Preparation of Porous Carbon Materials as Adsorbent Materials from Phosphorus-Doped Watermelon Rind
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation Method
2.3. Analysis Method
2.3.1. Scanning Electron Microscopy Test Analysis
2.3.2. FTIR Spectrum
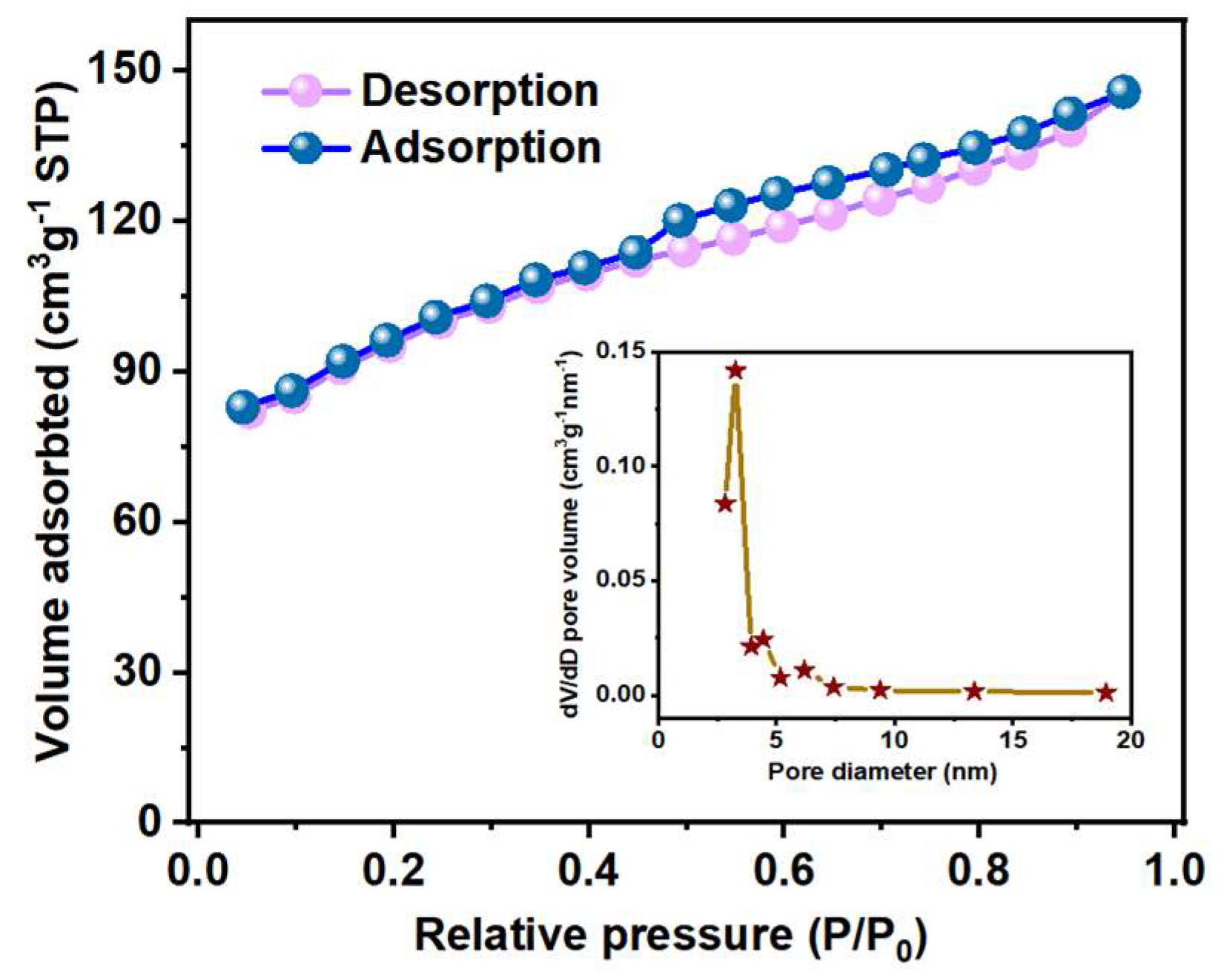
2.3.3. Analysis of Nitrogen Adsorption–Desorption Curve
2.3.4. Raman Spectroscopy
3. Results and Discussion
3.1. Characterization of WC-M Materials
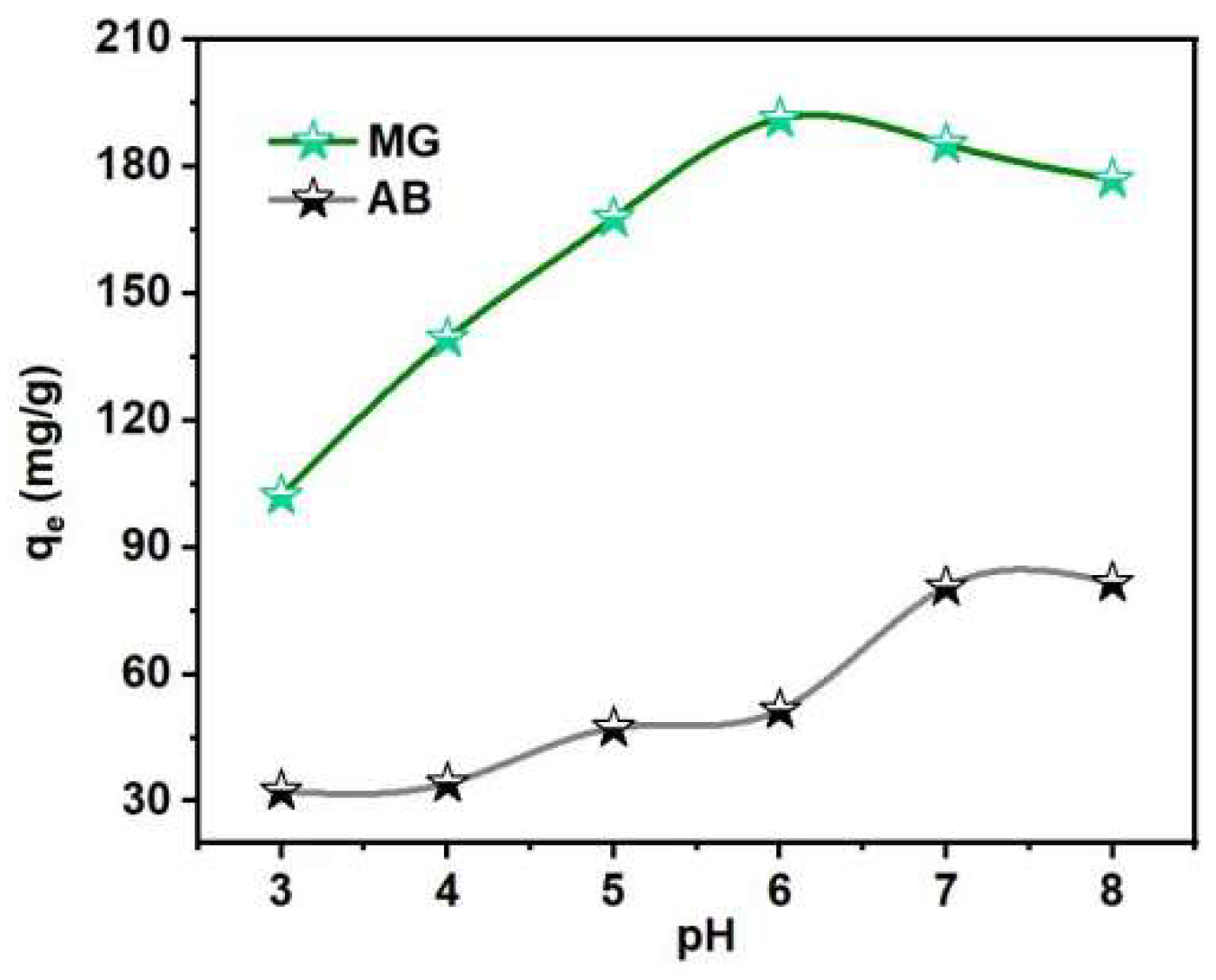
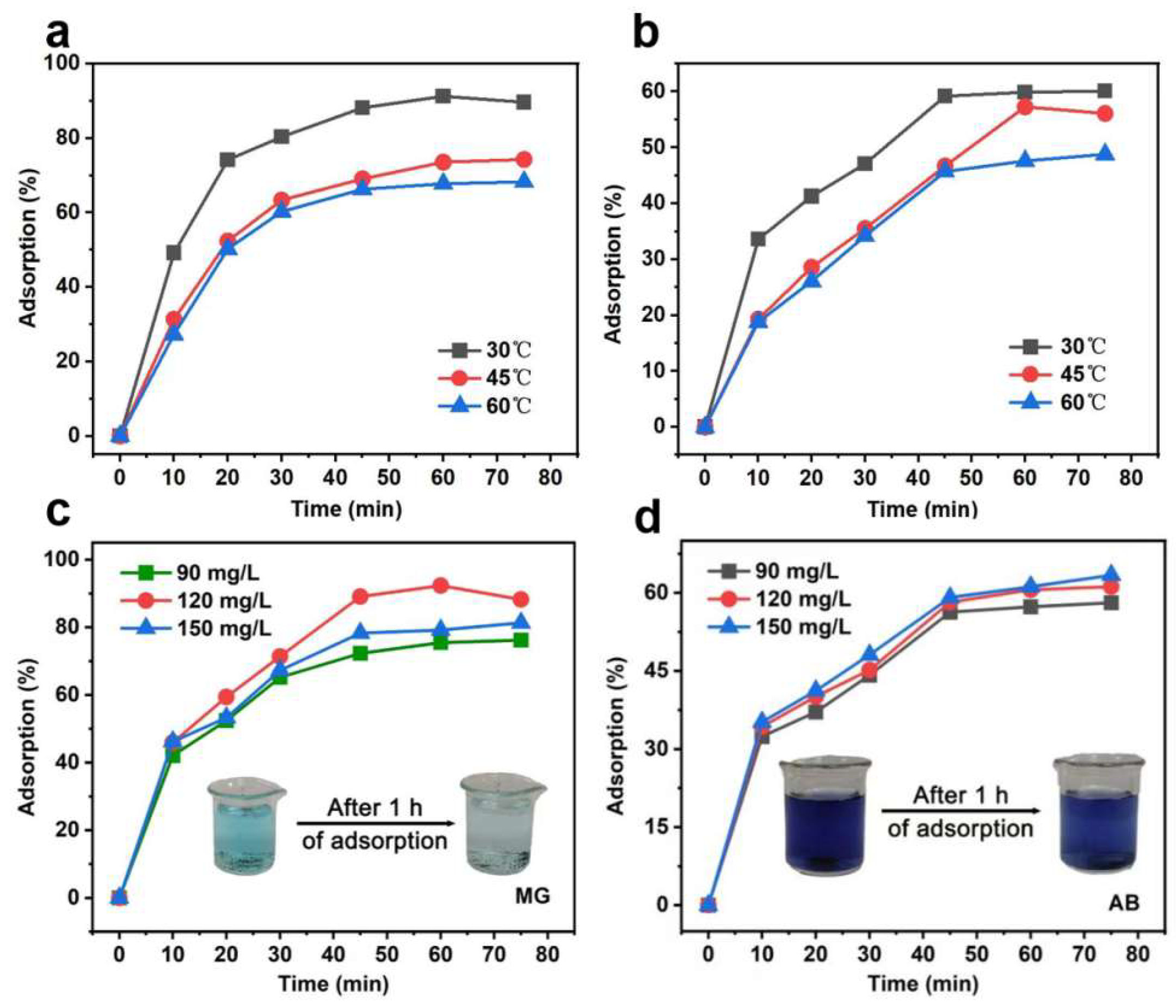
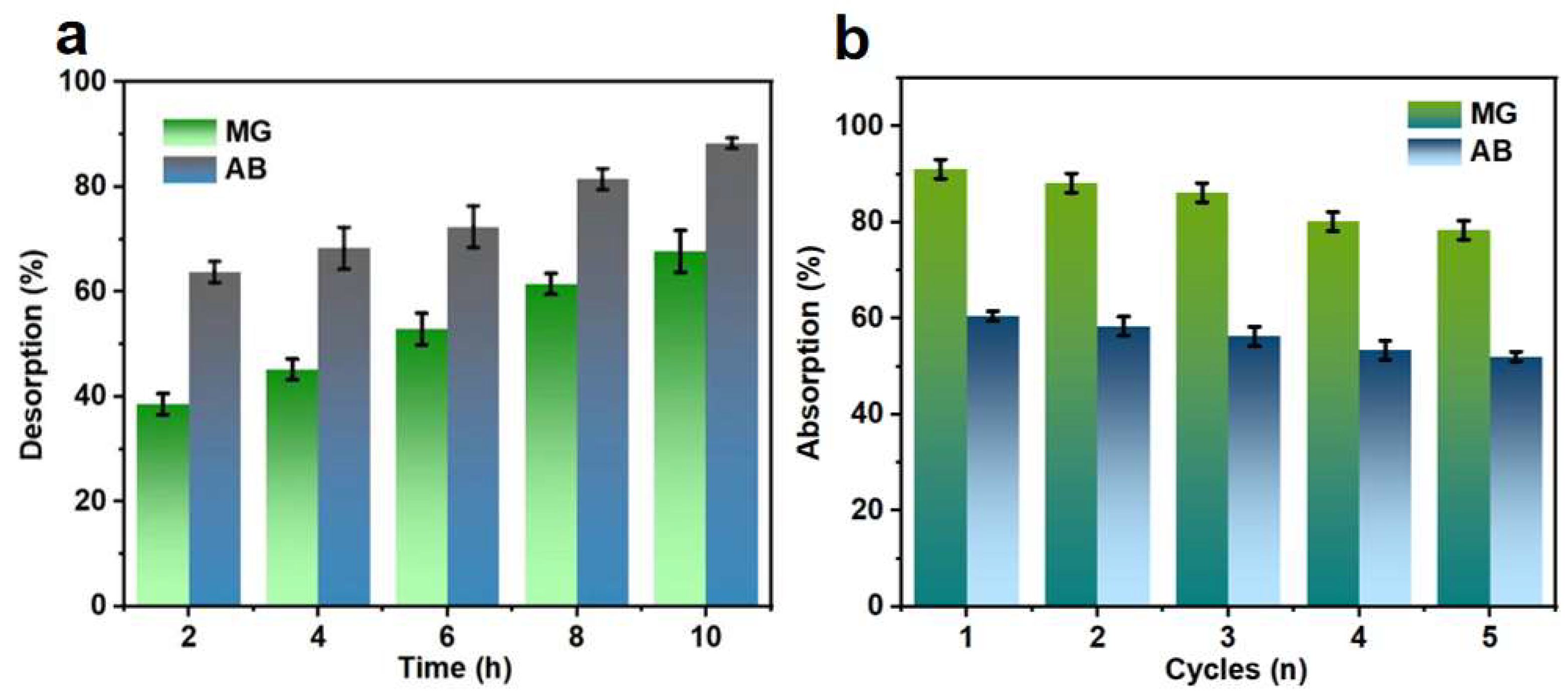
3.2. Thermodynamic and Kinetic Analysis of WC-M Material Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, K.C.; Wu, J.Y.; Liou, D.J.; Sz-Chwun, J.H. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003, 101, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Susana, R.C. Dye removal by immobilised fungi. Biotechnol. Adv. 2009, 27, 227–235. [Google Scholar]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid. Interface. Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Belgin, G.; Berkant, K.; Murat, A.G.; Arif, H. Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods. J. Hazard. Mater. 2009, 168, 129–136. [Google Scholar]
- Sittichok, K.; Mail, H. Remediation of wastewater from pulp and paper mill industry by the electrochemical technique. Chen. Eng. J. 2009, 151, 228–234. [Google Scholar]
- Anuradha, N.K.; Umesh, B.J.; Jyoti, P.J.; Vishwas, A.B.; Sanjay, P.G. Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii Hook. Bioresour. Technol. 2009, 100, 4104–4110. [Google Scholar]
- Wei, X.; Jiang, X.P.; Liu, X.; Zhou, W.M.; Garba, Z.N.; Lawan, L.; Wang, L.W.; Yuan, Z.H. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar]
- Zemskova, L.A.; Voit, A.V.; Sheveleva, I.V.; Mironova, L.N. The sorption properties of chitosan-carbon fibrous materials. Russ. J. Phys. Chem. 2007, 81, 1658–1661. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C. Biomass: A renewable source of fuels, chemicals and carbon materials. Molecules 2020, 25, 5217. [Google Scholar] [CrossRef]
