Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters from the Reshui Area, Dulan of Qinghai, China
Abstract
:1. Introduction
2. Geological Setting
3. Sampling and Analytical Methods
4. Results and Discussion
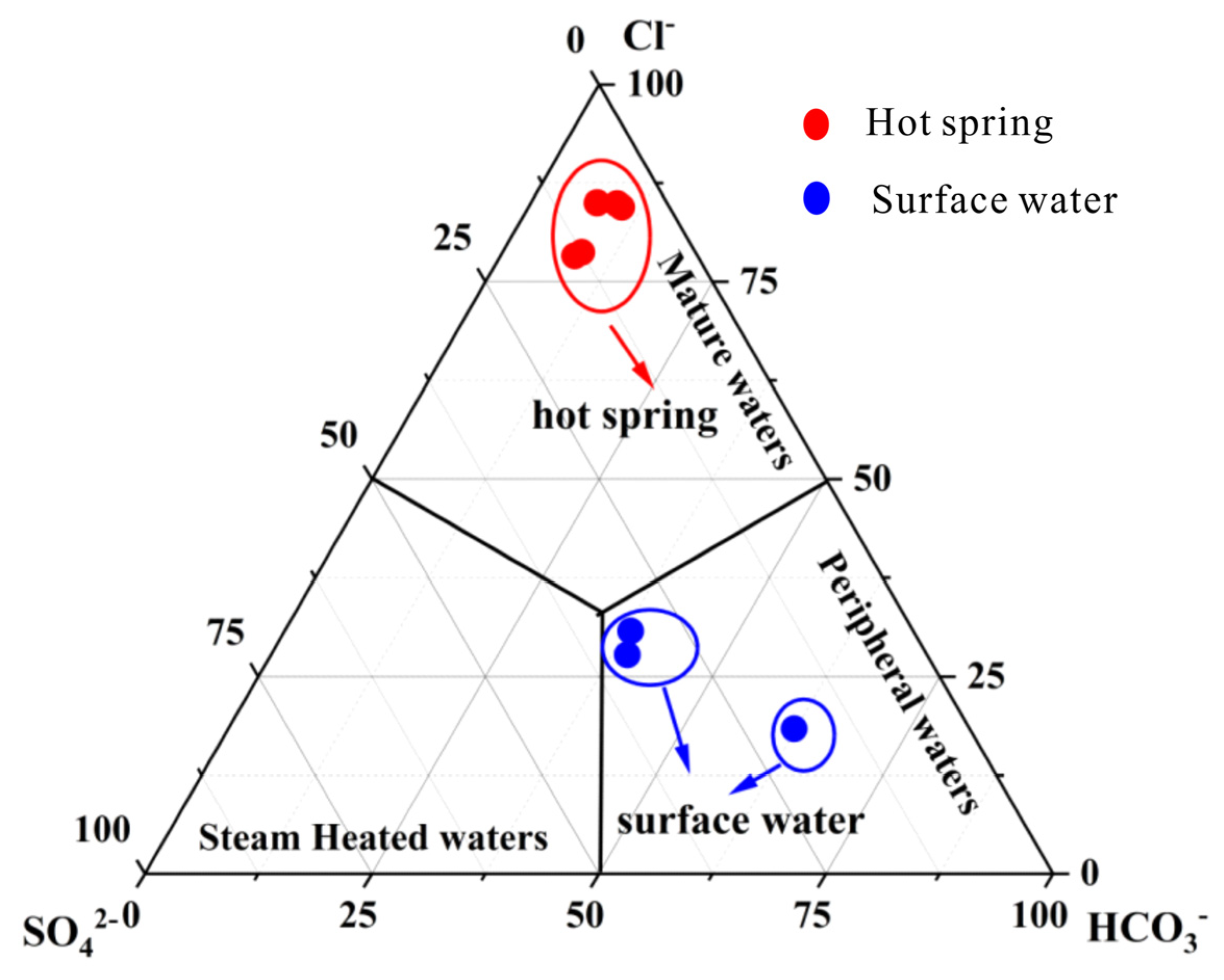
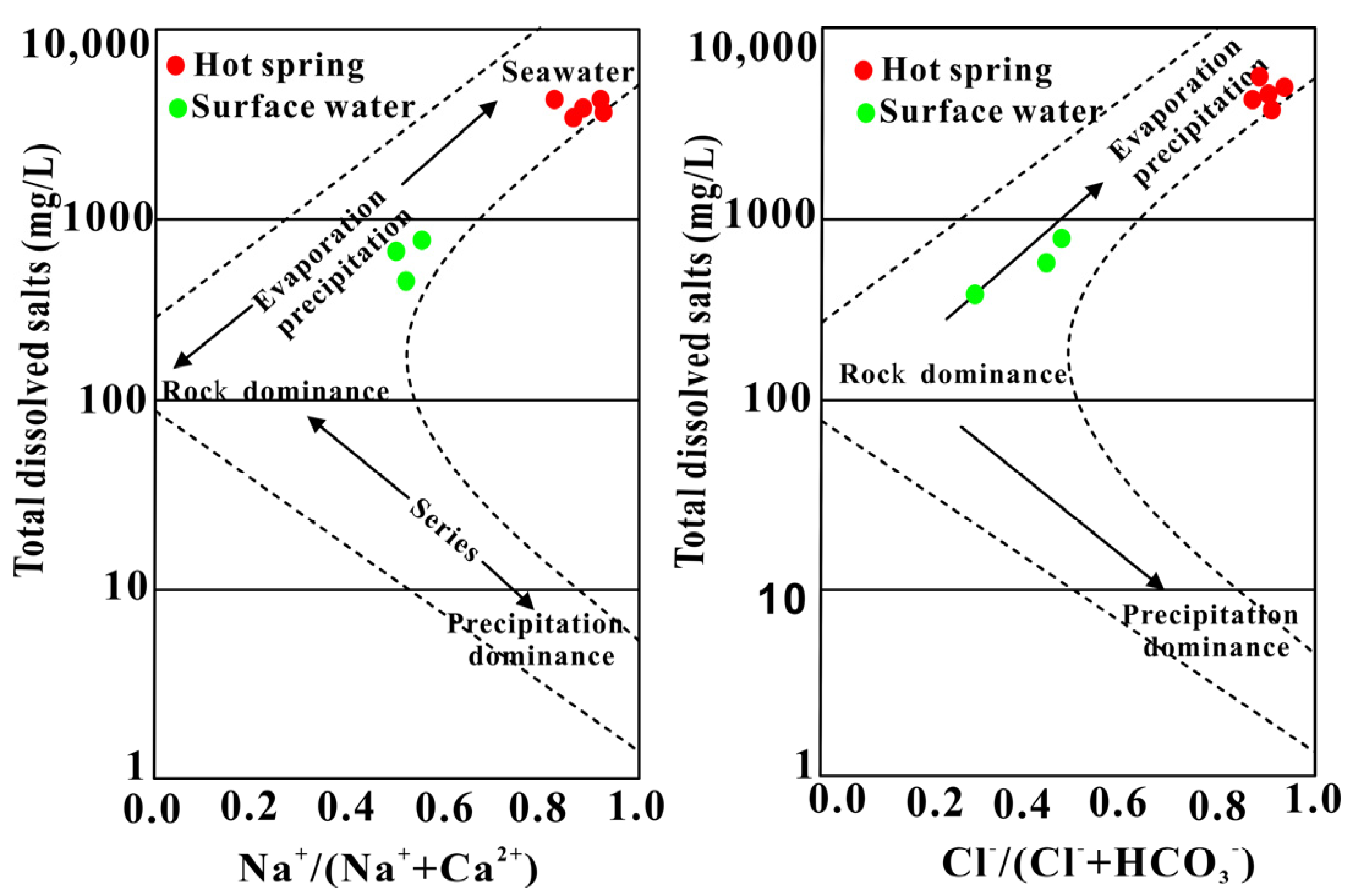
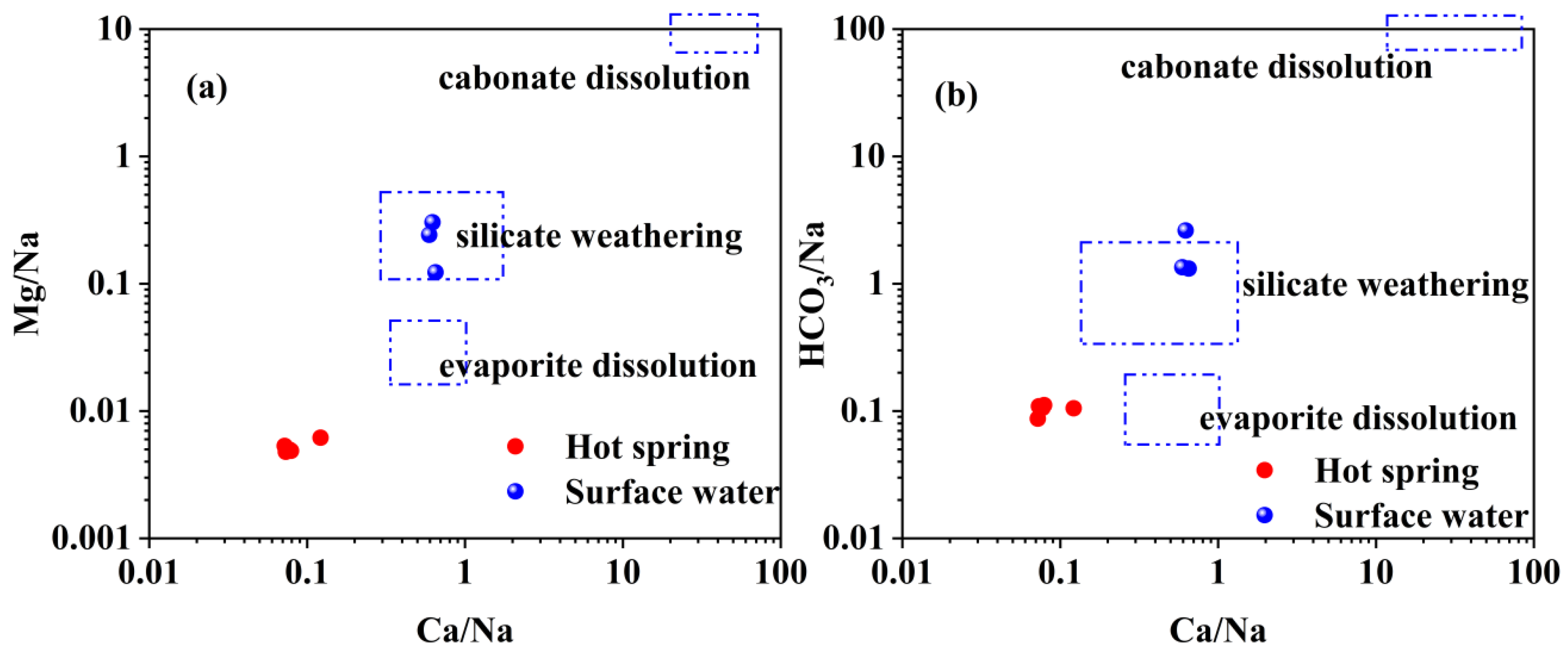
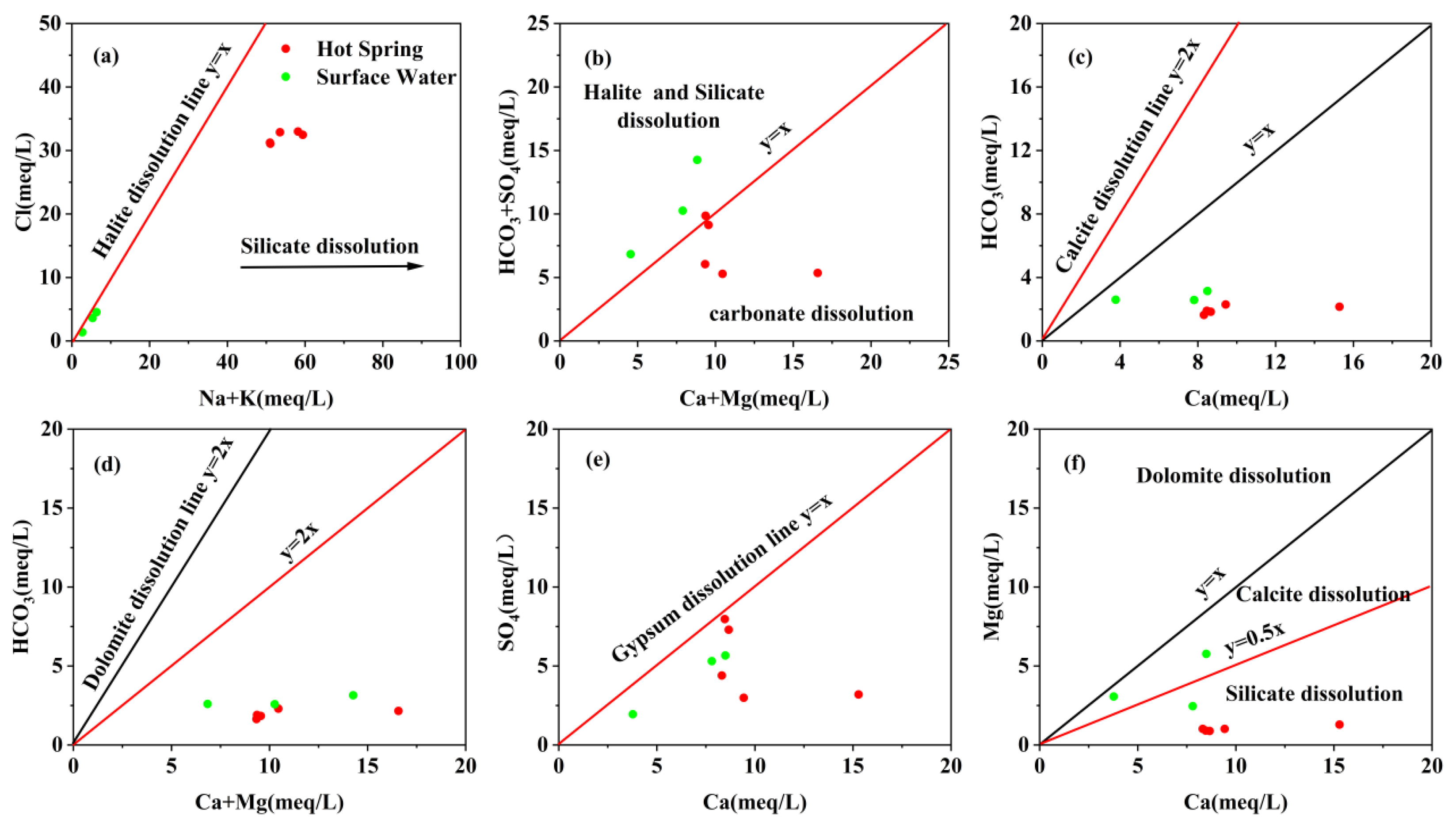
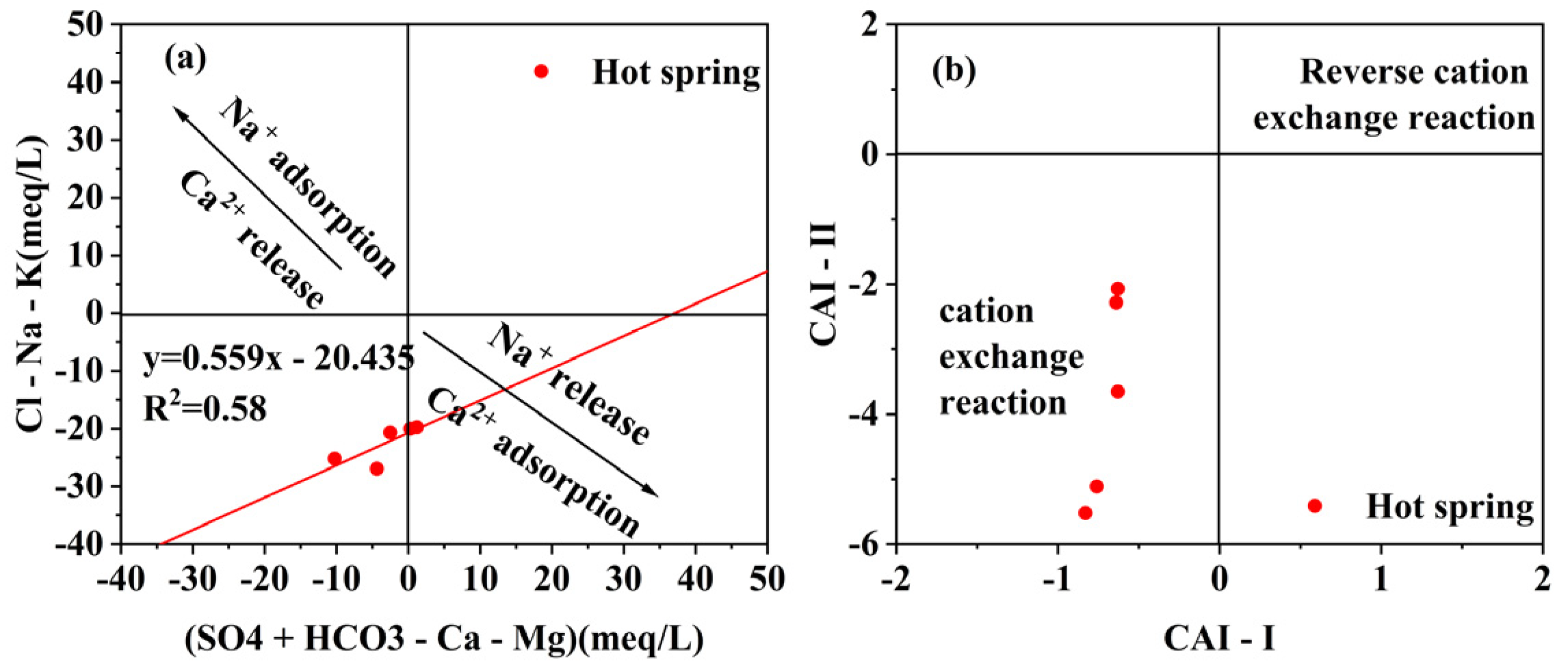
4.1. Hydrochemical Characteristics
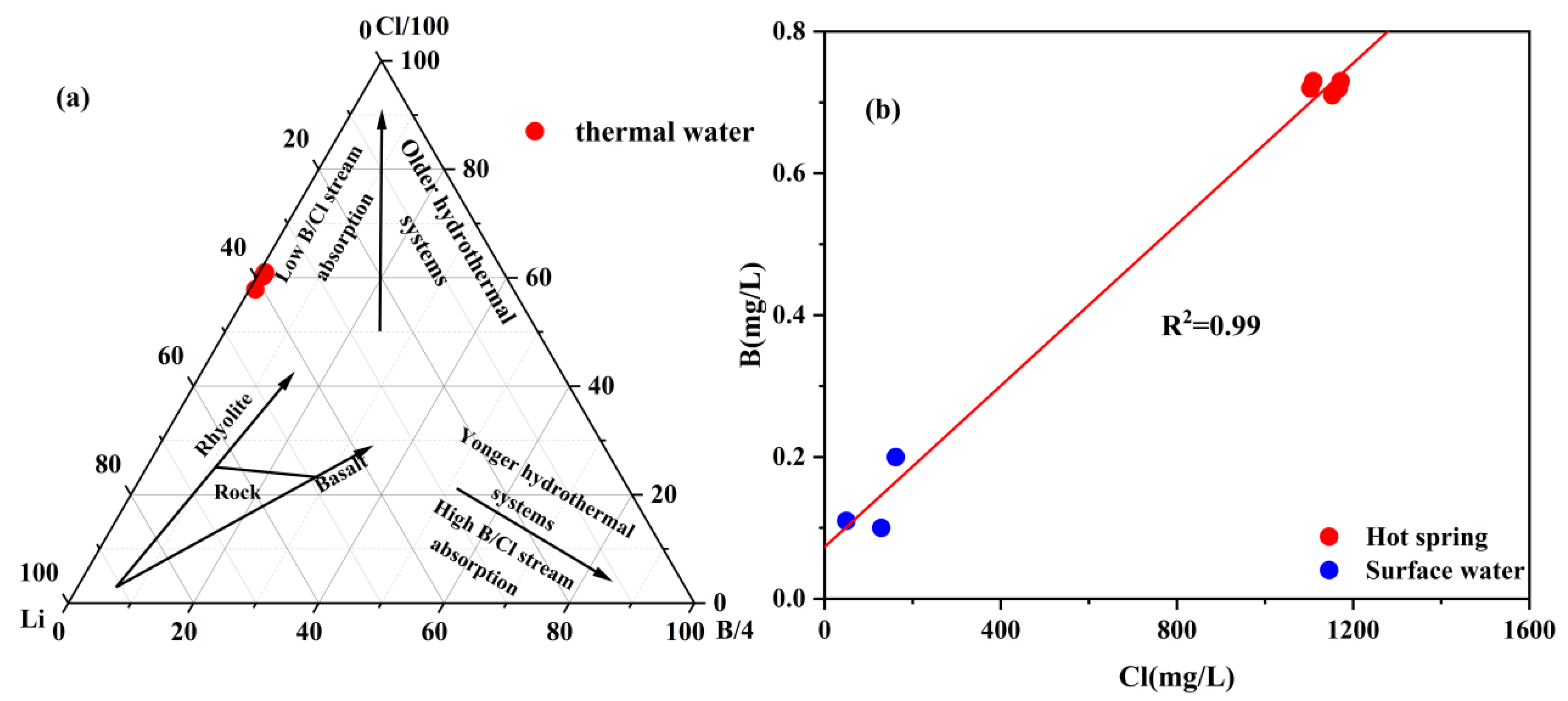
4.2. Characteristics of Trace Elements in Geothermal Water
4.3. Strontium Isotope Analysis
