 | 134 ± 18.2 | 140 | 13.5 | 739 | 152 ± 31.8 | 203 | 200 |
| Mg2+ | mg/L | 5.70 | 394 | 88.6 ± 10.2 | 78.3 | 5.20 | 1663 | 115 ± 44 | 281 | 150 |
| As | μg/L | 0.05 | 46.9 | 7.23 ± 1.36 | 10.4 | 0.50 | 66.98 | 11.2 ± 2.61 | 16.7 | 10 |
| Cl− | mg/L | 18.9 | 8258 | 501 ± 144 | 1108 | 4.96 | 18,403 | 1238 ± 536 | 3430 | 250 |
| SO42− | mg/L | 40.6 | 3646 | 616 ± 95.1 | 731 | 20.6 | 13,450 | 1032 ± 397 | 2543 | 250 |
| HCO3− | mg/L | 21.4 | 540 | 192 ± 17.7 | 136 | 36.6 | 412 | 156 ± 14.4 | 92.4 | 250 |
| CO32− | mg/L | 0 | 42.0 | 13.4 ± 1.20 | 9.23 | 0 | 51 | 11.5 ± 1.98 | 12.7 | 250 |
| F− | mg/L | 0.20 | 4.70 | 1.6 ± 0.14 | 1.04 | 0.32 | 5.30 | 1.62 ± 0.19 | 1.20 | 1.5 |
| TDS | mg/L | 200 | 18,112 | 1847 ± 346 | 2660 | 144 | 47,128 | 3525 ± 1385 | 8868 | 1000 |
| PH | / | 7.41 | 8.75 | 8.16 ± 0.05 | 0.35 | 7.26 | 8.69 | 8.17 ± 0.05 | 0.35 | 6.5–8.5 |
| TH | mg/L | 78.1 | 3443 | 695 ± 81.5 | 626 | 64.1 | 8668 | 853 ± 244 | 1563 | 300 |
| Index | PCA Method | |||||
|---|---|---|---|---|---|---|
| Groundwater (n = 59) | Surface Water (n = 41) | |||||
| F1 | F2 | F3 | F1 | F2 | F3 | |
| 1 | 2 | 3 | 1 | 2 | 3 | |
| K+ | 0.87 | −0.22 | 0.10 | 0.96 | 0.02 | 0.04 |
| Na+ | 0.90 | 0.33 | −0.17 | 0.96 | −0.10 | 0.14 |
| Ca2+ | 0.95 | 0.16 | −0.05 | 0.87 | −0.16 | −0.01 |
| Mg2+ | 0.89 | −0.24 | −0.05 | 0.95 | 0.06 | −0.02 |
| As | −0.11 | 0.37 | 0.66 | −0.16 | 0.46 | 0.71 |
| Cl− | 0.85 | 0.40 | −0.21 | 0.97 | −0.12 | 0.13 |
| SO42− | 0.95 | 0.02 | −0.04 | 0.99 | −0.01 | 0.06 |
| HCO3− | 0.51 | −0.75 | 0.23 | 0.57 | 0.56 | −0.42 |
| CO32− | 0.20 | −0.78 | −0.02 | 0.29 | 0.85 | −0.28 |
| F− | 0.51 | 0.31 | 0.60 | 0.50 | 0.18 | 0.36 |
| TDS | 0.95 | 0.24 | −0.14 | 0.98 | −0.08 | 0.11 |
| PH | −0.66 | 0.27 | −0.33 | −0.53 | 0.40 | 0.29 |
| TH | 0.98 | −0.04 | −0.06 | 0.98 | −0.01 | −0.02 |
| Eigenvalues | 7.75 | 1.93 | 1.06 | 8.33 | 1.50 | 1.02 |
| Variance (%) | 59.60 | 14.84 | 8.18 | 64.05 | 11.55 | 7.86 |
| Cumulative (%) | 59.60 | 74.44 | 82.62 | 64.05 | 75.59 | 83.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, F.; Wang, W.; He, J. Groundwater–Surface Water Exchange and Spatial Distribution of Arsenic in Arid and Semi-Arid Regions: The Case of Aksu River in Xinjiang, Northwestern China. Water 2023, 15, 2391. https://doi.org/10.3390/w15132391
Shao F, Wang W, He J. Groundwater–Surface Water Exchange and Spatial Distribution of Arsenic in Arid and Semi-Arid Regions: The Case of Aksu River in Xinjiang, Northwestern China. Water. 2023; 15(13):2391. https://doi.org/10.3390/w15132391
Chicago/Turabian StyleShao, Fengjun, Wenfeng Wang, and Jing He. 2023. "Groundwater–Surface Water Exchange and Spatial Distribution of Arsenic in Arid and Semi-Arid Regions: The Case of Aksu River in Xinjiang, Northwestern China" Water 15, no. 13: 2391. https://doi.org/10.3390/w15132391