- Rezazad, H.; Vahdati-Khajeh, S.; Eftekhari-Sis, B. Egg yolk biomass derived carbon material as a highly efficient and reusable hydrophobic oil-absorbent. J. Porous Mater. 2020, 27, 1439–1446. [Google Scholar] [CrossRef]
- Dai, Y.J.; Mihara, Y.; Tanaka, S.; Watanabe, K.; Terui, N. Nitrobenzene-adsorption capacity of carbon materials released during the combustion of woody biomass. J. Hazard. Mater. 2010, 174, 776–781. [Google Scholar] [CrossRef]
- Quan, G.X.; Yan, J.L.; Ding, C. Preparation and Characterization of Biomass Carbon Adsorbent from Rice Husk and Its Adsorption Properties on p-Chlorophenol; Iceesd 2011; Shanghai University of Electric Power: Shanghai, China, 2011; Volume 356–360, pp. 510–513. [Google Scholar]
- Jiang, L.L.; Sheng, L.Z.; Fan, Z.J. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Lyu, D.M.; Mahtab, N.; Ateeq, S.; Zhou, X.M.; Donald, L.S. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sust. Energ. Rev. 2021, 139, 110691. [Google Scholar]
- André, B.; Tim, K.; Jdo, M.; Bernd, M.; Anja, N.; Felix, R.; Thomas, R.; Roland, B.; Thomas, H.; Christian, B.; et al. How to measure the impact of biogenic residues, wastes and by-products: Development of a national resource monitoring based on the example of Germany. Biomass Bioenergy 2019, 127, 105275. [Google Scholar]
- Wang, Z.H.; Shen, D.K.; Wu, C.F.; Gu, H. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.C.; Liu, L.; Xiong, J.J.; Luo, Y.M.; Cai, L.C.; Qian, Y.; Wang, H.; Mu, L.W.; Feng, X.; Lu, X.H.; et al. Advanced material-oriented biomass precise reconstruction: A review on porous carbon with inherited natural structure and created artificial structure by post-treatment. Macromol. Biosci. 2022, 22, 2100479. [Google Scholar] [CrossRef]