4.4. Hydrogen and Oxygen Isotope Characteristic
4.4.1. Recharge Source of Geothermal Water
4.4.2. Recharge Elevation in Geothermal Water
4.4.3. Geothermal Water Recharge Temperature
4.5. Residence Time of Geothermal Water
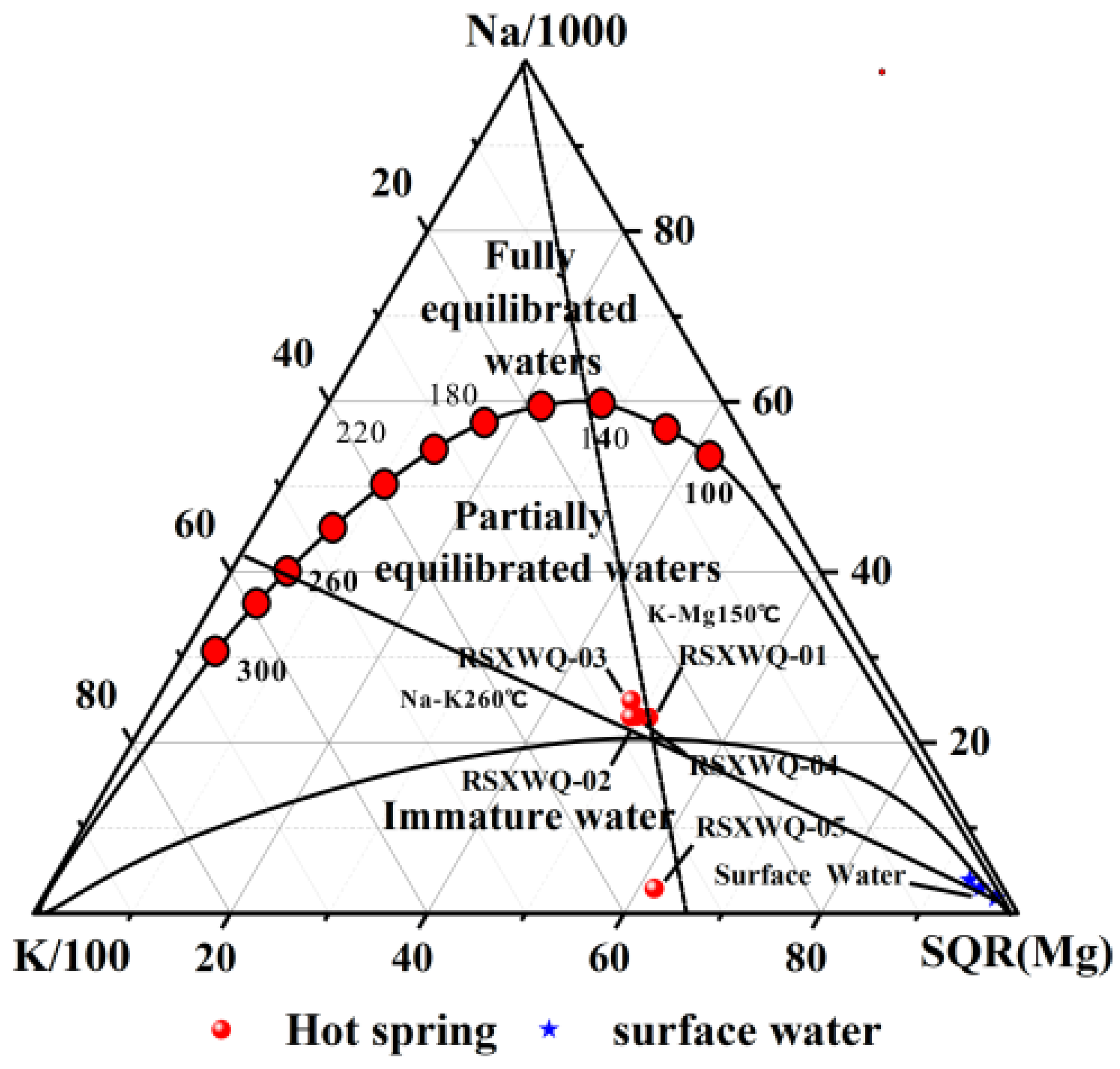
4.6. Estimation of Reservoir Temperatures
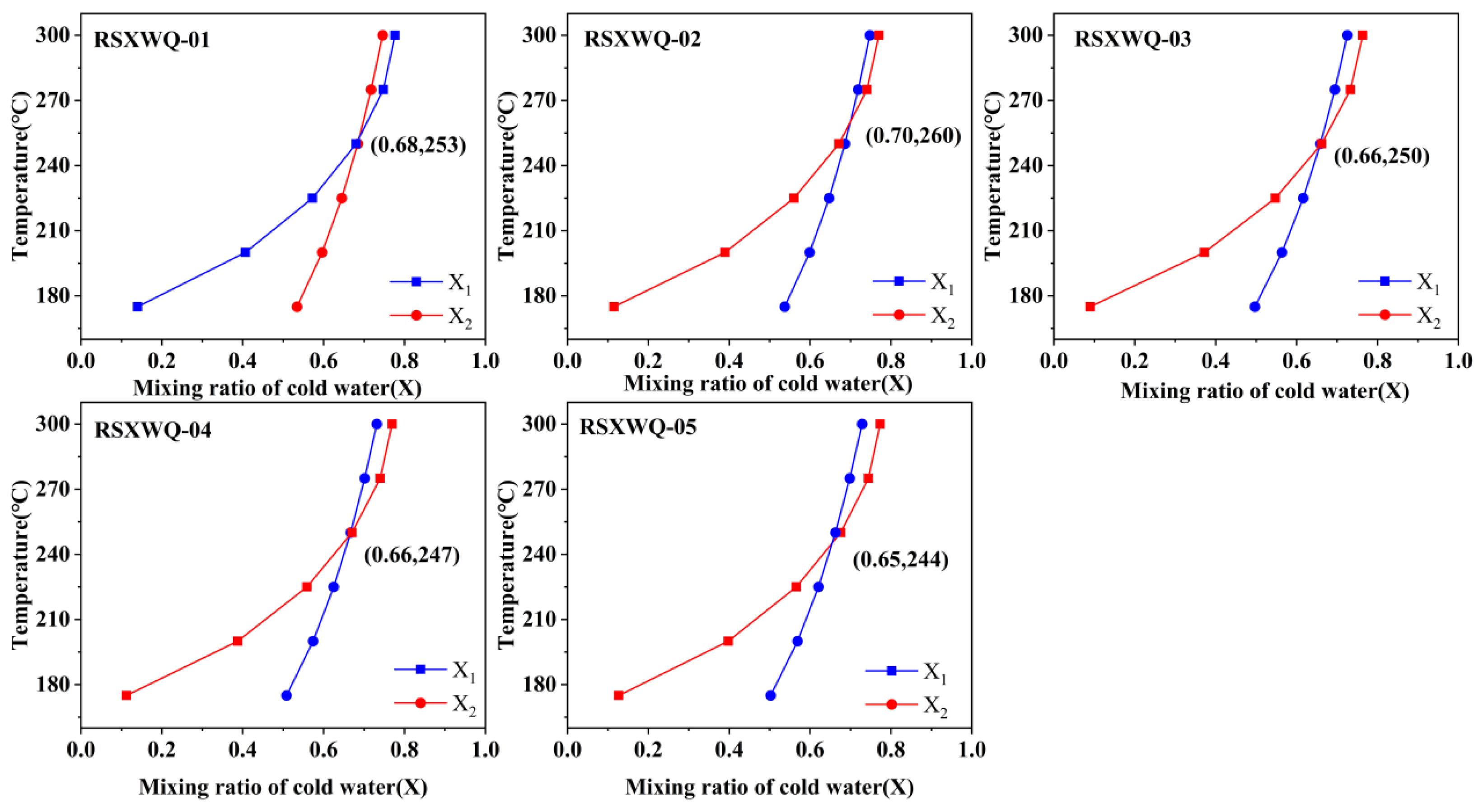
4.6.1. Mixing Ratio of Hot and Cold Water
4.6.2. SiO2 Geothermal Temperature Scale
4.6.3. Multi-Mineral Equilibrium Method
4.6.4. Other Thermometers
4.7. Geothermal Water Circulation Depth
4.8. Geological Genetic Model of the Geothermal Fluids
5. Conclusions
- (1)
- The geothermal water of Reshui area has a pH value ranging between 8.11 and 8.29, and TDS ranges from 2924 to 3140 mg/L. The hydrochemical type of hot spring is Cl-Na type, and its hydrochemical composition is mainly controlled by the interaction between water and rock, as well as ion exchange. Among the water–rock interactions, carbonate dissolution and evaporite dissolution are the most significant. The geothermal water in the research area contains a Li content of 7.08–7.85 mg/L and Sr content of 0.15–3.03 mg/L, meeting the standard for drinking natural mineral water and holding a certain development value.
