Vapour Multicycle Sorption of a Cement–Bentonite Cutoff Wall Material: Hysteresis Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Sample Preparation and Pre-Conditioning
2.2.2. Test Method
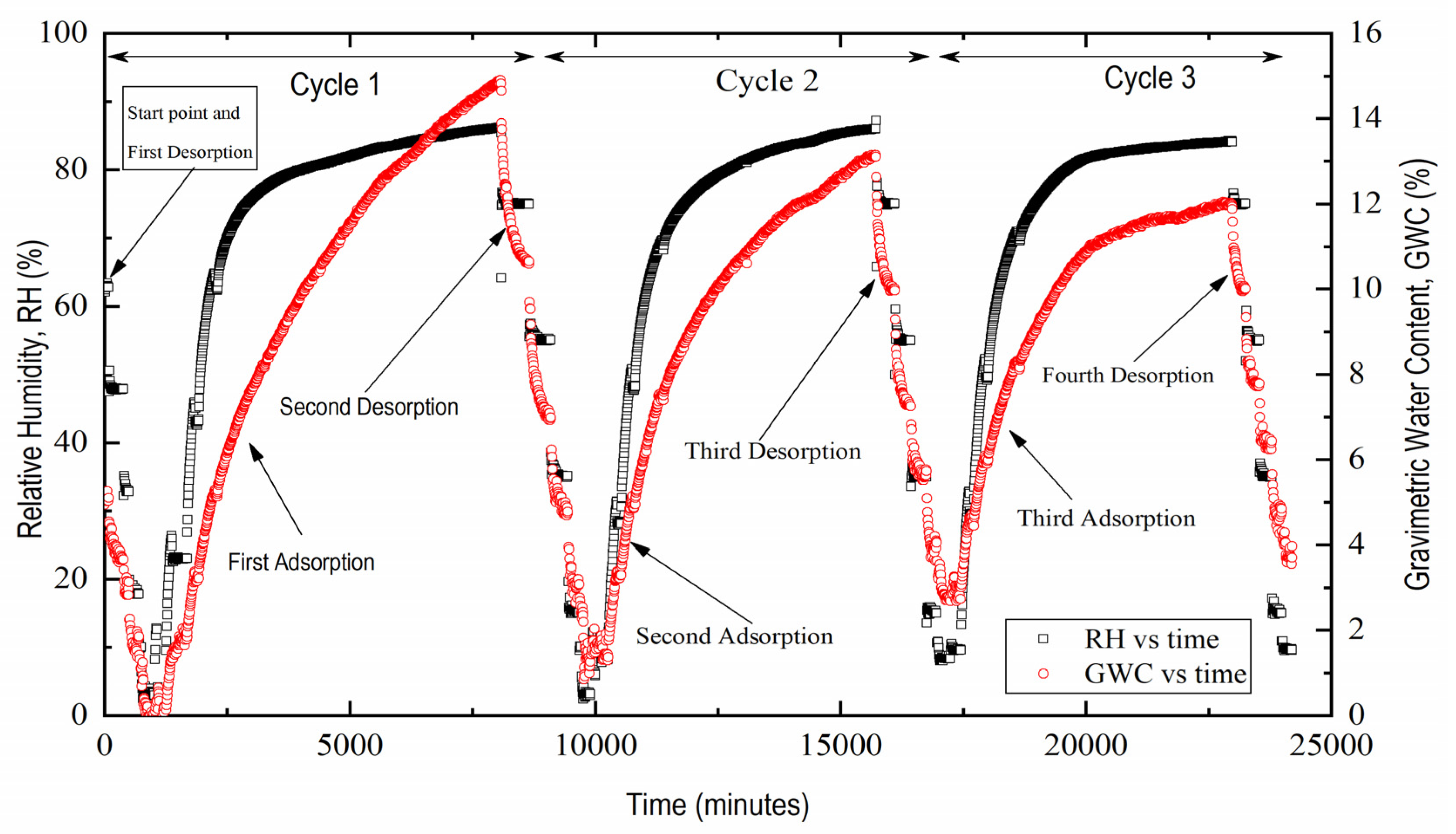
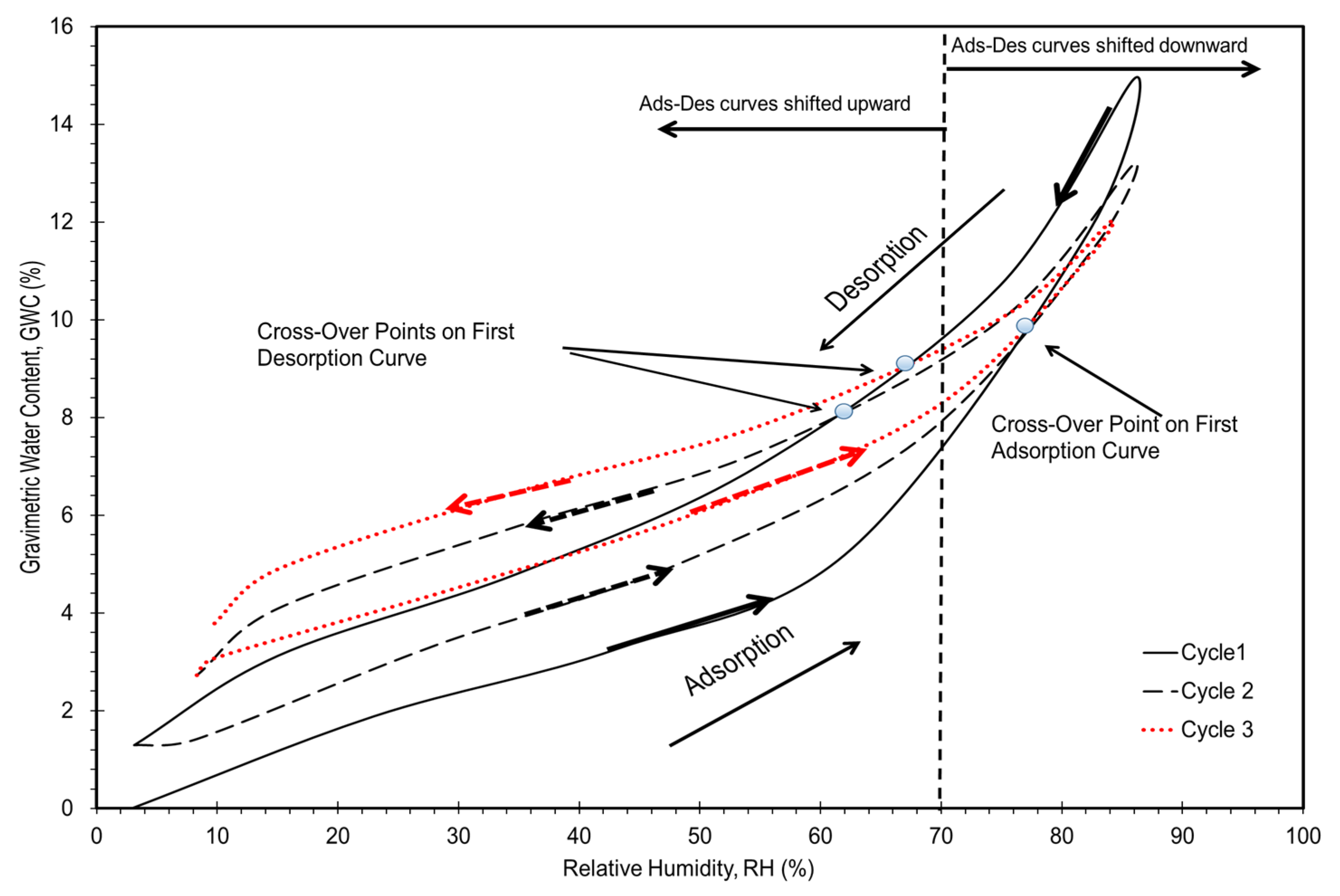
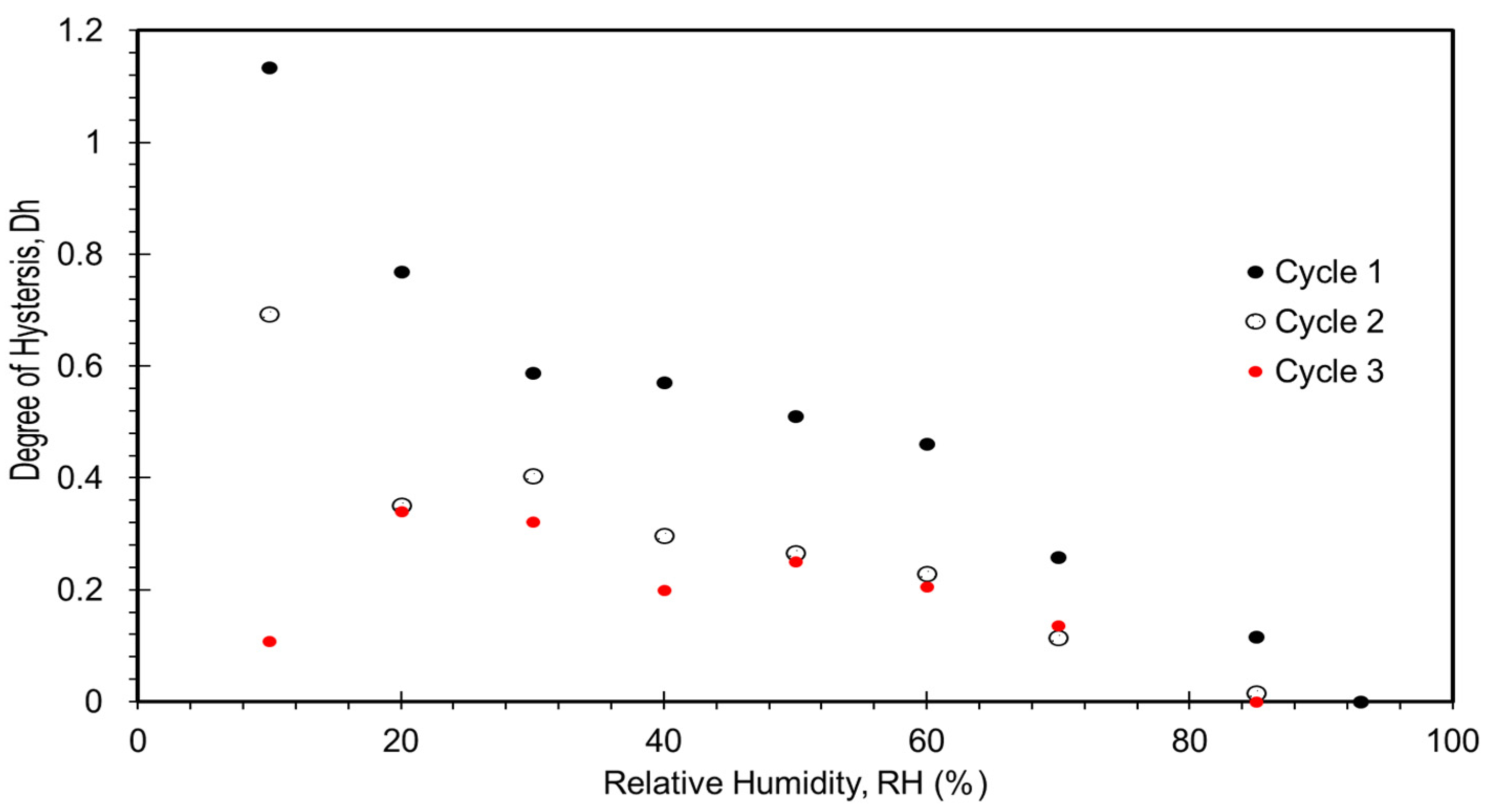
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ryan, C.R.; Day, S. Performance Evaluation of Cement-Bentonite Slurry Wall Mix Design; US EPA: Washington, DC, USA, 1986.