- Soumya, R.; Sajini, V. Sustainable carbon nanomaterials: Recent advances and its applications in energy and environmental remediation. J. Environ. Chem. Eng. 2016, 4, 835–856. [Google Scholar]
- Aumber, A.; Lim, T.M.; Anh, N.P. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–79. [Google Scholar]
- Ding, J.; He, W.Z.; Yu, C.; Liu, Z.L.; Deng, C.J.; Zhu, H.X. Waste biomass derived carbon supported Mo2C/C composite materials for heavy metal adsorption and hydrogen evolution reaction. Mater. Today Commun. 2022, 31, 103646. [Google Scholar] [CrossRef]
- Jia, Z.G.; Li, Z.Y.; Ni, T.; Li, S.B. Adsorption of low-cost absorption materials based on biomass (Cortaderia selloana flower spikes) for dye removal: Kinetics, isotherms and thermodynamic studies. J. Mol. Liq. 2017, 229, 285–292. [Google Scholar] [CrossRef]
- Lu, Y.C.; Kooh, M.R.R.; Lim, L.B.L.; Priyantha, N. Effective and simple NaOH-modification method to remove methyl violet dye via lpomoea aquatica roots. Adsorp. Sci. Technol. 2021, 2021, 5932222. [Google Scholar] [CrossRef]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chao, C.T.C.; Chau, Y.F.C.; Mahadi, A.H.; Chiang, H.P.; Hassan, N.H.H.; Thotagamuge, R. The use of gigantochloa bamboo-derived biochar for the removal of mMethylene blue from aqueous solution. Adsorp. Sci. Technol. 2022, 2022, 8245797. [Google Scholar]
- Hung, N.Y.; Nguyet, B.T.M.; Nghi, N.H.; Thanh, N.M.; Quyen, N.D.V.; Nguyen, V.T.; Nhiem, D.N.; Khieu, D.Q. Highly effective adsorption of organic dyes from aqueous solutions on longan seed-derived activated carbon. Environ. Eng. Res. 2023, 28, 220116. [Google Scholar] [CrossRef]
- Kumari, P.; Tripathi, K.M.; Awasthi, K.; Gupta, R. Biomass-derived carbon nano-onions for the effective elimination of organic pollutants and oils from water. Environ. Sci. Pollut. Res. 2023, 30, 71048–71062. [Google Scholar] [CrossRef] [PubMed]