- (2)
- Hydrogen and oxygen stable isotope characteristic findings indicate that the geothermal water is primarily recharged by atmospheric precipitation, with a supply altitude ranging from 6150 to 6255 m. The proportion of cold water mixing, calculated using the silica enthalpy equation, is estimated to be between 65% and 70%. By performing a comprehensive calculation using the silica enthalpy equation and the corrected quartz geothermal temperature scale, the geothermal reservoir temperature of the geothermal water within the area is determined to be 234.4–247.8 °C, and the calculated circulation depth is between 7385 and 7818 m.
- (3)
- Based on regional hydrogeological conditions and the results of hydrochemical and isotopic analyses, a conceptual model of the geothermal water cycle and evolution in the Reshui area was constructed. This model comprehensively reveals that geothermal water is influenced by underground heat conduction and diffusion under the control of faults, naturally exposed in fractured zones, and constantly affected by mixing with cold groundwater during its ascent, which influences its temperature and hydrochemical characteristics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, H.; Wang, G.; Zhang, W.; Ma, F.; Lin, W.; Ji, M. Predicting the Potential of China’s Geothermal Energy in Industrial Development and Carbon Emission Reduction. Sustainability 2023, 15, 7508. [Google Scholar]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar]
- Wang, G.L.; Zhang, W.; Ma, F.; Lin, W.J.; Liang, J.Y.; Zhu, X. Overview on hydrothermal and hot dry rock researches in China. China Geol. 2018, 1, 273–285. [Google Scholar] [CrossRef]
- Nicholson, K. Geothermal Fluids: Chemistry and Exploration Techniques; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tirrell, M.; Hubbard, S.; Sholl, D.; Peterson, E.; Tsapatsis, M.; Maher, K.; Runkles, K. Basic Research Needs for Energy and Water: Report of the Office of Basic Energy Sciences Basic Research Needs Workshop for Energy and Water; USDOE Office of Science: Germantown, MD, USA, 2017. [Google Scholar]
- Mao, X.; Zhu, D.; Ndikubwimana, I.; He, Y.; Shi, Z. The mechanism of high-salinity thermal groundwater in Xinzhou geothermal field, South China: Insight from water chemistry and stable isotopes. J. Hydrol. 2021, 593, 125889. [Google Scholar]
- Yuan, R.; Zhang, W.; Gan, H.; Liu, F.; Wei, S.; Liu, L. Hydrochemical Characteristics and the Genetic Mechanism of Low–Medium Temperature Geothermal Water in the Northwestern Songliao Basin. Water 2022, 14, 2235. [Google Scholar] [CrossRef]
- Pauwels, H.; Fouillac, C.; Fouillac, A.M. Chemistry and isotopes of deep geothermal saline fluids in the Upper Rhine Graben: Origin of compounds and water-rock interactions. Geochim. Cosmochim. Acta 1993, 57, 2737–2749. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, H.; Zhang, Y. Boron geochemistry from some typical Tibetan hydrothermal systems: Origin and isotopic fractionation. Appl. Geochem. 2015, 63, 436–445. [Google Scholar] [CrossRef]
- Procesi, M.; Marini, L.; Cinti, D.; Sciarra, A.; Basile, P.; Mazzoni, T.; Zarlenga, F. Preliminary fluid geochemical survey in Tete Province and prospective development of geothermics in Mozambique. Geotherm. Energy 2022, 10, 1–27. [Google Scholar]
- Apollaro, C.; Fuoco, I.; Gennaro, E.; Giuliani, L.; Iezzi, G.; Marini, L.; Vespasiano, G. Advanced argillic alteration at Cave di Caolino, Lipari, Aeolian Islands (Italy): Implications for the mitigation of volcanic risks and the exploitation of geothermal resources. Sci. Total Environ. 2023, 889, 164333. [Google Scholar]
- Kebede, S.; Travi, Y.; Alemayehu, T.; Ayenew, T. Groundwater recharge, circulation and geochemical evolution in the source region of the Blue Nile River, Ethiopia. Appl. Geochem. 2005, 20, 1658–1676. [Google Scholar] [CrossRef]
- Bretzler, A.; Osenbrück, K.; Gloaguen, R.; Ruprecht, J.S.; Kebede, S.; Stadler, S. Groundwater origin and flow dynamics in active rift systems–A multi-isotope approach in the Main Ethiopian Rift. J. Hydrol. 2011, 402, 274–289. [Google Scholar]
- Cheng, G.; Jin, H. Permafrost and groundwater on the Qinghai-Tibet Plateau and in northeast China. Hydrogeol. J. 2013, 21, 5. [Google Scholar]