- Jefferis, S. Grouts and slurries. In Construction Materials Reference Book; Doran, D., Cather, B., Eds.; Routledge: Abingdon, UK; Taylor and Francis: Abingdon, UK, 2013; pp. 165–194. [Google Scholar]
- Evans, J.C.; Dawson, A.R.; Opdyke, S. Slurry walls for groundwater control: A comparison of U and US practice. In Proceedings of the ASCE/PENNDOT Central PA Geotechnical Conference, Philadelphia, PA, USA, 13–15 May 2002; pp. 1–9. [Google Scholar]
- Opdyke, S.M.; Evans, J.C. Slag-cement-bentonite slurry walls. J. Geotech. Geoenviron. Eng. 2005, 11, 673–681. [Google Scholar] [CrossRef]
- Jefferis, S. Cement-bentonite slurry systems. In Proceedings of the 4th International Conference on Grouting and Deep Mixing, New Orleans, LA, USA, 15–18 February 2012; pp. 1–24. [Google Scholar]
- Soga, K.; Joshi, K.D.; Evans, J.C. Cement bentonite cutoff walls for polluted sites. In Proceedings of the 1st International Symposium on Coupled Phenomena in Environmental Geotechnics, Torino, Italy, 1–3 July 2013; pp. 149–165. [Google Scholar]
- ICE (Institution of Civil Engineers). Specification for Construction of Slurry Trench Cut-Off Walls as Barriers to Pollution Migration; Thomas Telford: London, UK, 1999. [Google Scholar]
- Cermak, J.; Law, M. Cut-off performance of a soil-cement bentonite wall. In Proceedings of the 14th Pan-American Conference on Soil Mechanics and Geotechnical Engineering, Toronto, ON, Canada, 2–6 October 2011. [Google Scholar]
- Cermak, J.; Evans, J.C.; Tamaro, G.J. Evaluation of Soil-Cement-Bentonite Wall Performance-Effects of Backfill Shrinkage. In Proceedings of the 4th International Conference on Grouting and Deep Mixing, New Orleans, LA, USA, 15–18 February 2012; pp. 502–511. [Google Scholar]
- Joshi, K.D. Long-Term Engineering Performance and in-Situ Assessment of Cement-Bentonite Cut-Off Walls. Ph.D. Thesis, The University of Cambridge, Cambridge, UK, 2010. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- Espinosa, R.M.; Franke, L. Influence of the age and drying process on pore structure and sorption isotherms of hardened cement paste. Cem. Concr. Res. 2006, 36, 1969–1984. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W.; Bauer, A. Morphology of cementitious material during early hydration. Cem. Concr. Res. 2018, 107, 85–100. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; Volume 540. [Google Scholar]
- Zhang, Z.; Scherer, G.W. Physical and chemical effects of isopropanol exchange in cement-based materials. Cem. Concr. Res. 2021, 145, 106461. [Google Scholar] [CrossRef]
- Kumar, A.; Ketel, S.; Vance, K.K.; Oey, T.; Neithalath, N.; Sant, G. Water vapor sorption in cementitious material-Measurement, modeling and interpretation. Transp. Porous Media 2014, 103, 69–98. [Google Scholar] [CrossRef]
- Jennings, H.M.; Kumar, A.; Sant, G. Quantitative discrimination of the nano-pore-structure of cement paste during drying: New insights from water sorption isotherms. Cem. Concr. Res. 2015, 76, 27–36. [Google Scholar] [CrossRef]
- Jennings, H.M. Refinements to colloid model of CSH in cement: CM-II. Cem. Concr. Res. 2008, 38, 275–289. [Google Scholar] [CrossRef]