- Ratnakaram, V.N.; Rao, C.G.P.; Sree, S. Simultaneous Saccharification and Fermentation of Watermelon Waste for Ethanol Production; Itsfew; Vellore Institute of Technology: Vellore, India, 2018; Volume 2, pp. 196–202. [Google Scholar]
- Li, L.; Wu, Z.H.; Zhang, Z.; Zhao, D.Y. Watermelon peel-derived nitrogen-doped porous carbon as a superior oxygen reduction electrocatalyst for zinc-air batteries. ChemElectroChem 2021, 8, 4790–4796. [Google Scholar] [CrossRef]
- Wang, J.M.; Wang, C.W. Watermelon-like metallic Co/graphene-like nanohybrids from electrochemical exfoliation of anthracite coal as superior oxygen reduction reaction electrocatalyst. ACS Sustain. Chem. Eng. 2019, 7, 12457–12463. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, J.T.; Meng, Y.F.; Liu, Z.X.; Yu, N. Watermelon flesh-derived carbon aerogel with hierarchical porous structure for interfacial solar steam generation. Sol. Rrl. 2022, 6, 2200270. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.C.; Cheng, J.X.; Ding, C.X.; Chen, C.H. Watermelon used as a novel carbon source to improve the rate performance of iron oxide electrodes for lithium ion batteries. Electrochim. Acta 2013, 102, 306–311. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, J.H.; Guo, Z.Y.; Wong, S.L. Watermelon peel-derived heteroatom-doped hierarchical porous carbon as a high-performance electrode material for supercapacitors. ChemElectroChem 2021, 8, 1196–1203. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Preparation and characterization of mesoporous activated carbons from waste watermelon rind by using the chemical activation method with zinc chloride. Arab. J. Chem. 2019, 12, 3621–3627. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Barbosa, J.J.; López-Velandia, C.; Maldonado, A.P. Removal of lead(II) and zinc(II) ions from aqueous solutions by adsorption onto activated carbon synthesized from watermelon shell and walnut shell. Adsorption 2013, 19, 675–685. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified removal of copper from waste water using activated watermelon based biosorbent in the presence of ultrasound. Ultrason Sonochem. 2016, 30, 116–122. [Google Scholar] [CrossRef]