- Zou, F.; Wu, C.; Deng, L.; Gao, D.; Gao, Y. Petrological and geochemical constraints on the petrogenesis of granitoids in the Gonghe geothermal basin, western Qinling (China). Appl. Geochem. 2022, 136, 105176. [Google Scholar]
- Liu, S.; Tang, X.; Han, X.; Zhang, D.; Wang, G. Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System. Water 2023, 15, 1971. [Google Scholar]
- Bai, L.; Li, J.; Zeng, Z.; Wang, K. Analysis of the Geothermal Formation Mechanisms in the Gonghe Basin Based on Thermal Parameters Inversion. Geophysics 2023, 88, 1–64. [Google Scholar]
- Hang, X.; Hao, H.; Liu, K.; Mao, W.; Xiao, Y.; Zhang, W. Hydrogeochemical characteristics and genetic model of Oiga Graben Geothermal Waters System in Tibet. Geol. China 2020, 47, 1702–1714, (In Chinese with English Abstract). [Google Scholar]
- Zhou, R.; Zhou, X.; Li, Y.; He, M.; Li, J.; Dong, J.; Luo, Z. Hydrogeochemical and Isotopic Characteristics of the Hot Springs in the Litang Fault Zone, Southeast Qinghai–Tibet Plateau. Water 2022, 14, 1496. [Google Scholar]
- Zhang, C.; Huang, R.; Qin, S.; Hu, S.; Zhang, S.; Li, S.; Wang, Z. The high-temperature geothermal resources in the Gonghe-Guide area, northeast Tibetan plateau: A comprehensive review. Geothermics 2021, 97, 102264. [Google Scholar] [CrossRef]
- Saibi, H.; Batir, J.F.; Pocasangre, C. Hydrochemistry and geothermometry of thermal waters from UAE and their energetic potential assessment. Geothermics 2021, 92, 102061. [Google Scholar]
- Ayadi, R.; Trabelsi, R.; Zouari, K.; Saibi, H.; Itoi, R.; Khanfir, H. Hydrogeological and hydrochemical investigation of groundwater using environmental isotopes (18O, 2H, 3H, 14C) and chemical tracers: A case study of the intermediate aquifer, Sfax, southeastern Tunisia. Hydrogeol. J. 2018, 26, 983–1007. [Google Scholar]
- Cheng, F.; Jolivet, M.; Guo, Z.; Wang, L.; Zhang, C.; Li, X. Cenozoic evolution of the Qaidam basin and implications for the growth of the northern Tibetan plateau: A review. Earth-Sci. Rev. 2021, 220, 103730. [Google Scholar]
- Song, S.; Yang, J.; Liou, J.G.; Wu, C.; Shi, R.; Xu, Z. Petrology, geochemistry and isotopic ages of eclogites from the Dulan UHPM Terrane, the North Qaidam, NW China. Lithos 2003, 70, 195–211. [Google Scholar] [CrossRef]
- Xiao, W.; Windley, B.F.; Yong, Y.; Yan, Z.; Yuan, C.; Liu, C.; Li, J. Early Paleozoic to Devonian multiple-accretionary model for the Qilian Shan, NW China. J. Asian Earth Sci. 2009, 35, 323–333. [Google Scholar]
- Yu, S.; Li, S.; Zhang, J.; Peng, Y.; Somerville, I.; Liu, Y.; Wang, Z.; Li, Z.; Yao, Y.; Li, Y. Multistage anatexis during tectonic evolution from oceanic subduction to continental collision: A review of the North Qaidam UHP Belt, NW China. Earth-Sci. Rev. 2019, 191, 190–211. [Google Scholar]
- Xie, X. Numerical Simulation and Resource Evaluation of Groundwater in the Duolanchong Alluvial Plain; China University of Geosciences: Beijing, China, 2015. [Google Scholar]
- Cheng, F.; Guo, Z.; Jenkins, H.S.; Fu, S.; Cheng, X. Initial rupture and displacement on the Altyn Tagh fault, northern Tibetan Plateau: Constraints based on residual Mesozoic to Cenozoic strata in the western Qaidam Basin. Geosphere 2015, 11, 921–942. [Google Scholar]
- Wu, C.; Gao, Y.; Li, Z.; Lei, M.; Qin, H.; Li, M.; Wooden, J.L. Zircon SHRIMP U-Pb dating of granites from Dulan and the chronological framework of the North Qaidam UHP belt, NW China. Sci. China Earth Sci. 2014, 57, 2945–2965. [Google Scholar]
- Qu, H.; Li, P.; Dong, Y.; Yang, B.; Chen, S.; Han, X.; Wang, K.; He, M. Development and distribution rules of the main Neoproterozoic source and reservoir strata in the Yangtze Block, Southern China. Precambrian Res. 2020, 350, 105915. [Google Scholar]
- He, D.; Dong, Y.; Liu, X.; Yang, Z.; Sun, S.; Cheng, B. Tectono-thermal events in East Kunlun, Northern Tibetan Plateau: Evidence from zircon U–Pb geochronology. Gondwana Res. 2016, 30, 179–190. [Google Scholar]
- Yu, M.; Dick, J.M.; Feng, C.; Li, B.; Wang, H. The tectonic evolution of the East Kunlun Orogen, northern Tibetan Plateau: A critical review with an integrated geodynamic model. J. Asian Earth Sci. 2020, 191, 104168. [Google Scholar]