- Pinson, M.B.; Masoero, E.; Bonnaud, P.A.; Manzano, H.; Ji, Q.; Yip, S.; Thomas, J.J.; Bazant, M.Z.; Van Vliet, K.J.; Jennings, H.M. Hysteresis from multiscale porosity: Modeling water sorption and shrinkage in cement paste. Phys. Rev. Appl. 2015, 3, 064009. [Google Scholar] [CrossRef]
- Wu, Z.; Wong, H.S.; Buenfeld, N.R. Transport properties of concrete after drying-wetting regimes to elucidate the effects of moisture content, hysteresis and microcracking. Cem. Concr. Res. 2017, 98, 136–154. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V. Water vapour sorption experiments on hardened cementitious materials: Part I: Essential tool for analysis of hygral behaviour and its relation to pore structure. Cem. Concr. Res. 2007, 37, 414–437. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V. Water vapour sorption experiments on hardened cementitious materials. Part II: Essential tool for assessment of transport properties and for durability prediction. Cem. Concr. Res. 2007, 37, 438–454. [Google Scholar] [CrossRef]
- Wei, X.; Xiao, L.; Li, Z. Electrical measurement to assess hydration process and the porosity formation. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 761766. [Google Scholar] [CrossRef]
- Powers, T. Properties of cement paste and concrete. In Proceedings of the 4th International Symposium on the Chemistry of Cement, Washington, DC, USA, 2–7 October 1960; pp. 577–613. [Google Scholar]
- Sant, G.; Bentz, D.; Weiss, J. Capillary porosity depercolation in cement-based materials: Measurement techniques and factors which influence their interpretation. Cem. Concr. Res. 2011, 41, 854–864. [Google Scholar] [CrossRef]
- Lu, N.; Khorshidi, M. Mechanisms for soil-water retention and hysteresis at high suction range. J. Geotech. Geoenviron. Eng. 2015, 141, 04015032. [Google Scholar] [CrossRef]
- Bouazza, A.; Rouf, M.A. Hysteresis of the water retention curves of geosynthetic clay liners in the high suction range. Geotext. Geomembr. 2021, 49, 1079–1084. [Google Scholar] [CrossRef]

| Bulk Ca Saturated | <2 µm Fraction | |
|---|---|---|
| Quartz% | 12 | 3 |
| Cristobalite% | 1 | <1 |
| Montmorillonite% | 78 | 95 |
| Calcite% | <1 | - |
| Albite/Anorthite% | 7 | <1 |
| Kaolin% | 1 | 1 |
| Zeolite% | 1 | - |
| Particle size | 24.4 | 75.1 |
| CEC (cmol/kg) by Ba and XRF | - | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Baiaty, S.; Bouazza, A. Vapour Multicycle Sorption of a Cement–Bentonite Cutoff Wall Material: Hysteresis Effects. Water 2023, 15, 2469. https://doi.org/10.3390/w15132469
Al-Baiaty S, Bouazza A. Vapour Multicycle Sorption of a Cement–Bentonite Cutoff Wall Material: Hysteresis Effects. Water. 2023; 15(13):2469. https://doi.org/10.3390/w15132469
Chicago/Turabian StyleAl-Baiaty, Sarah, and Abdelmalek Bouazza. 2023. "Vapour Multicycle Sorption of a Cement–Bentonite Cutoff Wall Material: Hysteresis Effects" Water 15, no. 13: 2469. https://doi.org/10.3390/w15132469
APA StyleAl-Baiaty, S., & Bouazza, A. (2023). Vapour Multicycle Sorption of a Cement–Bentonite Cutoff Wall Material: Hysteresis Effects. Water, 15(13), 2469. https://doi.org/10.3390/w15132469