- Anandaraj, B.; Eswaramoorthi, S.; Rajesh, T.P.; Aravind, J.; Babu, P.S. Chromium(VI) adsorption by codium tomentosum: Evidence for adsorption by porous media from sigmoidal dose–response curve. Int. J. Environ. Sci. Technol. 2018, 15, 2595–2606. [Google Scholar] [CrossRef]
- Chigbundu, E.C.; Adebowale, K.O. Equilibrium and fractal-like kinetic studies of the sorption of acid and basic dyes onto watermelon shell (Citrullus vulgaris). Cellulose 2017, 24, 4701–4714. [Google Scholar] [CrossRef]
- Husein, D.Z.; Aazam, E.; Battia, M. Adsorption of cadmium(II) onto watermelon rind under microwave radiation and application into surface water from jeddah, saudi arabia. Arab. J. Sci. Eng. 2017, 42, 2403–2415. [Google Scholar] [CrossRef]
- Li, R.S.; Zheng, F.Y.; Zhang, X.H.; Hu, J.D.; Xu, C.Y.; Zhang, Y.C. Phosphorus and iron doped nitrogen-containing carbon derived from biomass for oxygen reduction under various pH conditions. Int. J. Hydrog. Energ. 2020, 45, 28651–28663. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Swaminathan, K.; Kuz, C.S.; Yun, S.E. Comparative studies on removal of Congo red by native and modified mycelial pellets of Trametes versicolor in various reactor modes. Bioresour. Technol. 2008, 99, 1080–1088. [Google Scholar] [CrossRef]
- Neeta, A.S. Decolorization of Erythrosine B by Rhizopus arrhizus biomass. Appl. Water Sci. 2018, 8, 205. [Google Scholar]
- Madhavakrishnan, S.; Manickavasagam, K.; Vasanthakumar, R.; Rasappan, K.; Mohanraj, R.; Pattabhi, S. Adsorption of crystal violet dye from aqueous solution using ricinus communis pericarp carbon as an adsorbent. E-J. Chem. 2009, 6, 1109–1116. [Google Scholar] [CrossRef] [Green Version]