- Oleiwi, A.S.; Al-Dabbas, M. Assessment of contamination along the Tigris River from Tharthar-Tigris canal to Azizziyah, middle of Iraq. Water 2022, 14, 1194. [Google Scholar] [CrossRef]
- Galley, A.G. Composite synvolcanic intrusions associated with Precambrian VMS-related hydrothermal systems. Miner. Depos. 2003, 38, 443–473. [Google Scholar]
- Haeri, A.; Porkhial, S.; Ashayeri, A.; Behrad, Y. Chemical analyses of hot springs in Sabalan Geothermal Area, NW Iran. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010. [Google Scholar]
- Xiao-Mei, H.; Jin, Y.; Chao, L.; Xiao-Jun, F.; Yuan, Z. Analysis of hydrochemical characteristics and genesis of water-deficient rivers in China: A case study of the Ciyao River Basin in Shanxi Province. Qual. Assur. Saf. Crops Foods 2023, 15, 32–43. [Google Scholar]
- Bouderbala, A.; Gharbi, B.Y. Hydrogeochemical characterization and groundwater quality assessment in the intensive agricultural zone of the Upper Cheliff plain, Algeria. Environ. Earth Sci. 2017, 76, 1–17. [Google Scholar]
- Zang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical characteristics of groundwater and dominant water–rock interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 2020, 12, 836. [Google Scholar] [CrossRef]
- Liu, B.; Yu, S.; Zhang, Q.; Ye, Y.; Qu, J.; Liu, X. Efficient Removal of Strontium from Simulated Radioactive Wastewater by Using Mechanically Modified Dolomite as a Novel Environment-friendly Adsorbent. Acta Microsc. 2019, 28, 455–466. [Google Scholar]
- Pengq, H.; Huangx; Sunm, L. Hydrochemicalcharacteristics and genetic mechanism of ChakaQuzhuomugeothermal in southern Tibet. Sediment. Geol. Tethyan Geol. 2023, 43, 283–294. [Google Scholar]
- Capo, R.C.; Stewart, B.W.; Chadwick, O.A. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma 1998, 82, 197–225. [Google Scholar]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou City, northwestern China. Urban Clim. 2022, 43, 101152. [Google Scholar]
- Zhang, L.; Guo, L.; Zhou, X.; Yang, Y.; Shi, D.; Liu, Y. Temporal variations in stable isotopes and synchronous earthquake-related changes in hot springs. J. Hydrol. 2021, 599, 126316. [Google Scholar]
- Keesari, T.; Goyal, M.K.; Gupta, B.; Kumar, N.; Roy, A.; Sinha, U.K.; Goyal, R.K. Big data and environmental sustainability based integrated framework for isotope hydrology applications in India. Environ. Technol. Innov. 2021, 24, 101889. [Google Scholar]
- Longinelli, A.; Sighinolfi, G.; de Michele, V.; Selmo, E. δ18O and chemical composition of Libyan Desert Glass, country rocks, and sands: New considerations on target material. Meteorit. Planet. Sci. 2011, 46, 218–227. [Google Scholar]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Chen, H.; Chen, T. Study of the water sources of Salsola abrotanoides under various precipitation conditions. J. Glaciol. Geocryol. 2015, 37, 1396–1405. [Google Scholar]
- Peng, H.; Mayer, B.; Harris, S.; Krouse, H.R. A 10-yr record of stable isotope ratios of hydrogen and oxygen in precipitation at Calgary, Alberta, Canada. Tellus B Chem. Phys. Meteorol. 2004, 56, 147–159. [Google Scholar] [CrossRef]
- Porowski, A.; Romanova, A.; Gebus-Czupyt, B.; Wach, B.; Radzikowska, M. Stable hydrogen and oxygen isotopic composition of bottled waters in Poland: Characterization in the context of different market categories and implications for the origin authentication and natural isotopic quality preservation. J. Geochem. Explor. 2021, 220, 106684. [Google Scholar]
- Yang, Q.; Wu, B.; Wang, S. Geology of Potassium Salt Deposit in Chaerhan Salt Lake; Geological Press: Beijing, China, 1993. [Google Scholar]
- Qian, Y.; Bihao, W.; Shengzu, W. Geology of Potassium Salt Deposit in Chaerhan Salt Lake; Collected Works of Chinese Academy of Geological Sciences; Chinese-English Bound Edition; Chinese Academy of Geological Sciences: Bejing, China, 1995. [Google Scholar]
- Yurtsever, Y. Worldwide Survey of Stable Isotopes in Precipitation; Rep. Sect. Isotope Hydrol.; IAEA: Vienna, Austria, 1975. [Google Scholar]
- Jieqing, W.; Xun, Z.; Xiaolu, L.; Mengmeng, W.; Ye, S.; Bin, F. Hydrochemistry and formation of the Yangchimi hot spring in the lanping Basin of Yunnan. Geoscience 2017, 31, 822. [Google Scholar]