- Kannamba, B.; Laxma Reddy, K.; AppaRao, B.V. Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J. Hazard. Mater. 2010, 175, 939–948. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, H.X.; Zhong, C.Q.; Wu, D.J. Biosorption of Cr(VI) by immobilized waste biomass from polyglutamic acid production. Sci. Rep. 2020, 10, 3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanafi, N.A.M.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yousef, T.A.; Duaij, O.K.A.; Alsaiari, N.S. Optimized removal process and tailored adsorption mechanism of crystal violet and methylene blue dyes by activated carbon derived from mixed orange peel and watermelon rind using microwave-induced ZnCl2 activation. Biomass Convers. Biorefin. 2022, 14, 1–13. [Google Scholar] [CrossRef]
- Aboua, K.N.; Yobouet, Y.A.; Yao, K.B.; Gone, D.L.; Trokourey, A. Investigation of dye adsorption onto activated carbon from the shells of Macoré fruit. J. Environ. Manag. 2015, 156, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef]
- El Nemr, A.; Shoaib, A.G.M.; El Sikaily, A.; Safaa; Mohamed, A. E.A.; Hassan, A.F. Utilization of green alga ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of Direct Red 23 dye from aquatic environment. Carbon Lett. 2022, 32, 153–168. [Google Scholar] [CrossRef]
- Panahi, M.; Behnam, S. Biosorption of Malachite Green dye by the brown alga Dictyota cervicornis: Kinetics and isotherm study. Color. Technol. 2018, 134, 292–298. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Kumar, A.; Naushad, M.; Du, B.; Ahamad, T.; Ghfar, A.A.; Alqadami, A.A.; Stadler, F.J. Honeycomb structured activated carbon synthesized from Pinus roxburghii cone as effective bioadsorbent for toxic malachite green dye. J. Water Process Eng. 2019, 32, 100931. [Google Scholar] [CrossRef]
- Ayan, E.M.; Toptas, A.; Kibrislioglu, G.; Yalcinkaya, E.E.S.; Yanik, J. Biosorption of dyes by natural and activated vine stem. Interaction between biosorbent and dye. Clean 2011, 39, 406–412. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Cortes-Arriagada, D.; Rojas-Mayorga, C.K.; Pineda-Urbina, K.; Muniz-Valencia, R.; Gonzalez, J. Importance of the interaction adsorbent-adsorbate in the dyes adsorption process and DFT modeling. J. Mol. Struct. 2020, 1203, 127398. [Google Scholar] [CrossRef]
- Brito, M.J.P.; Veloso, C.M.; Santos, L.S.; Renata, F.B.C.; Fontan, R.D.I. Adsorption of the textile dye Dianix (R) royal blue CC onto carbons obtained from yellow mombin fruit stones and activated with KOH and H3PO4: Kinetics, adsorption equilibrium and thermodynamic studies. Powder Technol. 2018, 339, 334–343. [Google Scholar] [CrossRef]
- Koyuncu, F.; Guzel, F. Use of new nanoporous carbon produced from Mandarin (Citrus reticulata) industrial processing waste to remove anionic and cationic dyes. Sep. Sci. Technol. 2021, 56, 1001–1013. [Google Scholar] [CrossRef]
- Ma, J.F.; Huang, D.Q.; Zou, J.; Li, L.Y.; Kong, Y.; Komarneni, S. Adsorption of methylene blue and Orange II pollutants on activated carbon prepared from banana peel. J. Porous Mater. 2015, 22, 301–311. [Google Scholar] [CrossRef]
- Valix, M.; Cheung, W.H.; McKay, G. Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption. Chemosphere 2004, 56, 493–501. [Google Scholar] [CrossRef]
- Rida, K.; Chaibeddra, K.; Cheraitia, K. Adsorption of cationic dye methyl green from aqueous solution onto activated carbon prepared from BrachychitonPopulneus fruit shell. Indian J. Chem. Techn. 2020, 27, 51–59. [Google Scholar]



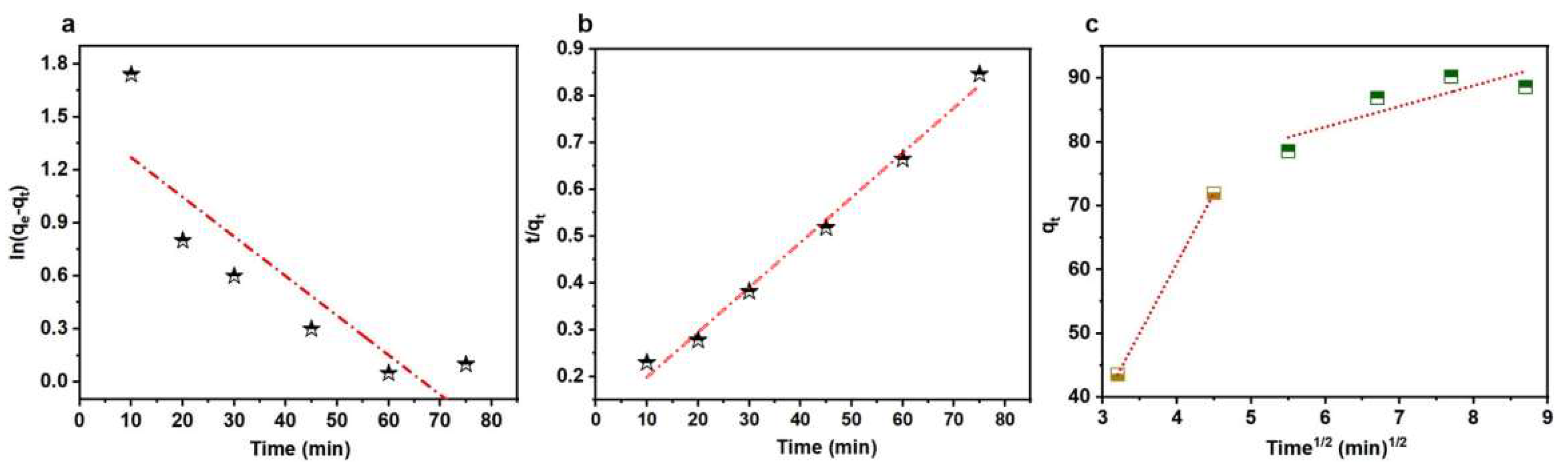
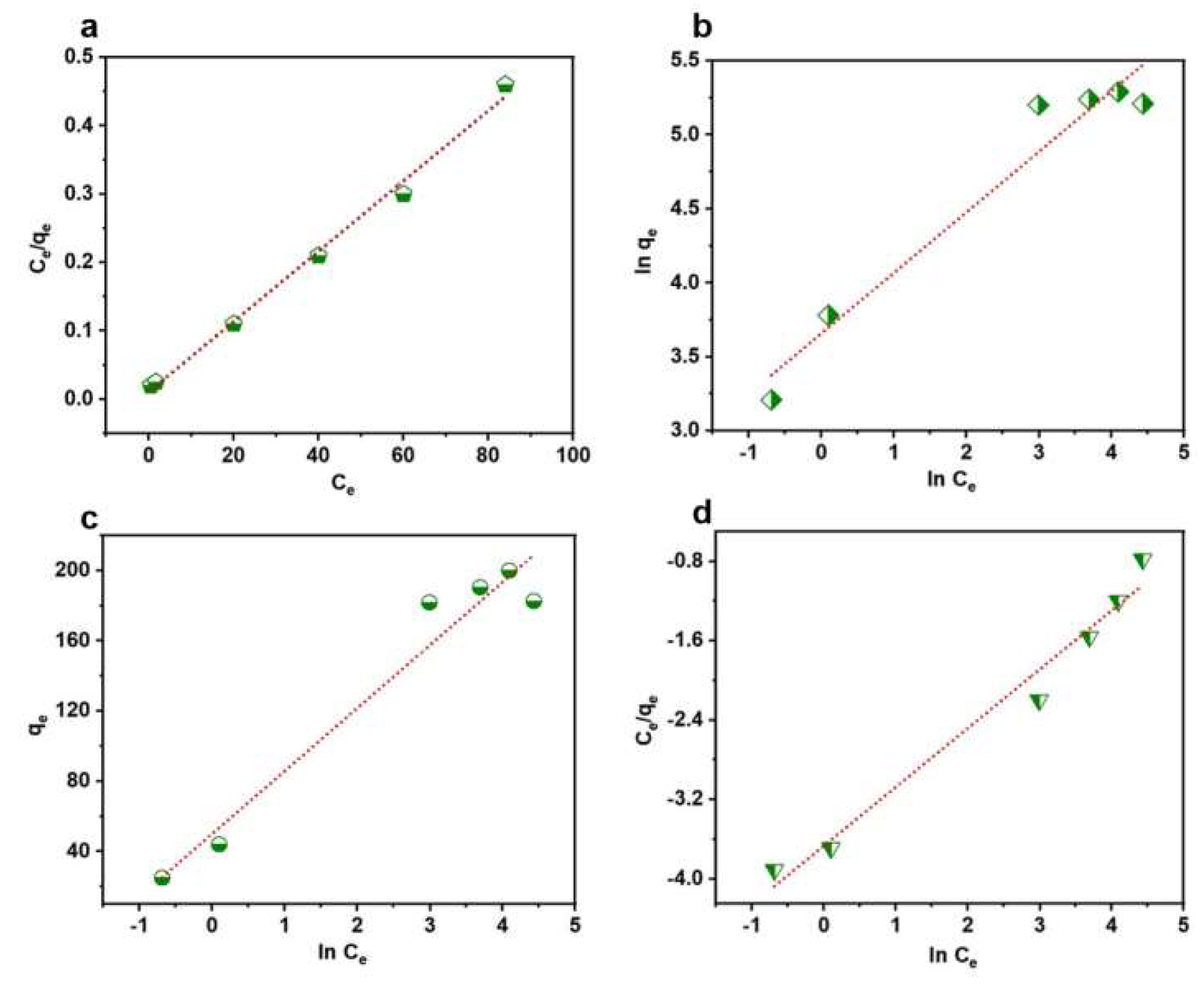
| Pseudo-First-Order | MG | Pseudo-Second-Order | MG |
|---|---|---|---|
| 3.12 | 0.0534 | ||
| 4.45 | 104.28 | ||
| 0.769 | 0.995 |
| Langmuir | MG | Freundlich | MG | Temkin | MG | Redlich-Peterson | MG |