- Ji, K.; Chen, X.; Aldahan, A.; Yi, P.; Murad, A.; Hussein, S.; Yu, Z. Chronology and characteristics of groundwater along the United Arab Emirates-Oman arid region: A guide for regional sustainability. J. Radioanal. Nucl. Chem. 2020, 323, 1055–1070. [Google Scholar] [CrossRef]
- Han, D.; Liang, X.; Currell, M.J.; Song, X.; Chen, Z.; Jin, M.; Han, Y. Environmental isotopic and hydrochemical characteristics of groundwater systems in Daying and Qicun geothermal fields, Xinzhou Basin, Shanxi, China. Hydrol. Process. 2010, 24, 3157–3176. [Google Scholar]
- Hamani, A.H.H.; Adamou, M.M.; Sandao, I.; Hado, H.A. Physico-Chemical and Isotopic (18O, 2H and 3H) Characterization of the Upper Dallol Maouri Watershed (Niger). Open J. Geol. 2022, 12, 613–639. [Google Scholar] [CrossRef]
- Shah, M.; Sircar, A.; Shaikh, N.; Patel, K.; Sharma, D.; Vaidya, D. Comprehensive geochemical/hydrochemical and geo-thermometry analysis of Unai geothermal field, Gujarat, India. Acta Geochim. 2019, 38, 145–158. [Google Scholar]
- Giggenbach, W.F. Geothermal solute equilibria. derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar]
- Tang, J.; Zhou, X.; Zhang, Y.; Tian, J.; He, M.; Li, J.; Liu, K. Hydrogeochemistry of Fault-Related Hot Springs in the Qaidam Basin, China. Appl. Sci. 2023, 13, 1415. [Google Scholar]
- Liu, Y.; Zhou, X.; Deng, Z.; Fang, B.; Tsutomu, Y.; Zhao, J.; Wang, X. Hydrochemical characteristics and genesis analysis of the Jifei hot spring in Yunnan, southwestern China. Geothermics 2015, 53, 38–45. [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Arnorsson, S. Application of the silica geothermometer in low temperature hydrothermal areas in Iceland. Am. J. Sci. 1975, 275, 763–784. [Google Scholar] [CrossRef]
- Fournier, R.O. A revised equation for the Na/K geothermometer. Trans. Geotherm. Resour. Counc. 1979, 3, 221–224. [Google Scholar]
- Pirlo, M.C. Hydrogeochemistry and geothermometry of thermal groundwaters from the Birdsville Track Ridge, Great Artesian Basin, South Australia. Geothermics 2004, 33, 743–774. [Google Scholar] [CrossRef]
- Sasaki, K.; Morita, J.; Iwaki, C.; Ueda, A. Geochemical evaluation of geothermal resources in Toyama Prefecture, Japan, based on the chemical and isotopic characteristics of hot spring waters. Geothermic 2021, 93, 102071. [Google Scholar]
- Fan, Y.; Pang, Z.; Liao, D.; Tian, J.; Hao, Y.; Huang, T.; Li, Y. Hydrogeochemical characteristics and genesis of geothermal water from the Ganzi geothermal field, eastern Tibetan Plateau. Water 2019, 11, 1631. [Google Scholar] [CrossRef]
- Tole, M.P.; Ármannsson, H.; Zhong-He, P.; Arnórsson, S. Fluid/mineral equilibrium calculations for geothermal fluids and chemical geothermometry. Geothermics 1993, 22, 17–37. [Google Scholar] [CrossRef]
- Guo, Q.; Pang, Z.; Wang, Y.; Tian, J. Fluid geochemistry and geothermometry applications of the Kangding high-temperature geothermal system in eastern Himalayas. Appl. Geochem. 2017, 81, 63–75. [Google Scholar] [CrossRef]
- Xu, C.; Yu, D.; Luo, Z. Recharge sources and genetic model of geothermal water in Tangquan, Nanjing, China. Sustainability 2021, 13, 4449. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Liu, C. Preliminary Analysis of Present Geothermal Characteristics in the Qaidam Basin. Chin. J. Geol. Sci. Acad. 1987, 13, 1–16. [Google Scholar]
- Ma, Z.J.; Gao, X.L.; Song, Z.F. Analysis and tectonic interpretation to the horizontal-gradient map calculated from Bouguer gravity data in the China mainland. Chin. J. Geophys. 2006, 49, 95–106. [Google Scholar]
- Zhang, P.Z. A review on active tectonics and deep crustal processes of the Western Sichuan region, eastern margin of the Tibetan Plateau. Tectonophysics 2013, 584, 7–22. [Google Scholar]
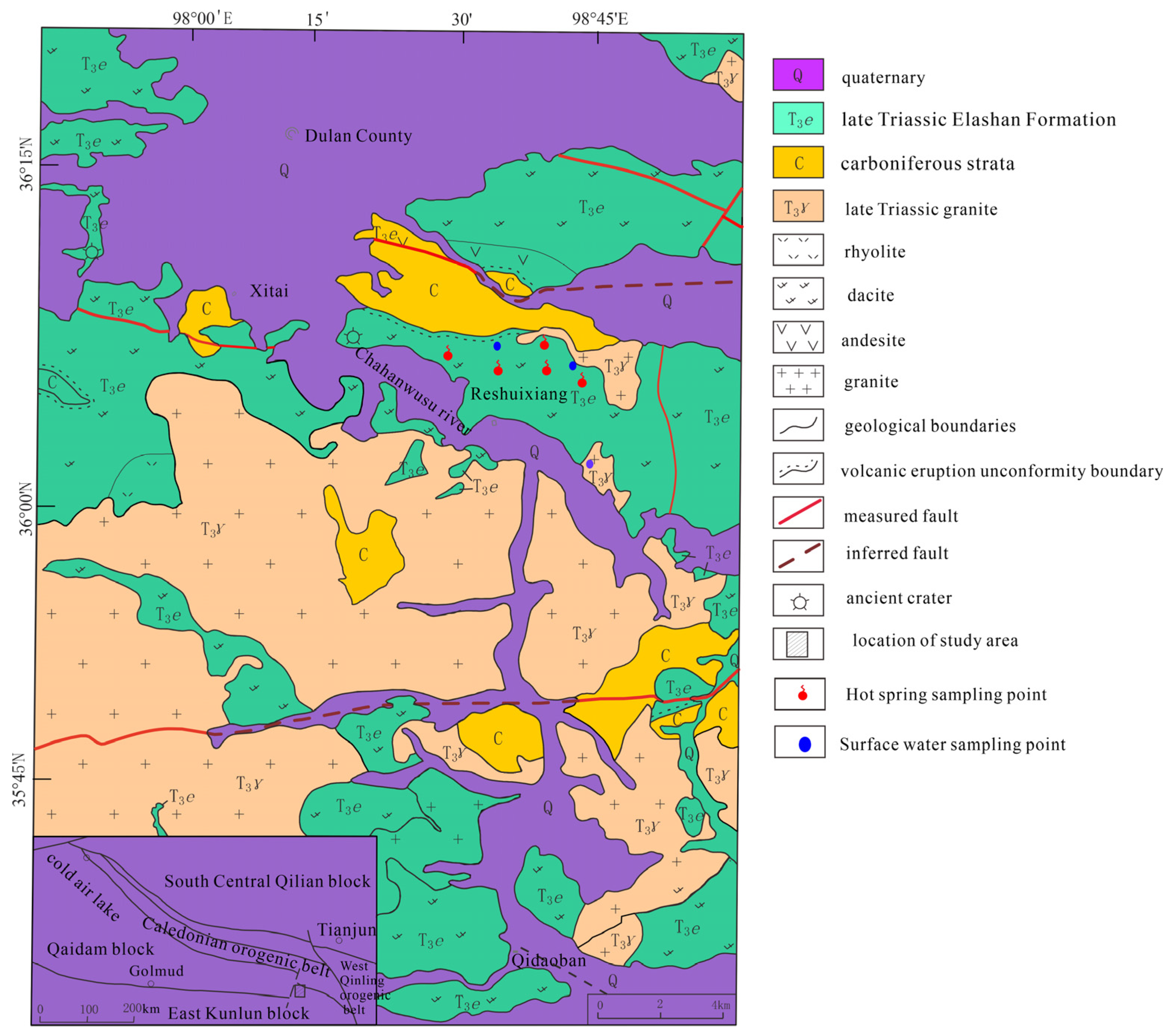
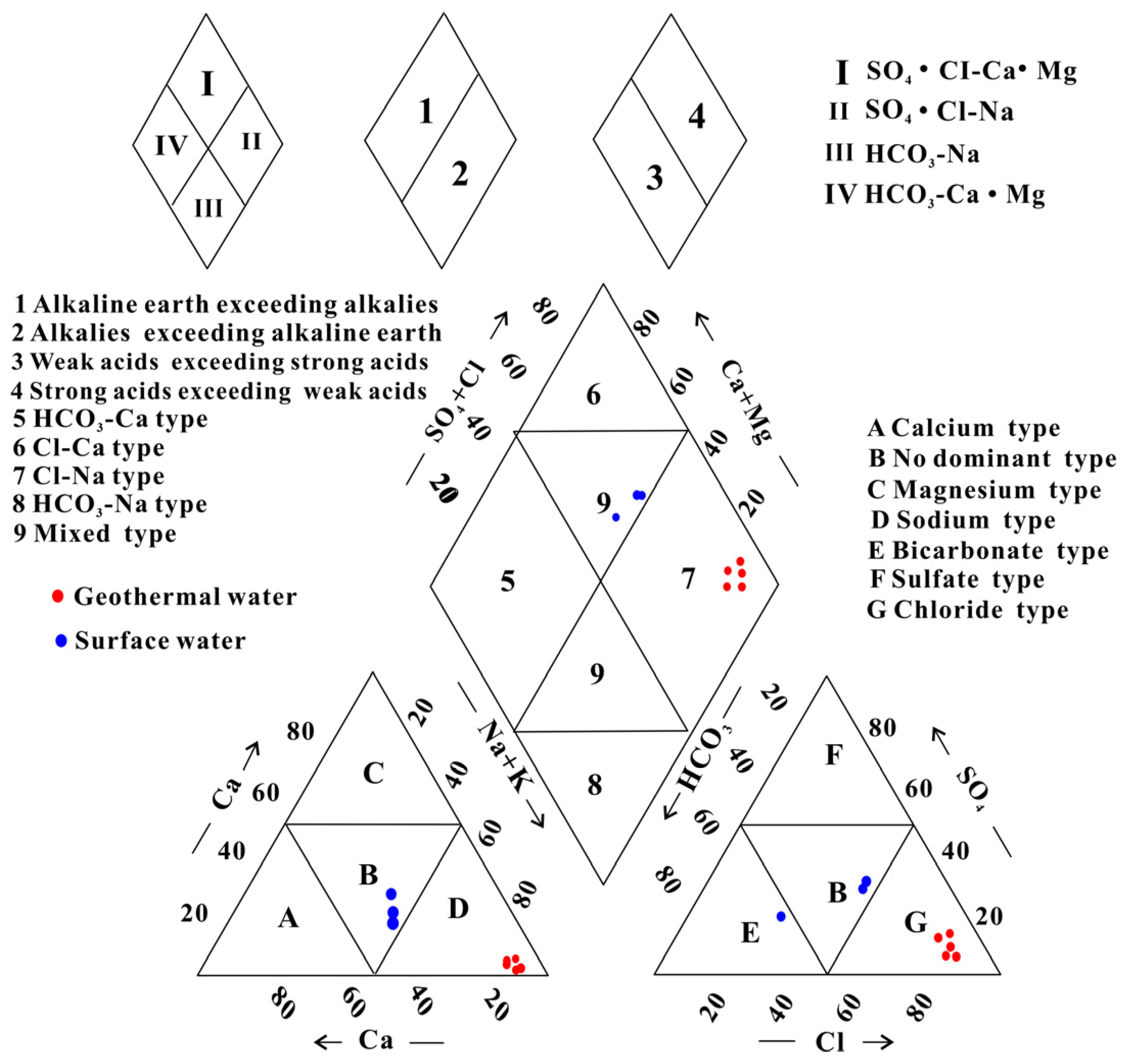
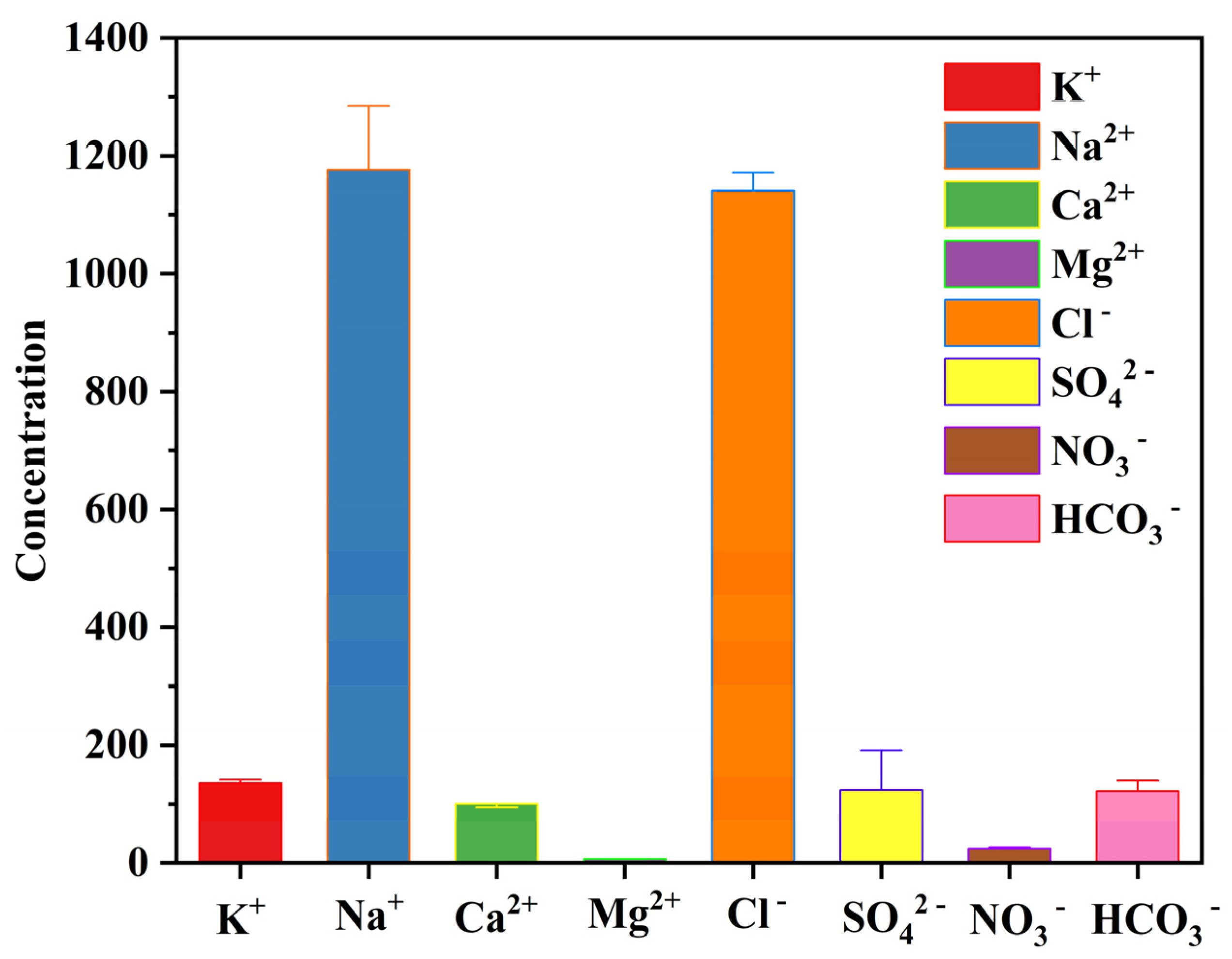
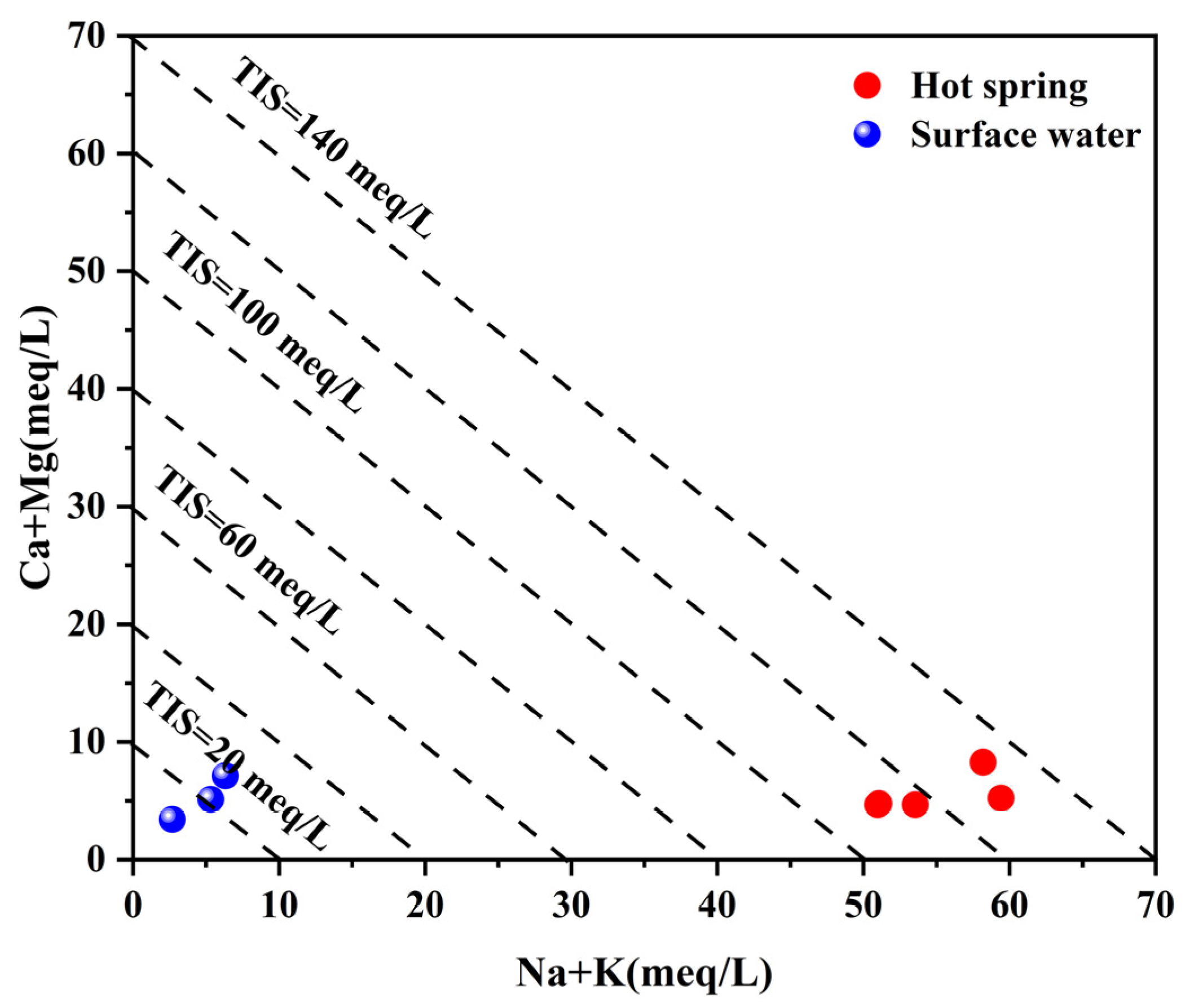
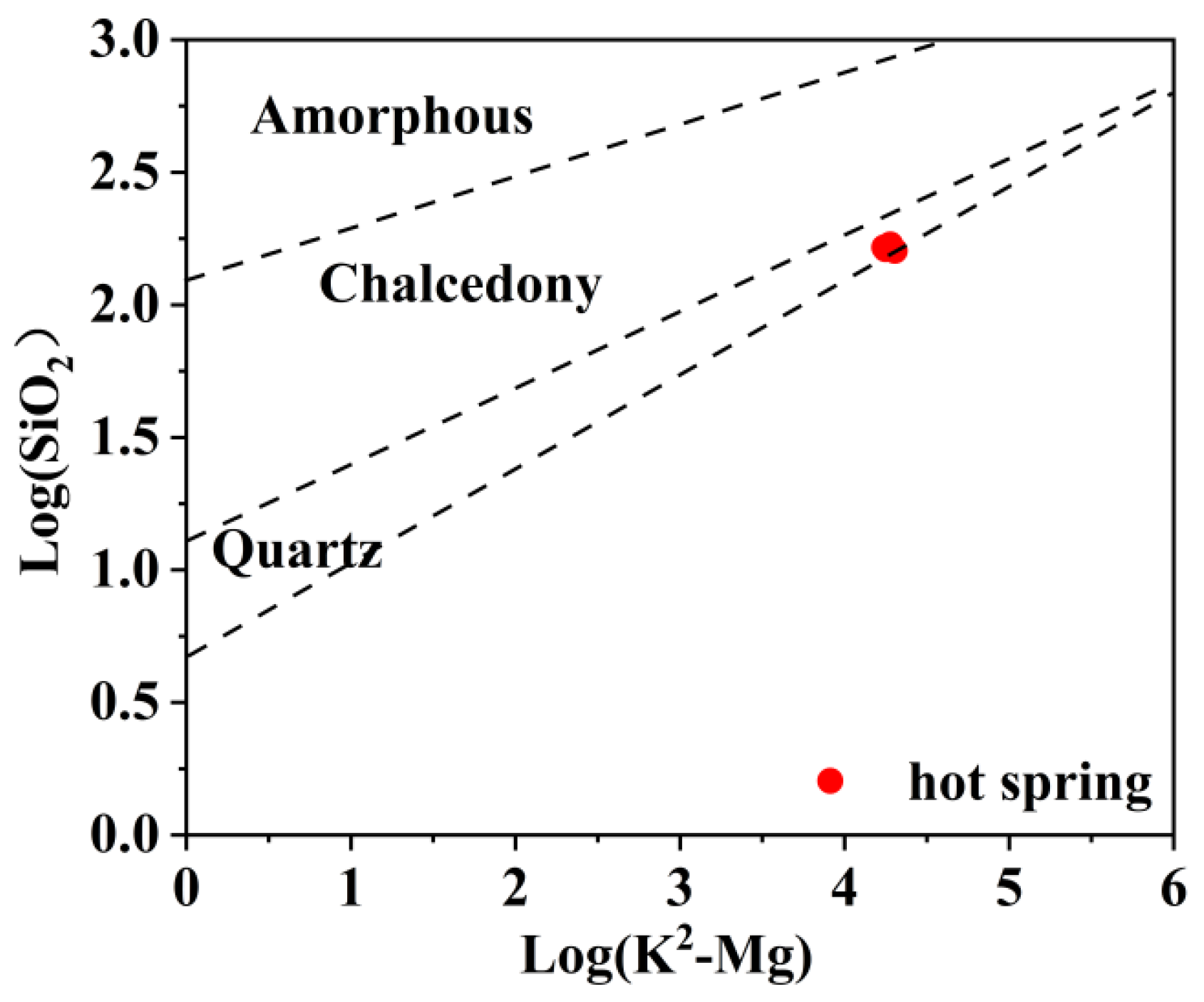
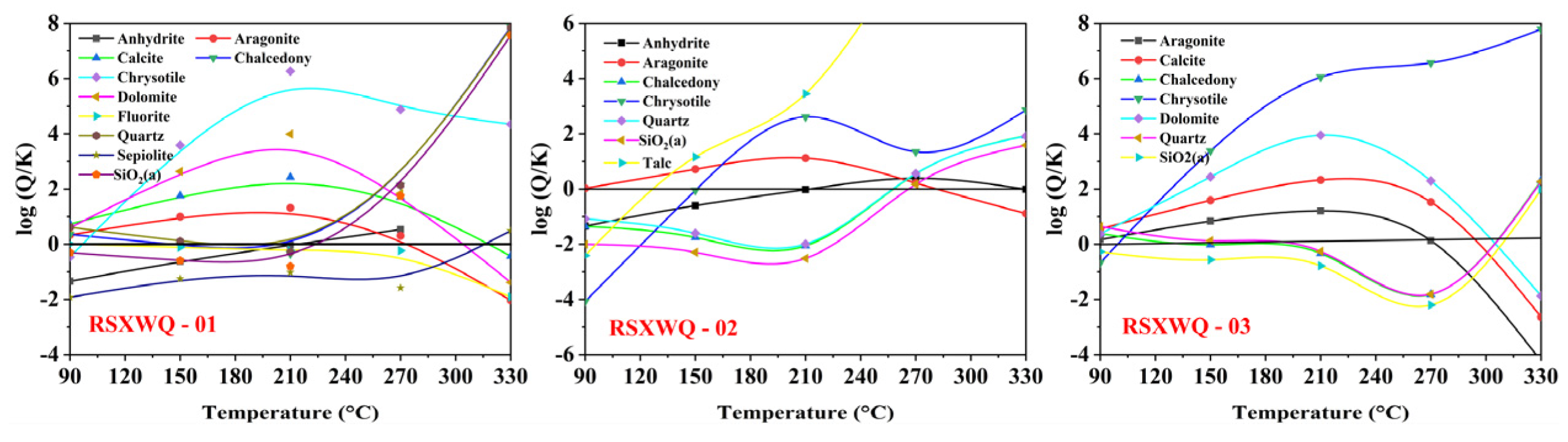

| Sample ID | Type | Latitude | Longitude | Altitude | T | pH | TDS | Ca2+ | Mg2+ | Na+ | K+ | HCO3− | Cl− | SO42− | NO3− | SiO2 | IB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ° | ° | m | °C | mg/L | (%) | ||||||||||||
| RSXWQ-01 | Hot Spring | 36.03130612 | 98.52656820 | 3996 | 85 | 8.29 | 3140 | 152.8 | 7.74 | 1254.5 | 142 | 131.8 | 1171.57 | 76.69 | 26.7 | 160.1 | 4.75 |