|---|---|---|---|---|---|---|---|
| 194.553 | 38.815 | Bt | 35.853 | 0.592 | |||
| 0.469 | 2.444 | KT | 4.038 | A | 39.017 | ||
| 0.997 | 0.937 | R2 | 0.943 | 0.970 |
| Adsorbent | Dye Adsorbate | Maximum Capacity (mg.g−1) | Reference |
|---|---|---|---|
| Vine stem | Acid red 111 | 58.82 | [50] |
| Peach endocarp shell | Brilliant green 1 | 49.61 | [51] |
| Yellow mombin fruit stones (CA) | Dianix® royal blue CC (The version number of the software is origin2019) | 147.47 | [52] |
| Sour cherry | Yellow 18 | 76.318 | [46] |
| Green alga ulva lactuca | Direct Red 23 | 149.26 | [47] |
| Ricinus communis pericarp | Crystal violet | 125.25 | [41] |
| Citrus | Methylene blue | 313 | [53] |
| Banana peel-activated | Orange II | 333 | [54] |
| Bagasse | Acid blue | 391 | [55] |
| Brachychiton populneus fruit shell | Methyl green | 67.93 | [56] |
| Watermelon rind | Malachite green | 182.68 | This study |
| Active black | 81.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, P.; Yang, C.; Li, X.; Yi, D.; Wu, W. Preparation of Porous Carbon Materials as Adsorbent Materials from Phosphorus-Doped Watermelon Rind. Water 2023, 15, 2433. https://doi.org/10.3390/w15132433
Wei Y, Li P, Yang C, Li X, Yi D, Wu W. Preparation of Porous Carbon Materials as Adsorbent Materials from Phosphorus-Doped Watermelon Rind. Water. 2023; 15(13):2433. https://doi.org/10.3390/w15132433
Chicago/Turabian StyleWei, Yumeng, Penghui Li, Chi Yang, Xiaoyu Li, Dairenjie Yi, and Wenjuan Wu. 2023. "Preparation of Porous Carbon Materials as Adsorbent Materials from Phosphorus-Doped Watermelon Rind" Water 15, no. 13: 2433. https://doi.org/10.3390/w15132433
APA StyleWei, Y., Li, P., Yang, C., Li, X., Yi, D., & Wu, W. (2023). Preparation of Porous Carbon Materials as Adsorbent Materials from Phosphorus-Doped Watermelon Rind. Water, 15(13), 2433. https://doi.org/10.3390/w15132433