| RSXWQ-02 | 36.01810039 | 98.82812566 | 3961 | 84 | 8.26 | 2955 | 83.13 | 6.12 | 1151.5 | 135.2 | 100.3 | 1167.3 | 105.71 | 24.5 | 164.5 | 0.79 | |
| RSXWQ-03 | 36.01835301 | 98.82851019 | 3961 | 91 | 8.45 | 3099 | 94.26 | 6.14 | 1285 | 137.7 | 140.1 | 1152.8 | 71.78 | 25.6 | 169 | 4.56 | |
| RSXWQ-04 | 36.01835301 | 98.82851019 | 3953 | 89 | 8.30 | 2934 | 84.58 | 5.44 | 1094.5 | 132 | 120.5 | 1109.18 | 191.22 | 20.2 | 165 | 4.36 | |
| RSXWQ-05 | 36.01808925 | 98.82882745 | 3953 | 90 | 8.25 | 2924 | 86.6 | 5.34 | 1095.5 | 133.2 | 130.5 | 1102.91 | 175.25 | 23.13 | 162.4 | 3.74 | |
| CHWSH | Surface Water | 36.01808925 | 98.82882745 | 3590 | 12.5 | 8.11 | 768 | 84.88 | 34.63 | 142.9 | 4.2 | 192.3 | 161.54 | 136.1 | - | 9.74 | 2.5 |
| RSXXS-01 | 35.96760524 | 98.69474768 | 3764 | 12 | 8.18 | 641 | 78.01 | 14.75 | 119.9 | 4.14 | 157.5 | 128.66 | 127.33 | 2.55 | 7.42 | 3.41 | |
| RSXXS-02 | 36.14865831 | 97.18424421 | 2874 | 8 | 8.37 | 380 | 37.74 | 18.41 | 60.54 | 1.8 | 158.3 | 49.07 | 46.73 | 2.84 | 4.11 | 3.63 | |
| Sample ID | Li | B | F | Sr | Br | 86Sr/87Sr | δ18O | δ2H | d | 3H | Recharge Elevation | Air Temperature | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | ‰ | ‰ | Tu | m | °C | ||||||||
| RSXWQ-01 | 7.41 | 0.73 | 5.37 | 3.03 | 0.025 | 0.712028 | −91 | −13.2 | 14.6 | 5.0 ± 0.5 | 6255 | 3.4 | 0.6 |
| RSXWQ-02 | 7.29 | 0.72 | 5.67 | 2.8 | 0.072 | 0.712102 | −89 | −13.0 | 15 | 7.5 ± 0.5 | 6151 | 3.8 | 0.9 |
| RSXWQ-03 | 7.42 | 0.71 | 6.37 | 2.57 | 0.048 | 0.712043 | −90 | −13.1 | 14.8 | 5.7 ± 0.5 | 6186 | 3.6 | 0.7 |
| RSXWQ-04 | 7.08 | 0.73 | 5.24 | 2.9 | 0.048 | 0.712054 | −89 | −13.2 | 15.8 | 6.1 ± 0.5 | 6178 | 3.8 | 0.9 |
| RSXWQ-05 | 7.85 | 0.72 | 6.28 | 2.82 | 0.032 | 0.712061 | −87 | −13.4 | 15.8 | 5.1 ± 0.5 | 6178 | 3.6 | 0.7 |
| Sample ID | Calibration Status | SiO2 (mg/L) | Tquartz-no | Tquartz-max | Tcalibrate silicon | Tchalcedony |
|---|---|---|---|---|---|---|
| RSXWQ-01 | Uncorrected State (cold water mixing effect is not eliminated) | 160.1 | 165.3 | 159.8 | 254.4 | 142 |
| RSXWQ-02 | 164.5 | 167 | 161.2 | 260.5 | 144 | |
| RSXWQ-03 | 169 | 168.8 | 162.7 | 266.7 | 146 | |
| RSXWQ-04 | 165 | 167.2 | 161.4 | 261.2 | 144.2 | |
| RSXWQ-05 | 162.4 | 166.2 | 160.5 | 257.6 | 143 | |
| 160.1–169 | 165.3–168.8 | 159.8–162.7 | 254.4–266.7 | 142–146 | ||
| RSXWQ-01 | Corrected state (cold water mixing effect is eliminated) | 500.3 | 254.4 | 230.7 | 681.6 | 245.2 |
| RSXWQ-02 | 548.3 | 260.9 | 237.4 | 738.4 | 255.3 | |
| RSXWQ-03 | 497.1 | 251.8 | 230.2 | 677.8 | 244.5 | |
| RSXWQ-04 | 485.3 | 249.6 | 228.5 | 663.8 | 241.8 | |
| RSXWQ-05 | 464 | 245.6 | 225.3 | 638.4 | 236.9 | |
| 464–500.3 | 245.6–260.9 | 225.3–237.4 | 638.4–738.4 | 236.9–255.3 |
| Sample ID | Na-K | Na-K-Ca | Quartz Temperature Scale | Multimineral Balance | Silicon Enthalpy Model | Na-Li |
|---|---|---|---|---|---|---|
| °C | ||||||
| XSWQ-01 | 227.84 | 206.46 | 254 | 107–333 | 253 | 255.50 |
| XSWQ-02 | 231.14 | 213.19 | 261 | 192–313 | 260 | 260.85 |
| XSWQ-03 | 222.98 | 208.51 | 252 | 125–345 | 250 | 253.78 |
| XSWQ-04 | 233.59 | 213.63 | 249 | - | 247 | 262.53 |
| XSWQ-05 | 234.33 | 213.91 | 246 | - | 244 | 270.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Qin, X.; Ren, E.; Feng, N.; Yang, S.; Li, W.; Li, G.; Jiang, Z. Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters from the Reshui Area, Dulan of Qinghai, China. Water 2023, 15, 3084. https://doi.org/10.3390/w15173084
Wang B, Qin X, Ren E, Feng N, Yang S, Li W, Li G, Jiang Z. Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters from the Reshui Area, Dulan of Qinghai, China. Water. 2023; 15(17):3084. https://doi.org/10.3390/w15173084
Chicago/Turabian StyleWang, Bing, Xiwei Qin, Erfeng Ren, Ning Feng, Sha Yang, Wei Li, Guorong Li, and Ziwen Jiang. 2023. "Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters from the Reshui Area, Dulan of Qinghai, China" Water 15, no. 17: 3084. https://doi.org/10.3390/w15173084
APA StyleWang, B., Qin, X., Ren, E., Feng, N., Yang, S., Li, W., Li, G., & Jiang, Z. (2023). Geochemical Characteristics and Formation Mechanisms of the Geothermal Waters from the Reshui Area, Dulan of Qinghai, China. Water, 15(17), 3084. https://doi.org/10.3390/w15173084








